HPLC Introduction DISCOVERY M S Tswett 1903 Warsaw





- Slides: 16

HPLC Introduction

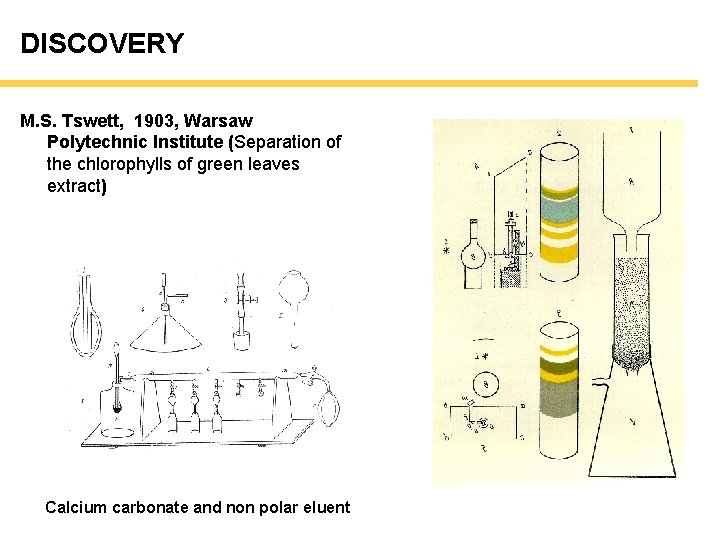
DISCOVERY M. S. Tswett, 1903, Warsaw Polytechnic Institute (Separation of the chlorophylls of green leaves extract) Calcium carbonate and non polar eluent

HPLC Introduction

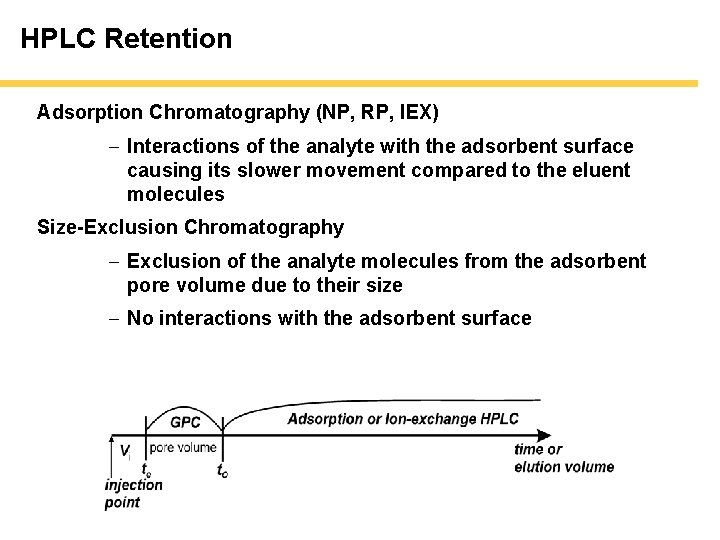
HPLC Retention Adsorption Chromatography (NP, RP, IEX) - Interactions of the analyte with the adsorbent surface causing its slower movement compared to the eluent molecules Size-Exclusion Chromatography - Exclusion of the analyte molecules from the adsorbent pore volume due to their size - No interactions with the adsorbent surface

NORMAL PHASE Principle: Adsorption of analytes on the polar, weakly acidic surface of silica gel. Stationary Phase. : Silica (p. H 2 -8), Alumina (p. H 2 - 12), Bonded Diol, and NH 2, or CN Mobile Phase: Non-polar solvents (Hexane, CHCl 3) Applications: Non-polar and semi-polar samples; hexane soluble; positional isomers. 1980 2004 30% 5% 47% 44% Silica Gel Silica based bonded phases • Diol • Amino • Cyano 3% 1% Alumina 20% 50% Chiral Bonded Phases

NP: SEPARATION PRINCIPLE Polar (specific but nonionic) interactions of analyte with polar adsorption sites (Si. OH, -NH 2, -CN, Diol) cause its retention Different sorption affinities between analytes result in their separation - More polar analytes retained longer - Analytes with larger number of polar functional group are retained longer - Structural isomers are often separated

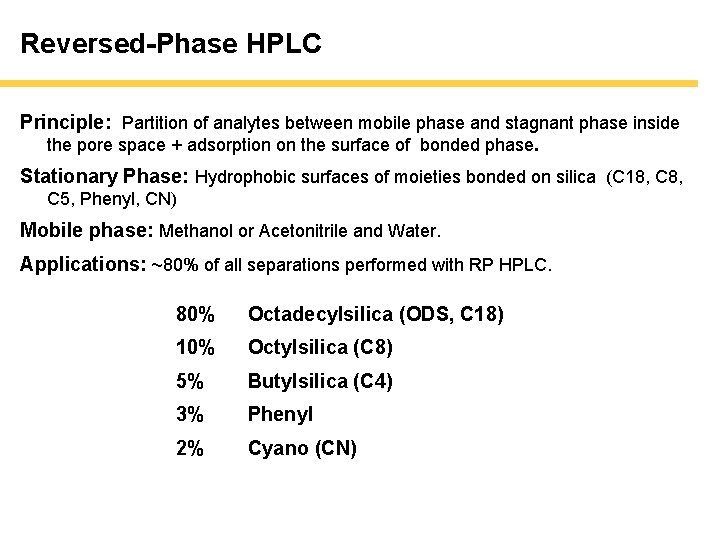
Reversed-Phase HPLC Principle: Partition of analytes between mobile phase and stagnant phase inside the pore space + adsorption on the surface of bonded phase. Stationary Phase: Hydrophobic surfaces of moieties bonded on silica (C 18, C 5, Phenyl, CN) Mobile phase: Methanol or Acetonitrile and Water. Applications: ~80% of all separations performed with RP HPLC. 80% Octadecylsilica (ODS, C 18) 10% Octylsilica (C 8) 5% Butylsilica (C 4) 3% Phenyl 2% Cyano (CN)

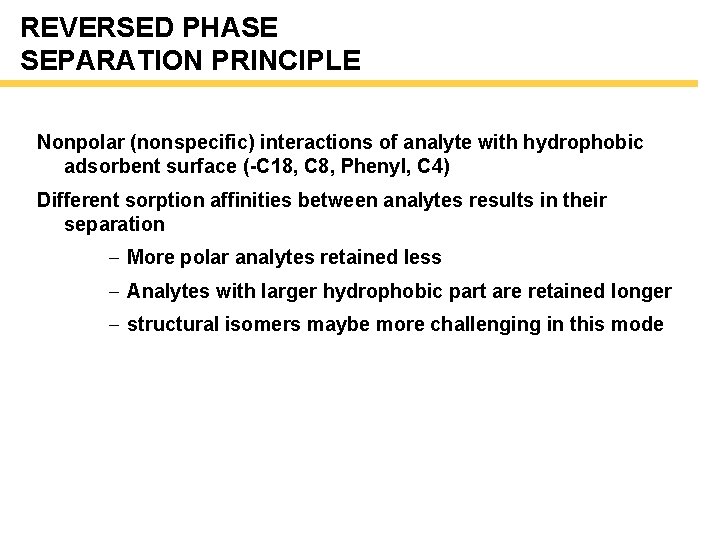
REVERSED PHASE SEPARATION PRINCIPLE Nonpolar (nonspecific) interactions of analyte with hydrophobic adsorbent surface (-C 18, C 8, Phenyl, C 4) Different sorption affinities between analytes results in their separation - More polar analytes retained less - Analytes with larger hydrophobic part are retained longer - structural isomers maybe more challenging in this mode

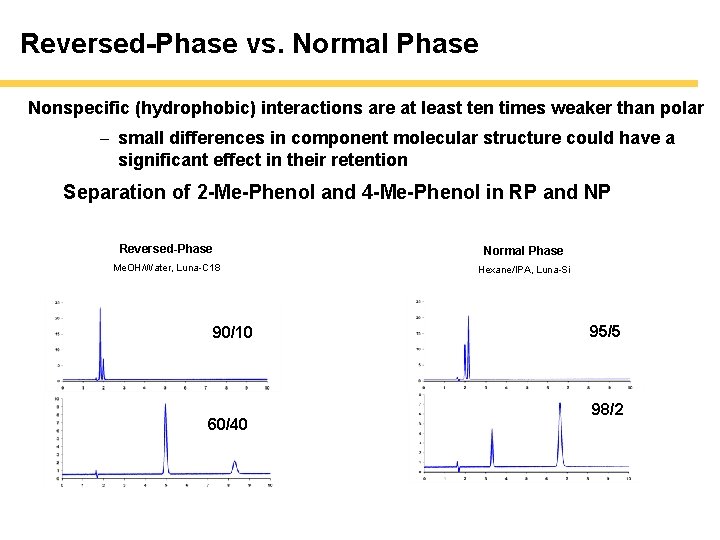
Reversed-Phase vs. Normal Phase Nonspecific (hydrophobic) interactions are at least ten times weaker than polar - small differences in component molecular structure could have a significant effect in their retention Separation of 2 -Me-Phenol and 4 -Me-Phenol in RP and NP Reversed-Phase Normal Phase Me. OH/Water, Luna-C 18 Hexane/IPA, Luna-Si 90/10 60/40 95/5 98/2

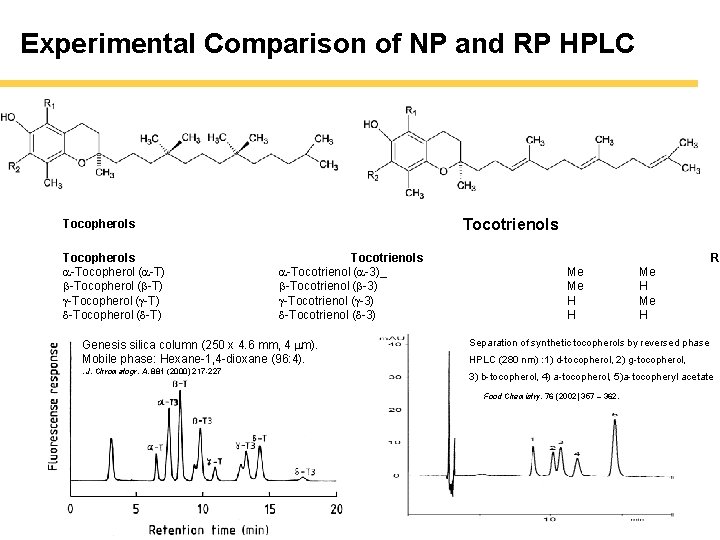
Experimental Comparison of NP and RP HPLC Tocotrienols Tocopherols a-Tocopherol (a-T) b-Tocopherol (b-T) g-Tocopherol (g-T) d-Tocopherol (d-T) Tocotrienols a-Tocotrienol (a-3)_ b-Tocotrienol (b-3) g-Tocotrienol (g-3) d-Tocotrienol (d-3) Me Me H H Me H R 1 Genesis silica column (250 x 4. 6 mm, 4 m). Mobile phase: Hexane-1, 4 -dioxane (96: 4). Separation of synthetic tocopherols by reversed phase . J. Chromatogr. A, 881 (2000) 217 -227 3) b-tocopherol, 4) a-tocopherol, 5)a-tocopheryl acetate HPLC (280 nm) : 1) d-tocopherol, 2) g-tocopherol, Food Chemistry, 76 (2002) 357 – 362.

Ion Exchange Principle: Reversible adsorption of ions on S. P. with oppositely charged functional groups. Anionic polymers are known as cation exchange resins and these resins can be strong or weak cation exchange resins which are strongly dependent upon the anionic group that is bonded to the polymer. Cationic polymers on the other hand are known as anion exchange resins and these resins can also be weak or strong anion exchange. Stationary Phase: · For cations (cation exchange) - SO 3 - (strong), CO 2 - (weak) Anionic polymer · For anions (anion exchange)- NR 4+ (strong), NH 3+ (weak) Cationic polymer Mobile Phase: Aqueous buffer with p. H and buffer strength carefully controlled. Applications: All ionic compounds common anions, cations, sugars, amines, etc.

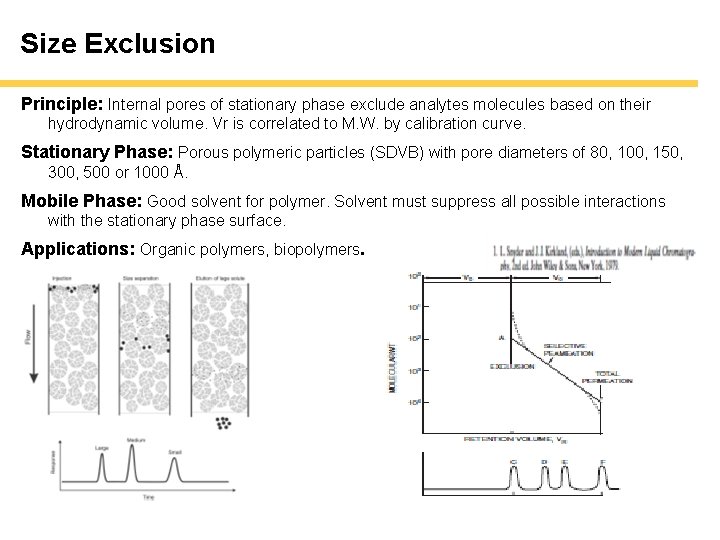
Size Exclusion Principle: Internal pores of stationary phase exclude analytes molecules based on their hydrodynamic volume. Vr is correlated to M. W. by calibration curve. Stationary Phase: Porous polymeric particles (SDVB) with pore diameters of 80, 100, 150, 300, 500 or 1000 Å. Mobile Phase: Good solvent for polymer. Solvent must suppress all possible interactions with the stationary phase surface. Applications: Organic polymers, biopolymers.

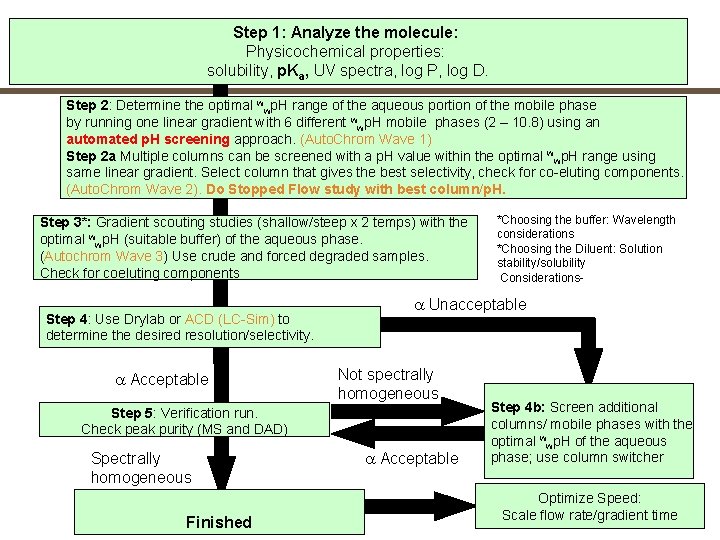
Step 1: Analyze the molecule: Physicochemical properties: solubility, p. Ka, UV spectra, log P, log D. Step 2: Determine the optimal wwp. H range of the aqueous portion of the mobile phase by running one linear gradient with 6 different wwp. H mobile phases (2 – 10. 8) using an automated p. H screening approach. (Auto. Chrom Wave 1) Step 2 a Multiple columns can be screened with a p. H value within the optimal wwp. H range using same linear gradient. Select column that gives the best selectivity, check for co-eluting components. (Auto. Chrom Wave 2). Do Stopped Flow study with best column/p. H. Step 3*: Gradient scouting studies (shallow/steep x 2 temps) with the optimal wwp. H (suitable buffer) of the aqueous phase. (Autochrom Wave 3) Use crude and forced degraded samples. Check for coeluting components Step 4: Use Drylab or ACD (LC-Sim) to determine the desired resolution/selectivity. a Acceptable a Unacceptable Not spectrally homogeneous Step 5: Verification run. Check peak purity (MS and DAD) Spectrally homogeneous Finished *Choosing the buffer: Wavelength considerations *Choosing the Diluent: Solution stability/solubility Considerations- a Acceptable Step 4 b: Screen additional columns/ mobile phases with the optimal wwp. H of the aqueous phase; use column switcher Optimize Speed: Scale flow rate/gradient time

Published Jan. 2, 2007

PART I HPLC THEORY AND PRACTICE. 1 Introduction (Yuri Kazakevich and Rosario Lo. Brutto). 2 HPLC Theory (Yuri Kazakevich). 3 Stationary Phases (Yuri Kazakevich and Rosario Lo. Brutto). 4 Reversed-Phase HPLC (Rosario Lo. Brutto and Yuri Kazakevich). 5 Normal-Phase HPLC (Yong Liu and Anant Vailaya). 6 Size-Exclusion Chromatography (Yuri Kazakevich and Rosario Lo. Brutto). 7 LC/MS: Theory, Instrumentation, and Applications to Small Molecules (Guodong Chen, Li-Kang Zhang, and Birendra N. Pramanik). 8 Method Development (Rosario Lo. Brutto). . 9 Method Validation (Rosario Lo. Brutto and Tarun Patel). . 10 Computer-Assisted HPLC and Knowledge Management (Yuri Kazakevich, Michael Mc. Brien, and Rosario Lo. Brutto). PART II HPLC IN THE PHARMACEUTICAL INDUSTRY. 11 The Expanding Role of HPLC in Drug Discovery (Daniel B. Kassel). 12 Role of HPLC in Preformulation (Irina Kazakevich). 13 The Role of Liquid Chromatography–Mass Spectrometry in Pharmacokinetics and Drug Metabolism (Ray Bakhtiar, Tapan K. Majumdar, and Francis L. S. Tse). 14 Role of HPLC in Process Development (Richard Thompson and Rosario Lo. Brutto). . 15 Role of HPLC During Formulation Development (Tarun S. Patel and Rosario Lo. Brutto). 16 The Role of HPLC in Technical Transfer and Manufacturing (Joseph Etse). PART III HYPHENATED TECHNIQUES AND SPECIALIZED HPLC SEPARATIONS. 17 Development of Fast HPLC Methods (Anton D. Jerkovich and Richard V. Vivilecchia). 18 Temperature as a Variable in Pharmaceutical Applications (Roger M. Smith). 19 LC/MS Analysis of Proteins and Peptides in Drug Discovery (Guodong Chen, Yan-Hui Liu, and Birendra N. Pramanik). 20 LC-NMR Overview and Pharmaceutical Applications (Maria Victoria Silva Elipe). 21 Trends in Preparative HPLC (Ernst Kuesters). 22 Chiral Separations (Nelu Grinberg, Thomas Burakowski, and Apryll M. Stalcup).

HPLC References • Lo. Brutto, R. *, Kazakevich, Y. V. * “Chaotropic effects in RP-HPLC” (Invited Review) for volume 44 of “The Advances in Chromatography” series, editors, Professor Eli Grushka and Nelu Grinberg (September 2005). • Lo. Brutto, R. Normal Phase Stationary Phases (Encyclopedia chapter), Cazes, J. , Editor, "Encyclopedia of Chromatography", New York, Marcel Dekker, 553 -556 (2001). • Grinberg, N. , Lo. Brutto, R. Efficiency in Chromatography, (Encyclopedia chapter), Cazes, J. , Editor, "Encyclopedia of Chromatography", New York, Marcel Dekker, 274 -276 (2001). • Lo. Brutto, R. , Kazakevich, Y. V. Retention of Ionizable Components in Reversed Phase HPLC (Book Chapter), Practical Problem Solving in HPLC, Wiley-VCH, 122 -158 (2000). • Jerkovich, A. D*, Lo. Brutto, R. , and Vivilecchia, R. V. , The Use of Acquity UPLC™ in Pharmaceutical Development, published in LC-GC, ( 2005 ) • Chan, F, Yeung, L. S, Lo. Brutto, R*, Kazakevich, Y*. Characterization of phenyl-type HPLC adsorbents • Journal of Chromatography A, 1069, Issue 2, April 2005, 217 -224. • Kazakevich, Y*. , Lo. Brutto, R* and Vivilecchia, R. Reversed-Phase HPLC Behavior of Chaotropic Counteranions, Journal of Chromatography, 1064, 9 -18, (2005) • Pan, L, Lo. Brutto, R. *, Kazakevich, Y. *, and Thompson, R. Influence of inorganic mobile phase additives on the retention, efficiency, and peak symmetry of protonated basic compounds in reversed phase liquid chromatography, Journal of Chromatography A, 1049, 63 -73 (2004). • Jones, A. , Lo. Brutto, R*. , Kazakevich, Y. V. * Effect of the counter-anion type and concentration on the HPLC retention of beta-blockers, Journal of Chromatography A, 964, 179 -187 (2002). • Rustamov, I. , Farcas, T. , Ahmed, F. , Chan, F. , Lo. Brutto, R. , Mc. Nair, H. M, . Kazakevich, Y. V. Geometry of Chemically Modified Silica, Journal of Chromatography A, 913, 49 – 63 (2001). • Kazakevich, Y. V. , Lo. Brutto, R. , Chan, F. , Patel, T. Interpretation of the excess adsorption isotherms of organic eluent components on the surface of reversed phase adsorbents: Effect on the analyte retention, Journal of Chromatography A, 913, 75 -87 (2001). • Lo. Brutto, R. , Jones, A. , Kazakevich, Y. V. Effect of counter-anion concentration on HPLC retention of protonated basic analytes, Journal of Chromatography A, 913, 189 – 196 (2001). • Lo. Brutto, R. , Jones, A. , Kazakevich, Y. V. , Mc. Nair, H. M. Effect of the Eluent p. H and Acidic Modifiers on the HPLC Retention of Basic Analytes, Journal of Chromatography A, 913, 173 -187 (2001).