Specific Heat HEAT SPECIFIC HEAT Specific heat PHASE






















- Slides: 34

Specific Heat HEAT SPECIFIC HEAT Specific heat PHASE CHANGES • Is different for different substances. • Is the amount of heat that raises the temperature Mr. Rex Angel G. Asuncion of 1 g of a substance by 1°C. • In the SI system has units of J/g C. • In the metric system has units of cal/g C. 1

Specific Heat (table 38 p. 223) • cp reflects that ability of a substance to absorb heat (defined as the amount of heat needed to raise the temperature of 1 gram by 1 degree Celsius) • cp of water = 1. 00 cal/g° C or 4. 185 J/g ° C • In most situations it is the temperature change of the surroundings that is measured (which equals the heat releases/absorbed 2 from the reaction itself)

Different Substances Absorb Different Amounts of Energy Objects with high specific heat absorb and store more heat energy with less increase in temperature than objects with a lower specific heat. This means an object with a higher specific heat changes temperature more S L O W L Y.

Specific Heat a. Some things heat up or cool down faster than others. Land heats up and cools down faster than water

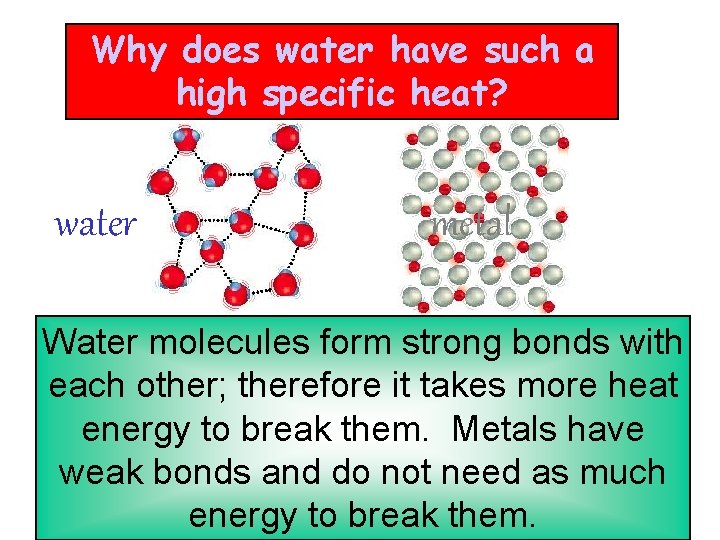
Why does water have such a high specific heat? water metal Water molecules form strong bonds with each other; therefore it takes more heat energy to break them. Metals have weak bonds and do not need as much energy to break them.
HEAT CAPACITY- amount of heat needed required to raise the temperature of a substance by one degree. It is the product of the mass of the substance and its sepecific heat. (cal/Co) *same objects=same specific heat but not heat capacity (dependent on mass). E. g. 100 g Al vs 300 g Al, C of Al = 0. 22 cal/g Co; [22 cal/g vs 66 cal/g]

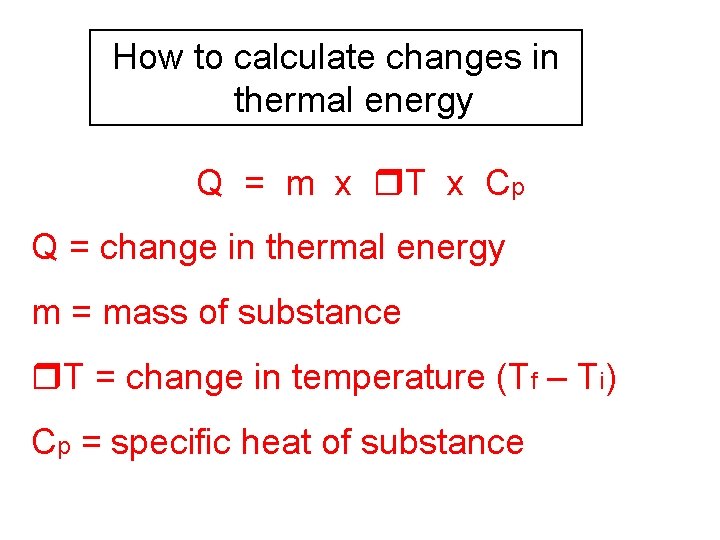
How to calculate changes in thermal energy Q = m x T x Cp Q = change in thermal energy m = mass of substance T = change in temperature (Tf – Ti) Cp = specific heat of substance

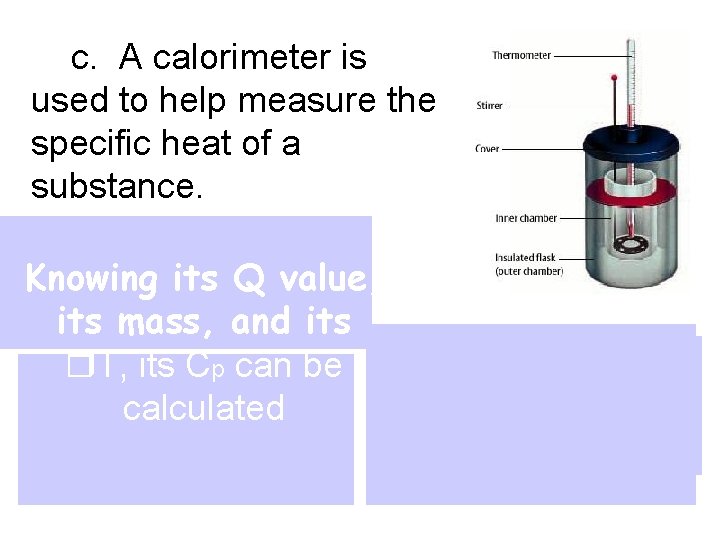
c. A calorimeter is used to help measure the specific heat of a substance. First, mass and Knowing its Q value, temperature of its mass, and its water are measured Then heated This gives the T, its Cp can be T is measured sample is put heat lost by the calculated for water to help inside and heat substance get its heat gain flows into water

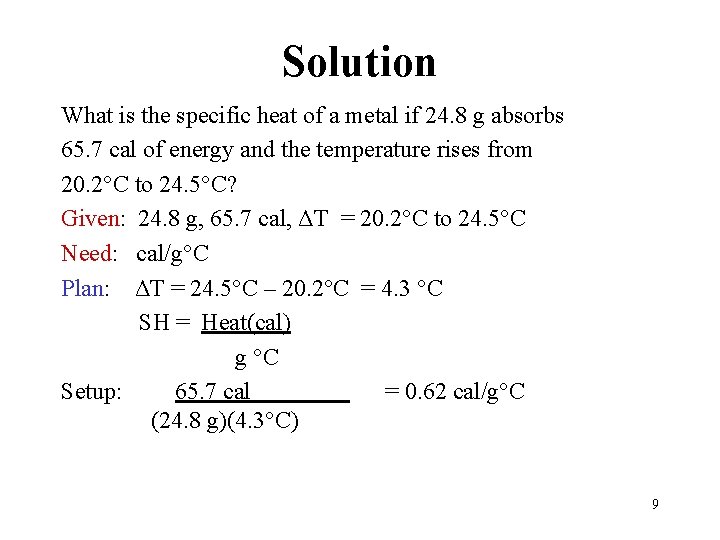
Solution What is the specific heat of a metal if 24. 8 g absorbs 65. 7 cal of energy and the temperature rises from 20. 2 C to 24. 5 C? Given: 24. 8 g, 65. 7 cal, ΔT = 20. 2 C to 24. 5 C Need: cal/g C Plan: ΔT = 24. 5 C – 20. 2 C = 4. 3 C SH = Heat(cal) g C Setup: 65. 7 cal = 0. 62 cal/g C (24. 8 g)(4. 3 C) 9

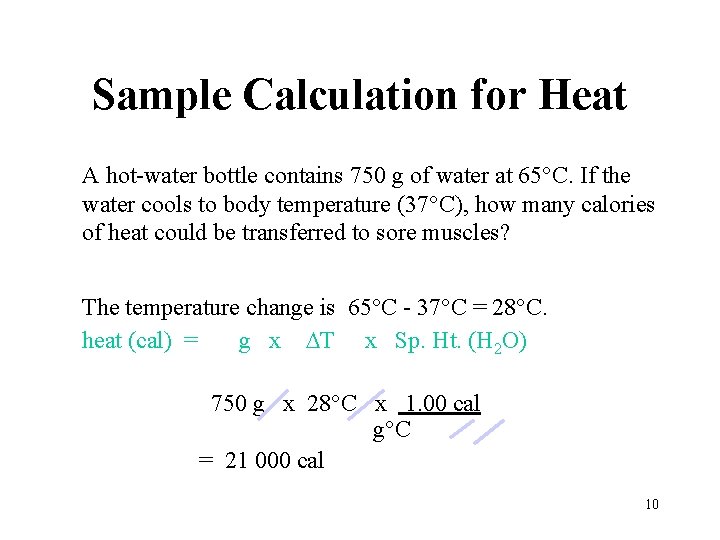
Sample Calculation for Heat A hot-water bottle contains 750 g of water at 65°C. If the water cools to body temperature (37°C), how many calories of heat could be transferred to sore muscles? The temperature change is 65°C - 37°C = 28°C. heat (cal) = g x T x Sp. Ht. (H 2 O) 750 g x 28°C x 1. 00 cal g°C = 21 000 cal 10

EVALUATE The specific heat for a marble bench is 880 J/Kg˚C while the specific heat for a wooden bench is 1700 J/Kg˚C. Which bench would you rather sit on at 12 noon? Why?

Question The specific heat of sand is 835 J/Kg˚C. The specific heat of water is 4180 J/Kg˚C. Which substance would you rather touch your entire body at 12 noon? Why?

Question If the specific heat of water is 4180 J/Kg˚C and the specific heat of vegetable oil is 810 J/Kg˚C, what would happen if the ocean were made of vegetable oil?

HEAT OF PHASE CHANGE • When 2 or more objects initially at diff temp are placed in contact , heat will flow from the hot objects to the cold objects until thermal equil is established and all bodies have common temp.

Principle of Heat Exchange • The amount of heat lost by a substance is equal to the amount of heat gained by the substance to which it is transferred. • m x ∆t x cp = m x ∆t x cp heat lost heat gained *change in state is never accompanied by change in temperature. Latent heat- table 39 p. 226

Liquid-Solid Phase Change • Melting-freezing point – this is the same temperature at which a pure substance can change into solid from liquid or solid into liquid. • The solid-liquid phases are in equilibrium. 16

Melting-Freezing Point • When heat is added to a solid, the temp. will increase till it reaches the melting-freezing point. It will remain at that temp. until all the solid has melted and then the temp. can rise again according to its specific heat. 17

Liquid – Gas Phase Change • Boiling point is defined as the temperature at which the liquid’s vapor pressure is equal to outside (atmospheric pressure usually). • When the vapor pressure equal atmospheric pressure as many mlcl are leaving the surface as are re-entering the surface of the liquid. 18

Boiling Point • Boiling point varies with elevation. • Cooking times must adjust due to elevation. • Pressure cookers can cook food more rapidly due to increased pressure, resulting in high boiling points (which cooks food faster). 19

Sublimation • Sublimation is the direct change of a solid to a gas • Deposition is the change of a gas to a solid • Examples: moth balls (naphthalein), paradichlorobenzene, camphor, iodine crystals, CO 2 fire extinguishers 20

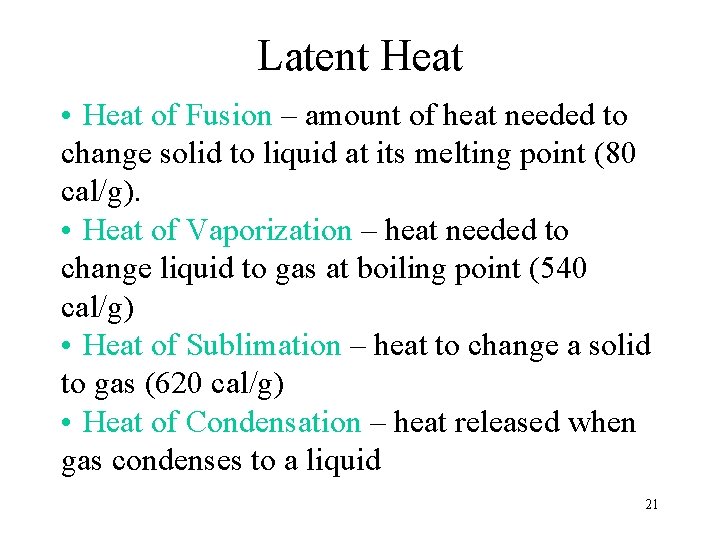
Latent Heat • Heat of Fusion – amount of heat needed to change solid to liquid at its melting point (80 cal/g). • Heat of Vaporization – heat needed to change liquid to gas at boiling point (540 cal/g) • Heat of Sublimation – heat to change a solid to gas (620 cal/g) • Heat of Condensation – heat released when gas condenses to a liquid 21

Examine this: • 1 g of ice at -10 o. C converted into a steam at 110 o. C Trace the transformation that occurs: Ice (-10 o. C) – ice (0 o. C)-water(0 o. C) –water (100 o. C ) – steam (100 o. C) – steam ( 110 o. C)

CONCEPT: Heat is given off during evaporation; hence it produces a cooling effect Steam burn is more damaging than a burn from boiling water of the same mass and temperature. Steam at 100 o. C can produce more severe burn than boiling water at 100 o. C because it is 540 calories hotter than boiling water.

PROBLEMS • Heat and Specific Heat

Learning Check A. A substance with a large specific heat 1) has a smaller increase in temperature 2) has a greater increase in temperature B. When ocean water cools, the surrounding air 1) cools 2) warms 3) stays the same C. Sand in the desert is hot in the day, and cool at night. Sand must have a 1) high specific heat 2) low specific heat 25

Solution A. A substance with a large specific heat 2) has a smaller increase in temperature B. When ocean water cools, the surrounding air 2) warms C. Sand in the desert is hot in the day, and cool at night. Sand must have a 2) low specific heat 26

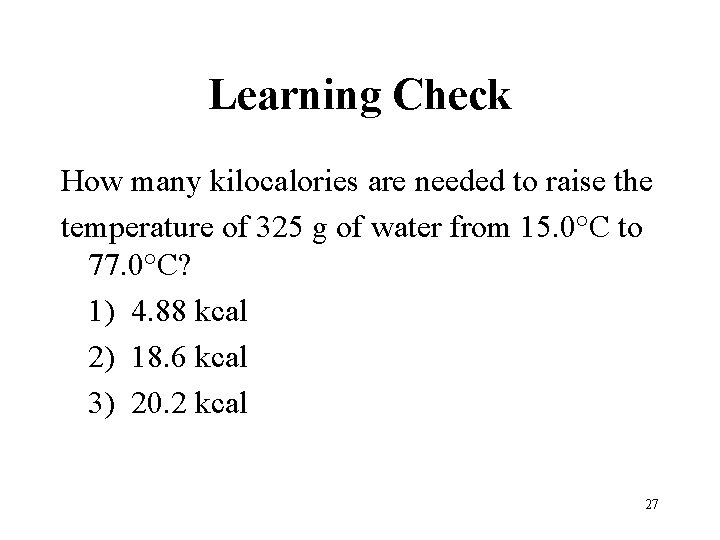
Learning Check How many kilocalories are needed to raise the temperature of 325 g of water from 15. 0°C to 77. 0°C? 1) 4. 88 kcal 2) 18. 6 kcal 3) 20. 2 kcal 27

Problems How much heat is needed to raise the temperature of 5 kg of water 10 K? What is the temperature of the mixture if 0. 4 kg of water at 85 o. C is added to 0. 3 kg of water at 15 o. C in a polysterene cup?

Problems What is the mass of a piece of copper if it loses 368 cal as it cools from 75. 3 o. C to 24. 2 o. C? How much energy is needed to melt 2 kg of ice at its melting point?

Problems: Suppose your body has a mass of 60 kg and that your daily food intake is 1500 cal. If this energy was all used to heat your body, how much would it raise the temperature of your body in a day? For heat capacity purposes, your body behaves much like water.

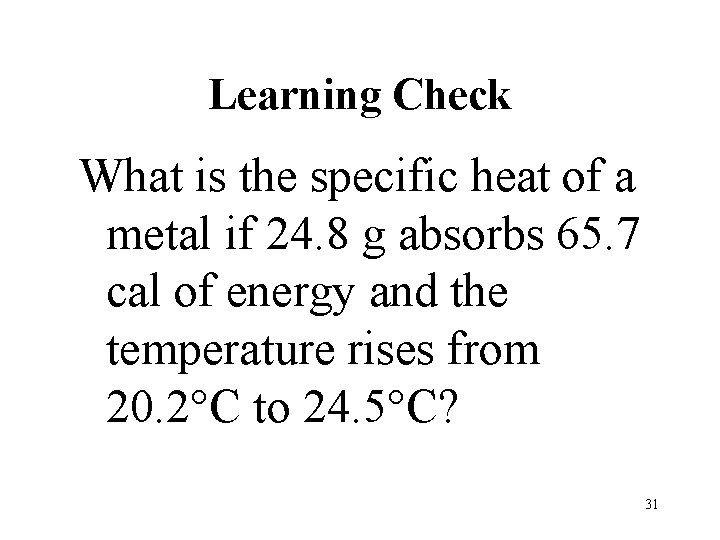
Learning Check What is the specific heat of a metal if 24. 8 g absorbs 65. 7 cal of energy and the temperature rises from 20. 2 C to 24. 5 C? 31

Method of Mixtures A glass weighing 30 g contains 85 g of water at 25 o. C. How much ice at 0 o. C will be needed to bring the temperature down to 5 o. C? The specific heat of glass is 0. 16 cal/g and that of water is 1. 00 cal/g

Method of Mixtures In a calorimetry experiment, 0. 225 kg of copper shot at 99. 5 o. C is added to 65. 5 g Aluminum cup which contains 0. 025 kg of water at 20. 5 o. C. What is the final temperature of the mixture? (Ccu= 385 J kg/K).

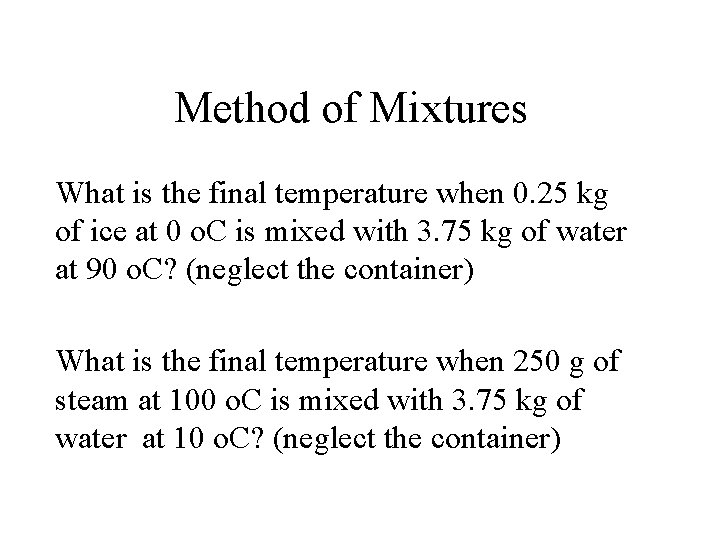
Method of Mixtures What is the final temperature when 0. 25 kg of ice at 0 o. C is mixed with 3. 75 kg of water at 90 o. C? (neglect the container) What is the final temperature when 250 g of steam at 100 o. C is mixed with 3. 75 kg of water at 10 o. C? (neglect the container)