Heat and Temperature What is Heat n Heat

























- Slides: 39

Heat and Temperature

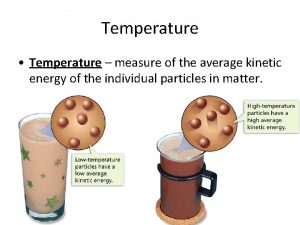
What is Heat? ? n Heat = Thermal Energy!! n Thermal Energy = the total energy of all of the particles in a material or object. n Throughout the ages people have invented a variety of devices to help create and capture heat for use.

Topic 1: Using Energy from Heat n What are some ways that we use heat? n n Cook food Warm buildings Dry clothes What are some ways Thermal Energy has been used throughout history?

Devices to generate, transfer, control or remove heat n Heat = Thermal energy n Can you think of any examples of devices that generate, transfer, control or remove heat?

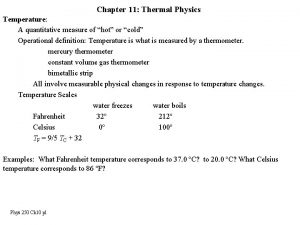
Topic 2: Measuring Temperature n Thermometer: Mechanical or electrical device for measuring temperature. Early thermometer was invented by Galileo. n Scale: A series of equally measured sections that are marked and numbered for use in measurement.

Celsius Scale n Celsius Scale: Most commonly used in Canada. Unit of temperature is called a degree. Based on the boiling and freezing points of water. n Boiling Point: The temperature at which water boils. 100 o C at sea level. n Freezing Point: The temperature at which water freezes. 0 o C at sea level.

Another Scale… n n Kelvin is another way of measuring temperature. Scientists use Kelvin to explain the behaviour of gases. “Absolute Zero” is measured in Kelvin – which is the coldest possible temperature 0 Kelvin = -273 ºC

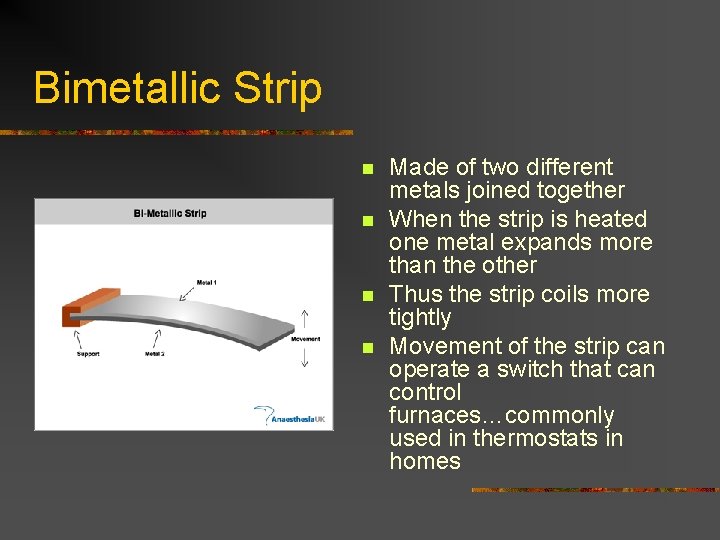
Bimetallic Strip n n Made of two different metals joined together When the strip is heated one metal expands more than the other Thus the strip coils more tightly Movement of the strip can operate a switch that can control furnaces…commonly used in thermostats in homes

Your Brain…(extra) n n Your brain has its own temperature sensor. It monitors your own internal temperature. If the temperature outside changes, the sensor signals your brain to release chemicals that will help your body adjust to normal temperature (37°C)

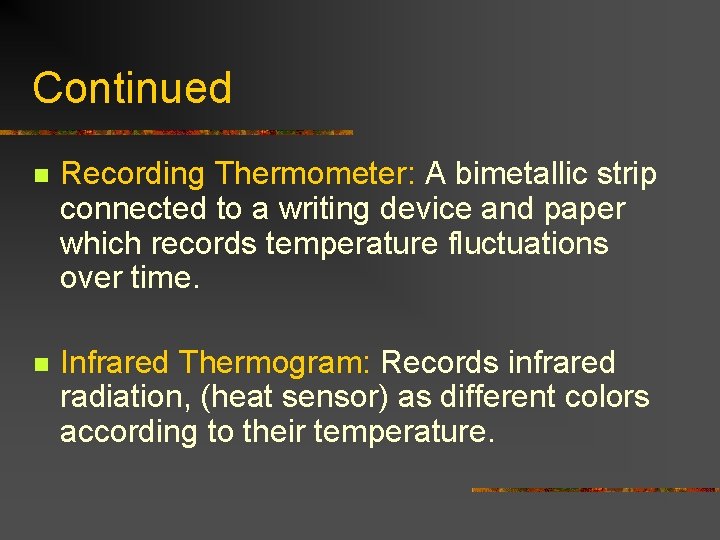
Continued n Recording Thermometer: A bimetallic strip connected to a writing device and paper which records temperature fluctuations over time. n Infrared Thermogram: Records infrared radiation, (heat sensor) as different colors according to their temperature.

Topic 3: Particle Model of Matter, Temperature and Thermal Energy n n Reminder: matter is anything that takes up space Three most important ideas of the model: n n n All substances are made of particles too small to see The particles are always in motion The particles have space between them

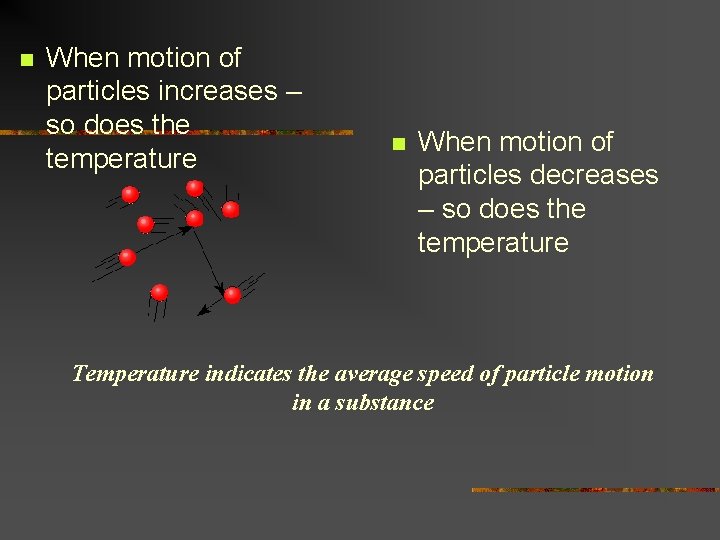
n When motion of particles increases – so does the temperature n When motion of particles decreases – so does the temperature Temperature indicates the average speed of particle motion in a substance

Energy n n n Energy is the ability to do work – in other words to cause change In order for something to change, there must be a transfer of energy from one thing to another Ex. Charged batteries run your i. Pod, dead batteries would not

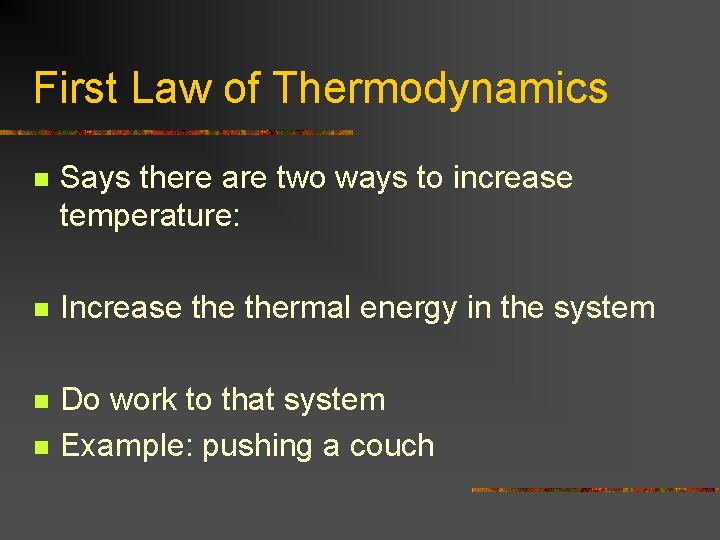
First Law of Thermodynamics n Says there are two ways to increase temperature: n Increase thermal energy in the system n Do work to that system Example: pushing a couch n

Second Law of Thermodynamics n Thermal energy spontaneously spreads from regions of high concentration to areas of low concentration. n Basically heat moves to cold places.

Entropy n The the measure of randomness in a system. n A lot of energy goes into a system, but very little energy comes out, it is transferred chaotically. Example: building a house of cards n

What Energy is…and is not n n Energy is not a substance. It cannot be weighed It does not take up space Energy describes a condition Law of Conservation of Energy: Energy cannot be created or destroyed. It can only be transformed from one type to another or passed from one object to another

Temperature vs. Thermal Energy n Temperature = A measure of the average energy of the particles in a material. n Thermal Energy = The total energy of all the particles in a material. What is the difference? ?

Topic 4: Expansion and Contraction n n Contract: Decrease in volume Expand: Increase in volume n Temperature changes cause things to expand contract n n Heated – usually causes expansion Cooled – usually causes contraction n Usually more drastic in gases, then liquids then solids

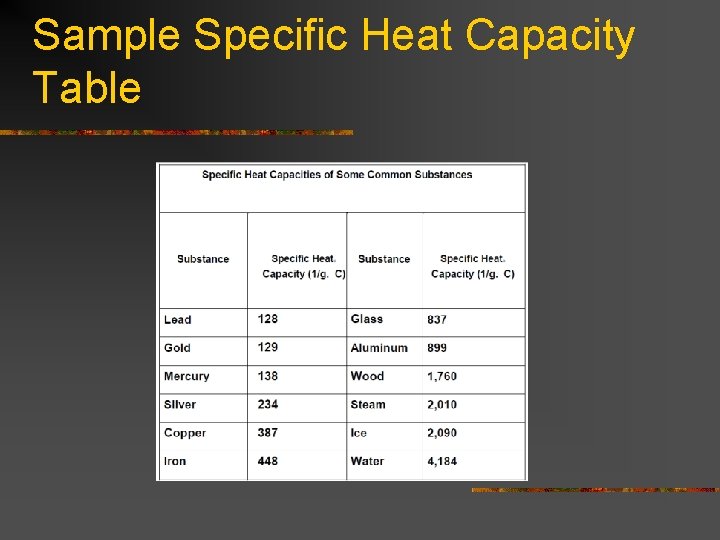
Specific Heat Capacity n Amount of thermal energy that warms or cools one gram of a material by one degree Celsius.

Sample Specific Heat Capacity Table

Solids n Solids have definite shape and volume n Cannot be compressed into smaller objects n When solids are heated – they expand When solids are cooled – they contract n

Liquids n Liquids have definite volume but no shape n Cannot be compressed (meaning if I have 1 litre of coke, I cannot make it fit into a pop can) n When liquids are heated – they expand When liquids are cooled – they contract n

Gases n Have no definite shape or size n Can be compressed n When heated, gases – expand When cooled, gases - contract n

Changes of State

Definitions: n Define, in your notes, each of the following: n Melt n Freeze n Evaporate n Condense n Sublimation

Continued n Evaporative Cooling: A process in which the faster moving particles on the surface of a liquid evaporate and escape into the air, the slower ones are left behind creating a lower average kinetic energy (cooling it) n Particles are more or less organized when they are hot or cold? ?

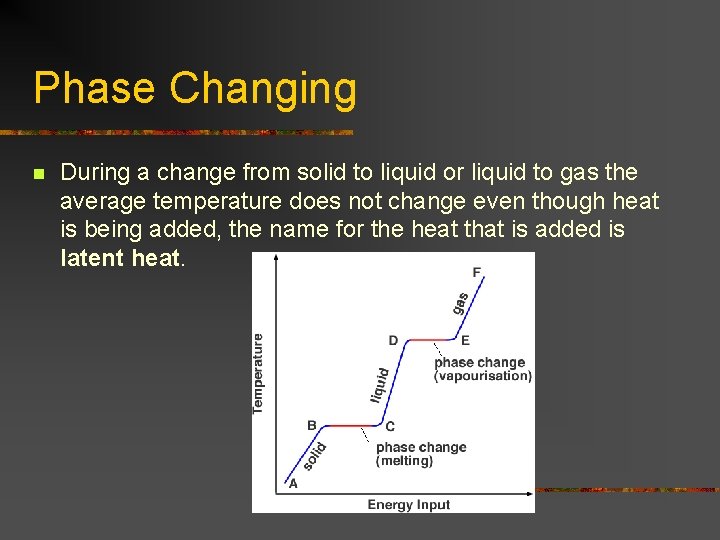
Phase Changing n During a change from solid to liquid or liquid to gas the average temperature does not change even though heat is being added, the name for the heat that is added is latent heat.

Radiant Energy All forms of radiant energy share several characteristics: - They behave like waves - They can be absorbed and reflected by objects - They travel across empty space at the same high speed of 300, 000 km/s n

Topic 7: Sources of Thermal Energy n Energy appears in many forms n Potential Energy: Stored energy n E. g. elastic pulled back and ready to be shot. n Kinetic Energy: Energy of motion n Examples? ?

Chemical Energy n Stored chemical energy is released in the form of thermal energy when it is burned.

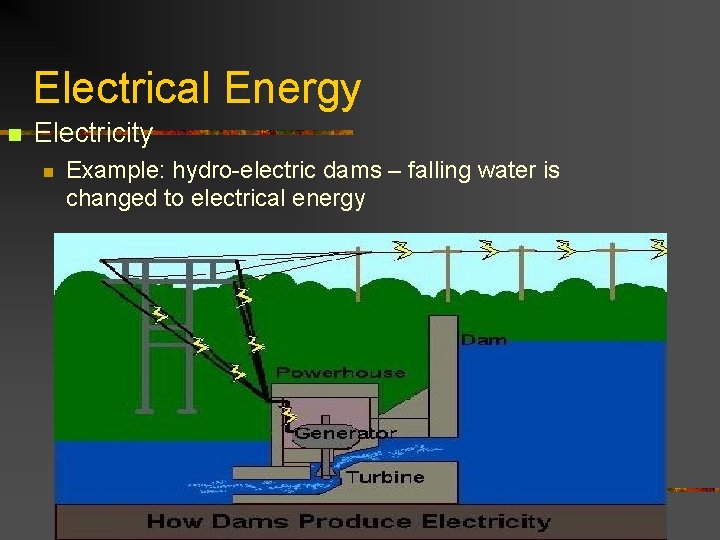
Electrical Energy n Electricity n Example: hydro-electric dams – falling water is changed to electrical energy

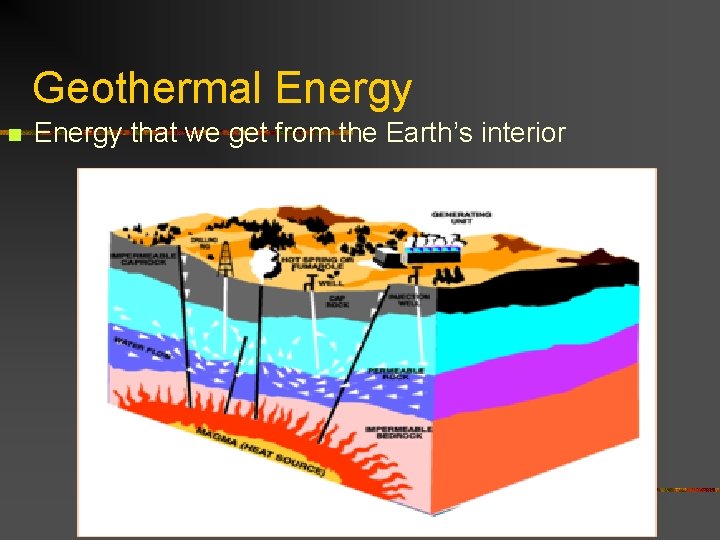
Geothermal Energy n Energy that we get from the Earth’s interior

Solar Energy n Energy from the sun

Passive Solar Heating n Uses materials in the structure to absorb, store, and release solar energy. n Example: a wall of windows

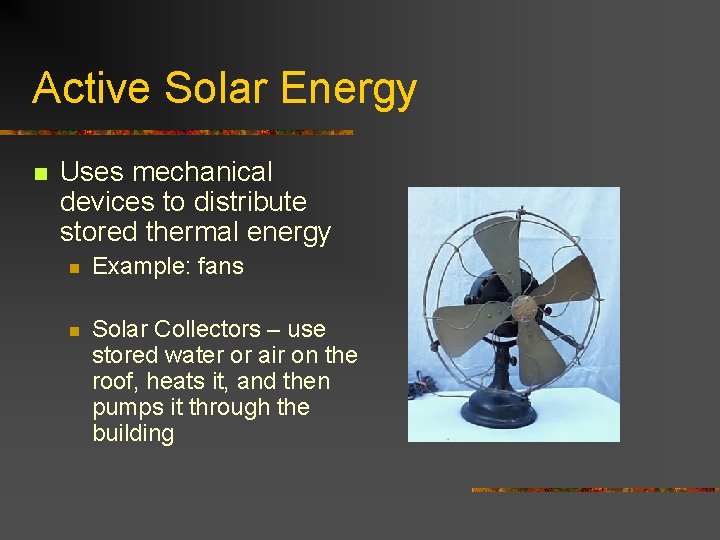
Active Solar Energy n Uses mechanical devices to distribute stored thermal energy n Example: fans n Solar Collectors – use stored water or air on the roof, heats it, and then pumps it through the building

Wind Energy n n Moving air Is a result of solar energy – as the sun heats the air, the warmer air rises and cools off. Cooler air falls, creating a convection current – this forms wind

Fossil Fuels n Chemicals made from plants and animals that died and decomposed millions of years ago and have preserved deep underground.

Energy Converters n Energy can be converted into another form. n For example a candle can convert chemical energy into heat and light energy. n Candles are energy converters (devices which convert or change energy from one form to another. ) n Other examples? ?
 Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
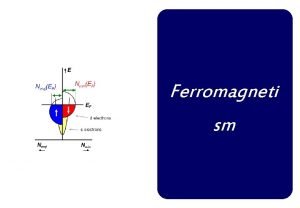
Difference between curie temperature and neel temperature Ferromagneti
Ferromagneti Heat thermal energy and temperature
Heat thermal energy and temperature Heat transfer types
Heat transfer types Chapter 21 temperature heat and expansion answer key
Chapter 21 temperature heat and expansion answer key Heat thermal energy and temperature
Heat thermal energy and temperature The difference between heat and temperature
The difference between heat and temperature Temperature and heat
Temperature and heat How to define heat
How to define heat Difference between heat and temperature
Difference between heat and temperature Chapter 15 temperature heat and expansion
Chapter 15 temperature heat and expansion Si unit of specific heat capacity
Si unit of specific heat capacity Chapter 21 temperature, heat and expansion answer key
Chapter 21 temperature, heat and expansion answer key Chapter 21 temperature heat and expansion answer key
Chapter 21 temperature heat and expansion answer key Heat and temperature relationship
Heat and temperature relationship Physics heat and temperature
Physics heat and temperature A double pipe parallel flow heat exchanger
A double pipe parallel flow heat exchanger Examples of convection
Examples of convection Heat and temperature venn diagram
Heat and temperature venn diagram Chapter 14 section 1 heat and temperature answers
Chapter 14 section 1 heat and temperature answers Chapter 21 temperature heat and expansion
Chapter 21 temperature heat and expansion Chapter 21 temperature heat and expansion
Chapter 21 temperature heat and expansion Differentiate between heat and temperature
Differentiate between heat and temperature Heat deflection temperature
Heat deflection temperature Heat vs temperature
Heat vs temperature Heat vs temperature
Heat vs temperature Thermal energy vs heat
Thermal energy vs heat Is temperature a quantitative measure of heat
Is temperature a quantitative measure of heat Steam cycle
Steam cycle Is temperature a measure of thermal energy
Is temperature a measure of thermal energy Define specific latent heat
Define specific latent heat Specific heat capacity of lead j/kg c
Specific heat capacity of lead j/kg c Typically involves high heat with 300°f or hotter
Typically involves high heat with 300°f or hotter Negative feedback and body temperature regulation
Negative feedback and body temperature regulation Temperature vs volume
Temperature vs volume Latitude and temperature relationship
Latitude and temperature relationship Charles law constant
Charles law constant N in ideal gas law
N in ideal gas law Axillary temperature abbreviation
Axillary temperature abbreviation