Specific Heat Part 1 Specific Heat Capacity Specific



- Slides: 12

Specific Heat Part 1

Specific Heat Capacity • Specific heat capacity is the amount of energy needed to change the temperature of 1 kg of a substance by 1ºC. • Energy transferred by heat cannot be measured directly. It must be calculated using specific heat, mass, and change in temperature.

Specific Heat Capacity • The amount of energy (in joules) required to raise 1 gram of a substance 1 degree Celsius (sometimes stated with kilojoules and kilograms) • The derived unit is J/go. C (or k. J/kgo. C) • Stated as “Joule per gram times degree Celsius” or “kilojoule per kilogram times degree Celsius” • Metals have low heat capacities – they heat up and cool down quickly • Water has a higher heat capacity – it heats up and cools down slowly

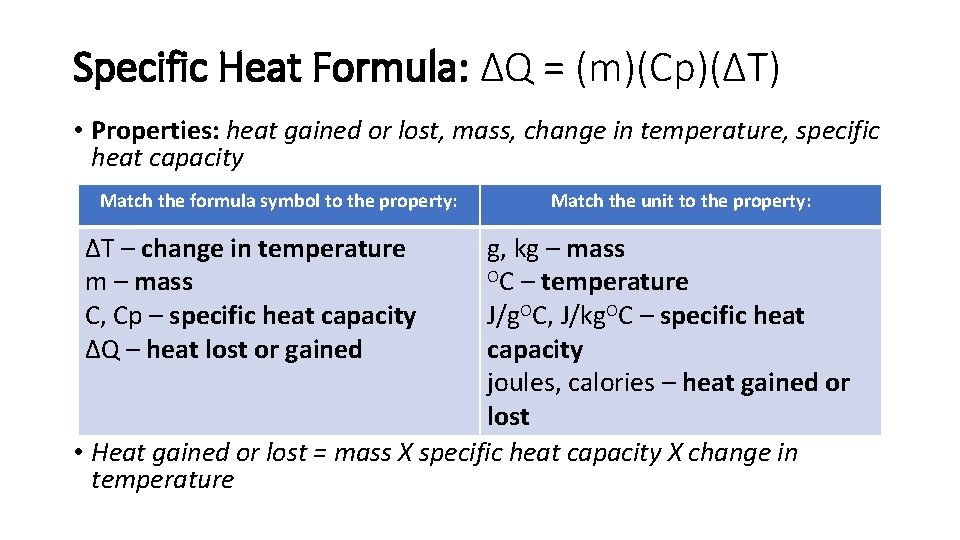
Specific Heat Formula: ΔQ = (m)(Cp)(ΔT) • Properties: heat gained or lost, mass, change in temperature, specific heat capacity Match the formula symbol to the property: ΔT – change in temperature m – mass C, Cp – specific heat capacity ΔQ – heat lost or gained Match the unit to the property: g, kg – mass OC – temperature J/g. OC, J/kg. OC – specific heat capacity joules, calories – heat gained or lost • Heat gained or lost = mass X specific heat capacity X change in temperature

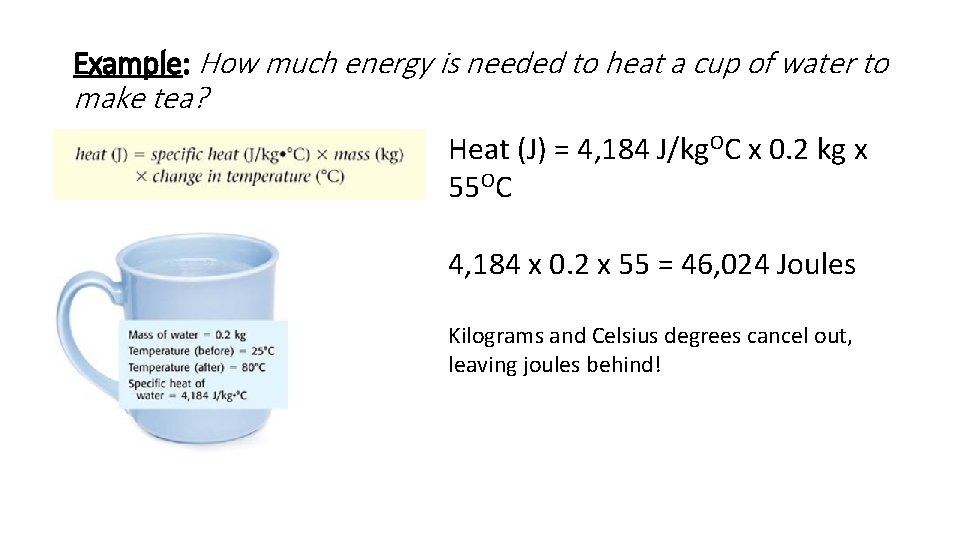
Example: How much energy is needed to heat a cup of water to make tea? Heat (J) = 4, 184 J/kg. OC x 0. 2 kg x 55 OC 4, 184 x 0. 2 x 55 = 46, 024 Joules Kilograms and Celsius degrees cancel out, leaving joules behind!

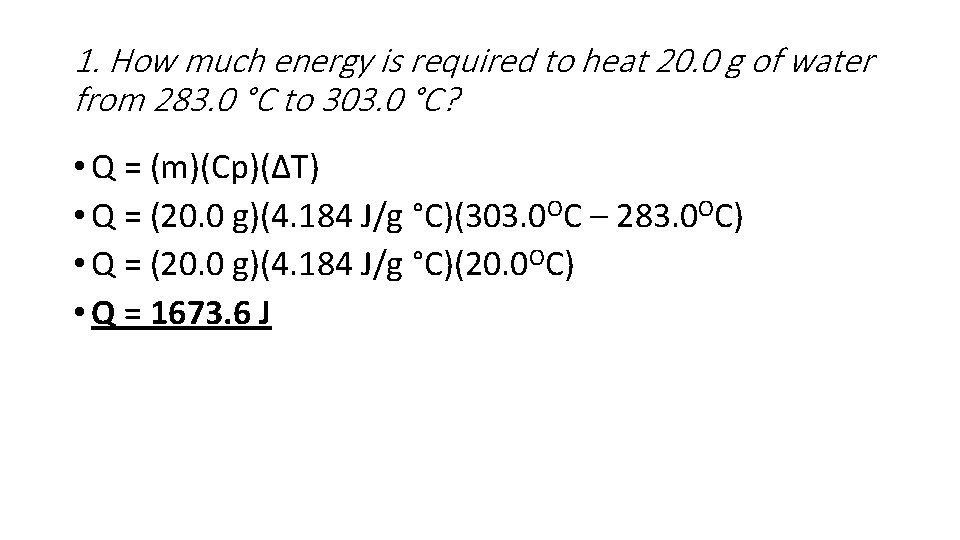
1. How much energy is required to heat 20. 0 g of water from 283. 0 °C to 303. 0 °C? • Q = (m)(Cp)(ΔT) • Q = (20. 0 g)(4. 184 J/g °C)(303. 0 OC – 283. 0 OC) • Q = (20. 0 g)(4. 184 J/g °C)(20. 0 OC) • Q = 1673. 6 J

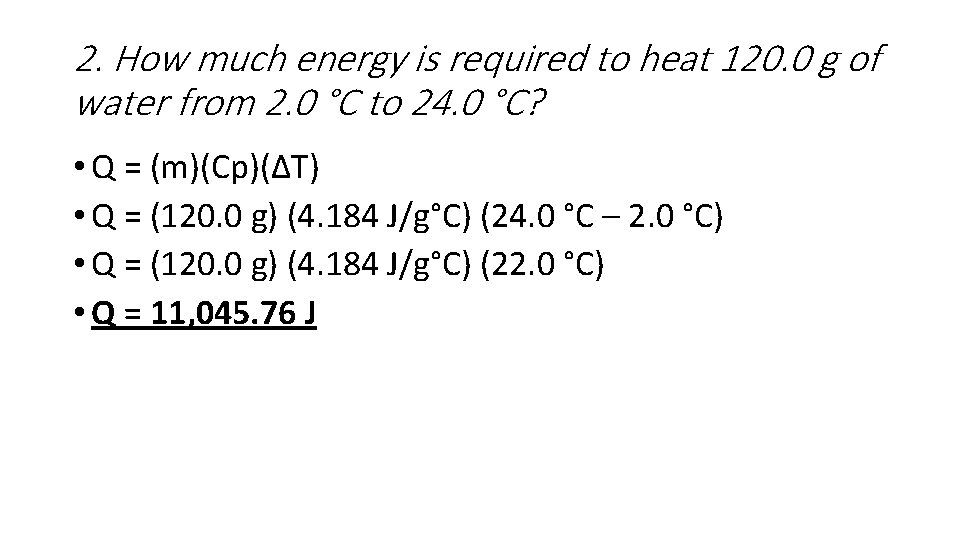
2. How much energy is required to heat 120. 0 g of water from 2. 0 °C to 24. 0 °C? • Q = (m)(Cp)(ΔT) • Q = (120. 0 g) (4. 184 J/g°C) (24. 0 °C – 2. 0 °C) • Q = (120. 0 g) (4. 184 J/g°C) (22. 0 °C) • Q = 11, 045. 76 J

3. How much energy is required to change 50. 0 grams of ice at -15. 0 °C to steam at 120. 0 °C? • Q = (m)(Cp)(ΔT) • Q = (50. 0 g) (4. 184 J/g °C) (120. 0 °C - -15. 0 °C) • Q = (50. 0 g) (4. 184 J/g °C) (135. 0 °C)*Careful! • Q = 28, 242 J

4. How much energy is lost when 15. 0 g of steam cools from 275. 0 °C to 250. 0 °C? • Q = (m)(Cp)(ΔT) • Q = (15. 0 g) (4. 184 J/g °C) (250. 0 °C – 275 °C) • Q = (15. 0 g) (4. 184 J/g °C) (– 25 °C) • Q = - 1569 J

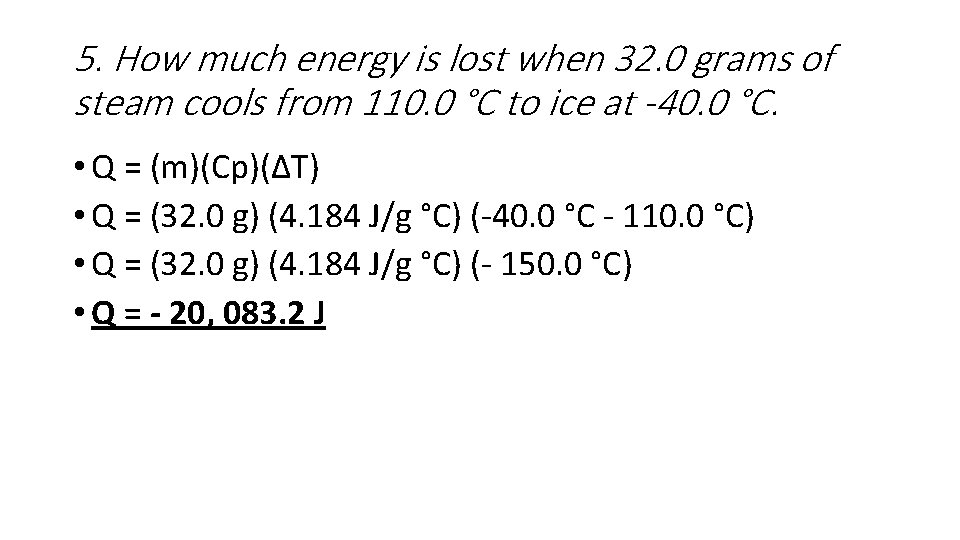
5. How much energy is lost when 32. 0 grams of steam cools from 110. 0 °C to ice at -40. 0 °C. • Q = (m)(Cp)(ΔT) • Q = (32. 0 g) (4. 184 J/g °C) (-40. 0 °C - 110. 0 °C) • Q = (32. 0 g) (4. 184 J/g °C) (- 150. 0 °C) • Q = - 20, 083. 2 J

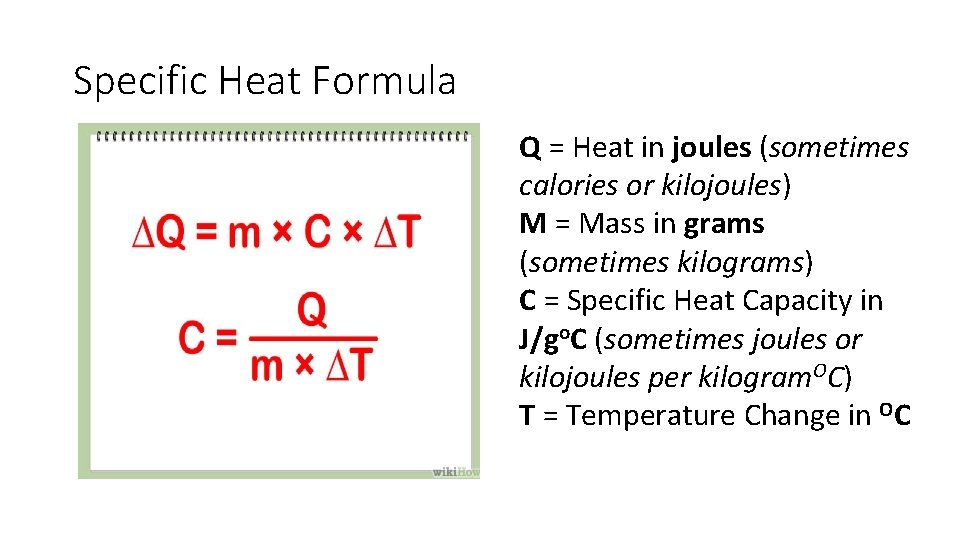
Specific Heat Formula Q = Heat in joules (sometimes calories or kilojoules) M = Mass in grams (sometimes kilograms) C = Specific Heat Capacity in J/go. C (sometimes joules or kilojoules per kilogram. OC) T = Temperature Change in OC

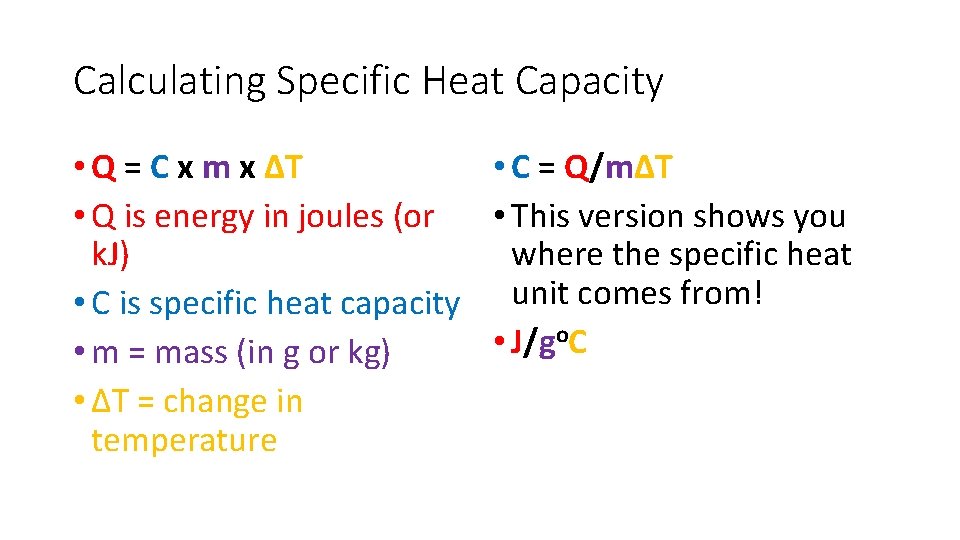
Calculating Specific Heat Capacity • Q = C x m x ΔT • C = Q/mΔT • Q is energy in joules (or • This version shows you k. J) where the specific heat • C is specific heat capacity unit comes from! o. C • J/g • m = mass (in g or kg) • ΔT = change in temperature