Introduction to Thermodynamics Heat Chemical Energy in Physical














- Slides: 70

Introduction to Thermodynamics Heat (Chemical Energy) in Physical & Chemical Changes Principles, Equations & Diagrams Dr. Ron Rusay

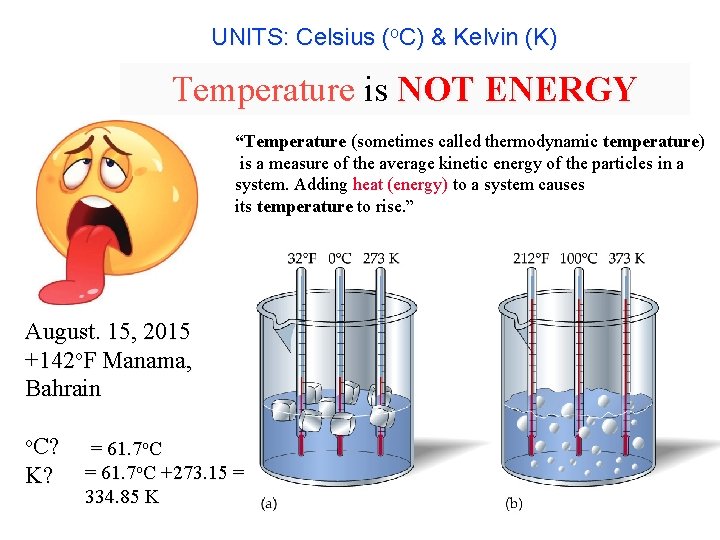
UNITS: Celsius (o. C) & Kelvin (K) Temperature Scales Temperature is NOT ENERGY “Temperature (sometimes called thermodynamic temperature) is a measure of the average kinetic energy of the particles in a system. Adding heat (energy) to a system causes its temperature to rise. ” August. 15, 2015 +142 o. F Manama, Bahrain o. C? K? = 61. 7 o. C +273. 15 = 334. 85 K

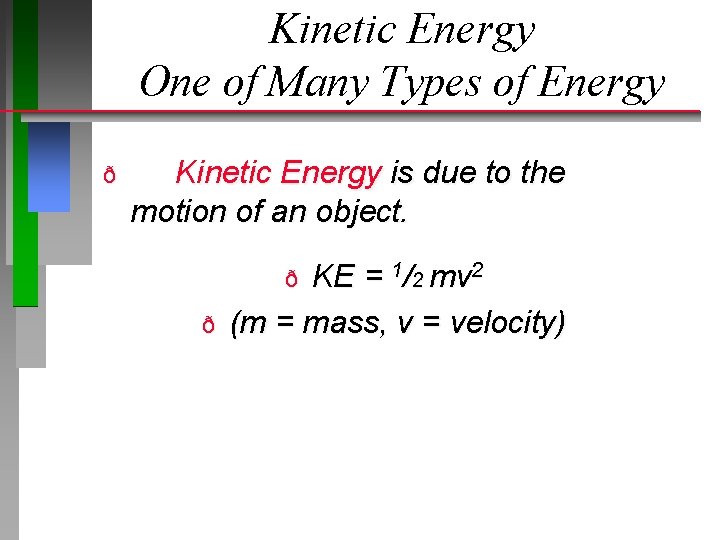
Kinetic Energy One of Many Types of Energy ð Kinetic Energy is due to the motion of an object. KE = 1/2 mv 2 (m = mass, v = velocity) ð ð

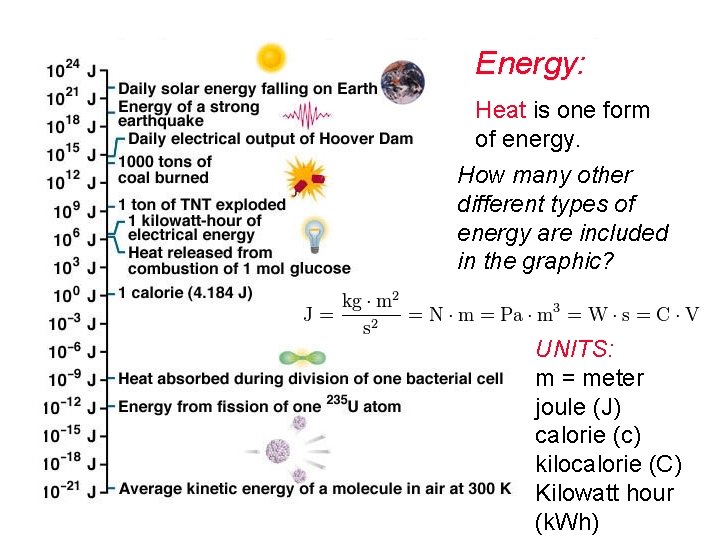
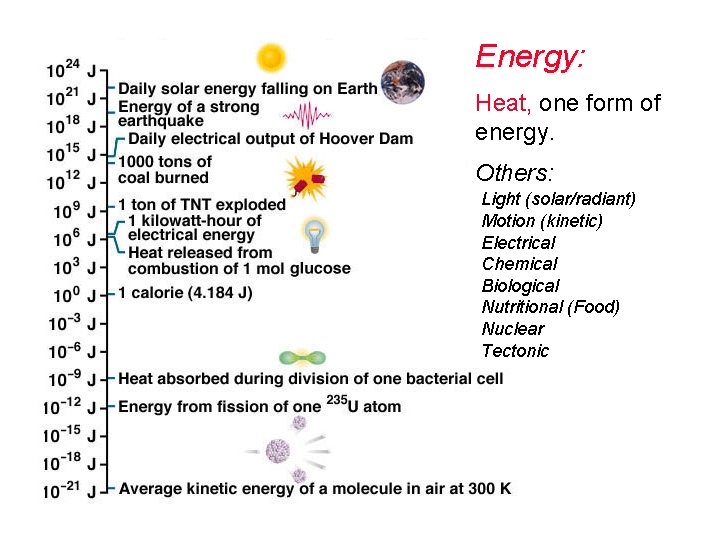
Energy: Heat is one form of energy. How many other different types of energy are included in the graphic? UNITS: m = meter joule (J) calorie (c) kilocalorie (C) Kilowatt hour (k. Wh)

Energy: Heat, one form of energy. Others: Light (solar/radiant) Motion (kinetic) Electrical Chemical Biological Nutritional (Food) Nuclear Tectonic

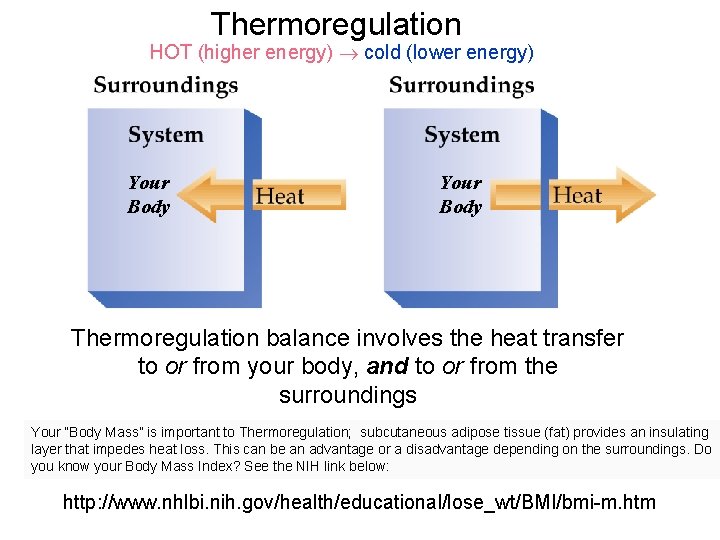
Thermoregulation HOT (higher energy) cold (lower energy) Your Body Thermoregulation balance involves the heat transfer to or from your body, and to or from the surroundings Your “Body Mass” is important to Thermoregulation; subcutaneous adipose tissue (fat) provides an insulating layer that impedes heat loss. This can be an advantage or a disadvantage depending on the surroundings. Do you know your Body Mass Index? See the NIH link below: http: //www. nhlbi. nih. gov/health/educational/lose_wt/BMI/bmi-m. htm

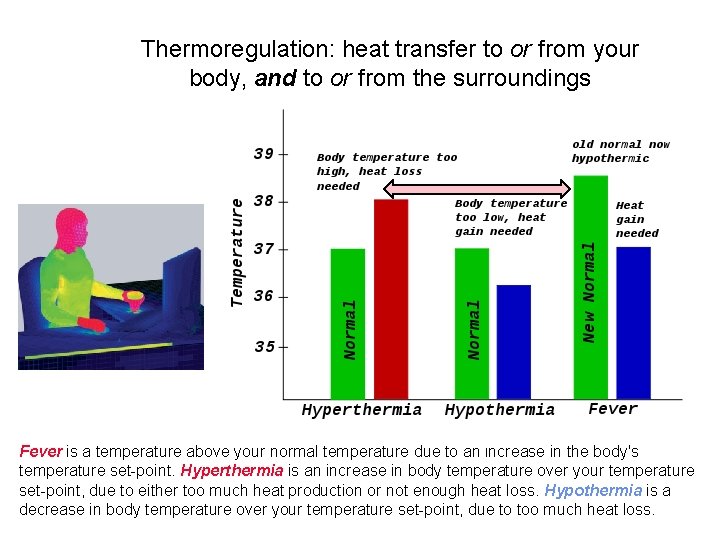
Thermoregulation: heat transfer to or from your body, and to or from the surroundings Fever is a temperature above your normal temperature due to an increase in the body's temperature set-point. Hyperthermia is an increase in body temperature over your temperature set-point, due to either too much heat production or not enough heat loss. Hypothermia is a decrease in body temperature over your temperature set-point, due to too much heat loss.

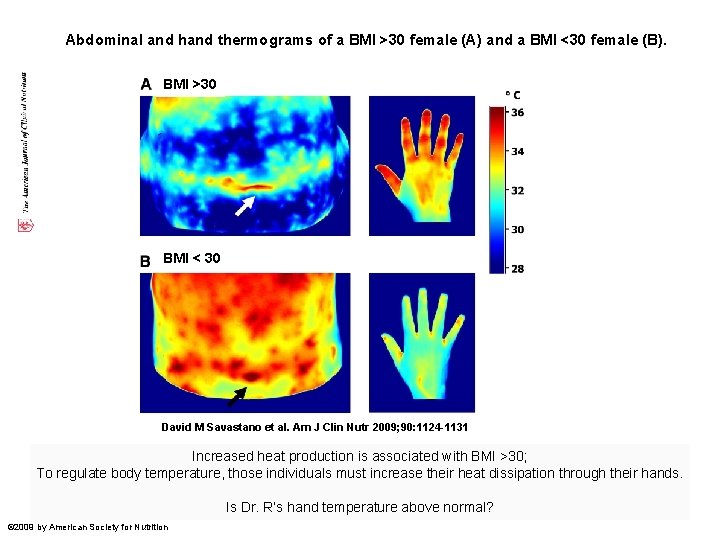
Abdominal and hand thermograms of a BMI >30 female (A) and a BMI <30 female (B). BMI >30 BMI < 30 David M Savastano et al. Am J Clin Nutr 2009; 90: 1124 -1131 Increased heat production is associated with BMI >30; To regulate body temperature, those individuals must increase their heat dissipation through their hands. Is Dr. R’s hand temperature above normal? © 2009 by American Society for Nutrition

Lying & Temperature Effects (2018) Dr Emilio Gómez Milán University of Granada

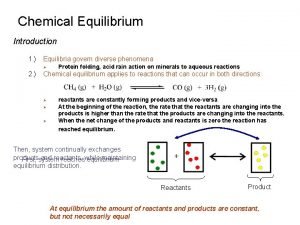
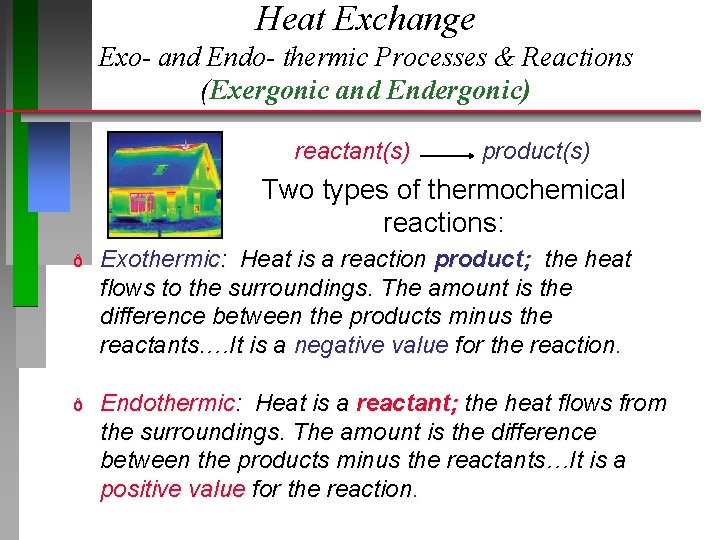
Heat Exchange Exo- and Endo- thermic Processes & Reactions (Exergonic and Endergonic) reactant(s) product(s) Two types of thermochemical reactions: ð Exothermic: Heat is a reaction product; the heat flows to the surroundings. The amount is the difference between the products minus the reactants. …It is a negative value for the reaction. ð Endothermic: Heat is a reactant; the heat flows from the surroundings. The amount is the difference between the products minus the reactants…It is a positive value for the reaction.

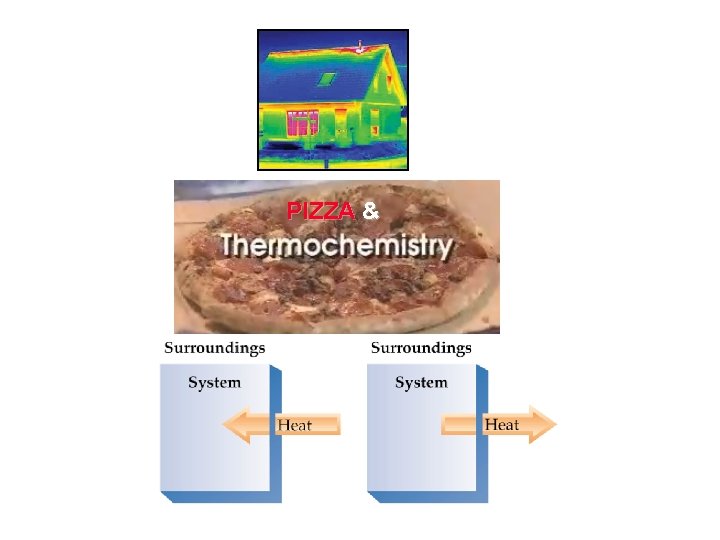
PIZZA &

Why can you burn the top of your mouth with hot pizza just out of the oven and not the bottom? (The top & bottom are at the same temperature!!!) http: //chemconnections. org/general/movies/Pizza-thermo%202. mp 4

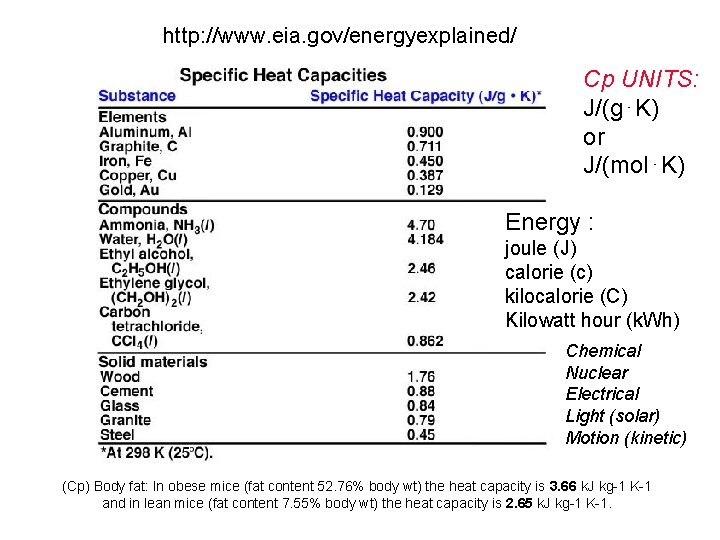
http: //www. eia. gov/energyexplained/ Cp UNITS: J/(g⋅K) or J/(mol⋅K) Energy : joule (J) calorie (c) kilocalorie (C) Kilowatt hour (k. Wh) Chemical Nuclear Electrical Light (solar) Motion (kinetic) (Cp) Body fat: In obese mice (fat content 52. 76% body wt) the heat capacity is 3. 66 k. J kg-1 K-1 and in lean mice (fat content 7. 55% body wt) the heat capacity is 2. 65 k. J kg-1 K-1.

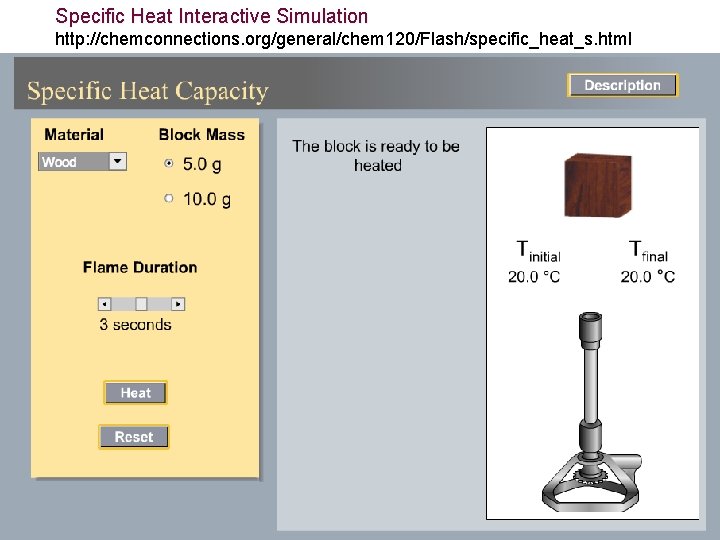
Specific Heat Interactive Simulation http: //chemconnections. org/general/chem 120/Flash/specific_heat_s. html

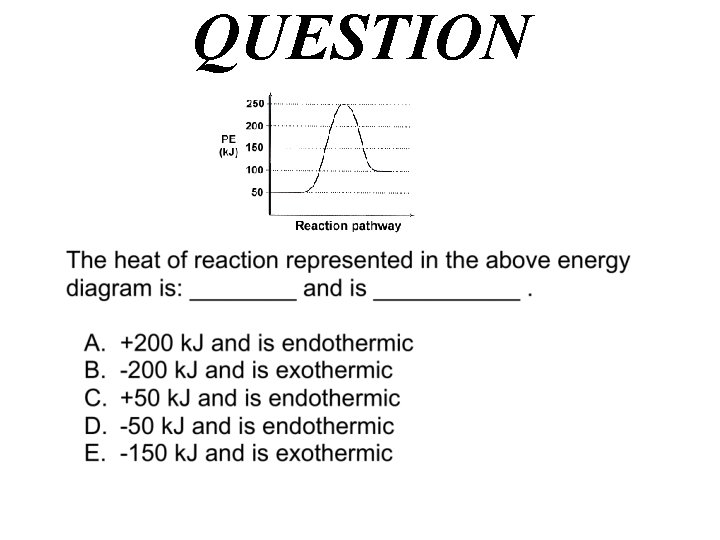
QUESTION

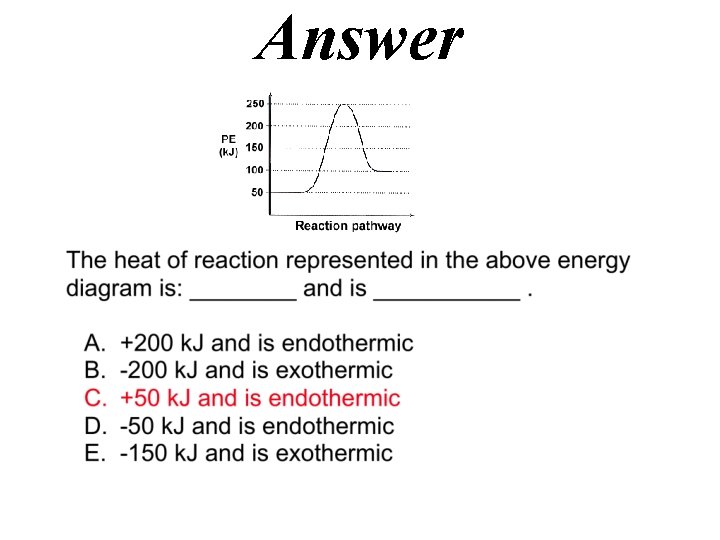
Answer 0. 13 J/g C = 48. 8 J / 15 grams. 25 C

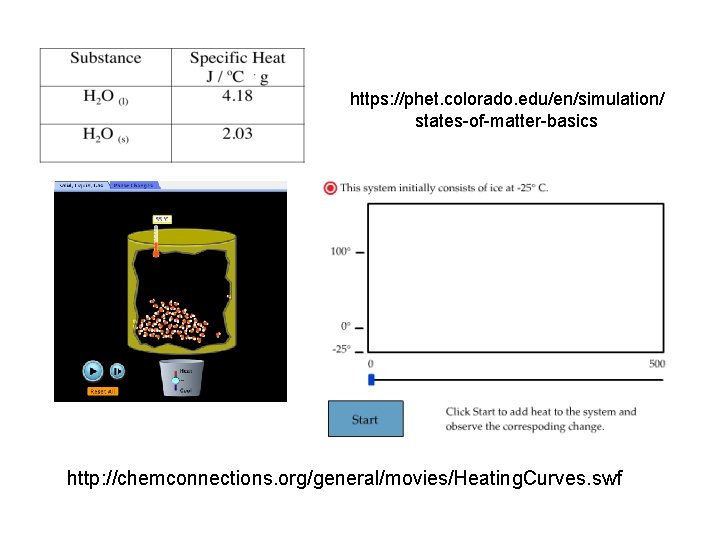
https: //phet. colorado. edu/en/simulation/ states-of-matter-basics http: //chemconnections. org/general/movies/Heating. Curves. swf

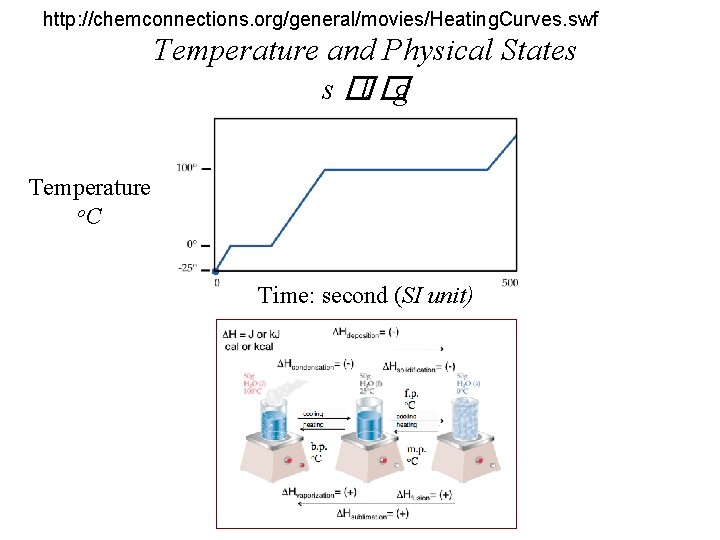
http: //chemconnections. org/general/movies/Heating. Curves. swf Temperature and Physical States s �l �g Temperature o. C Time: second (SI unit)

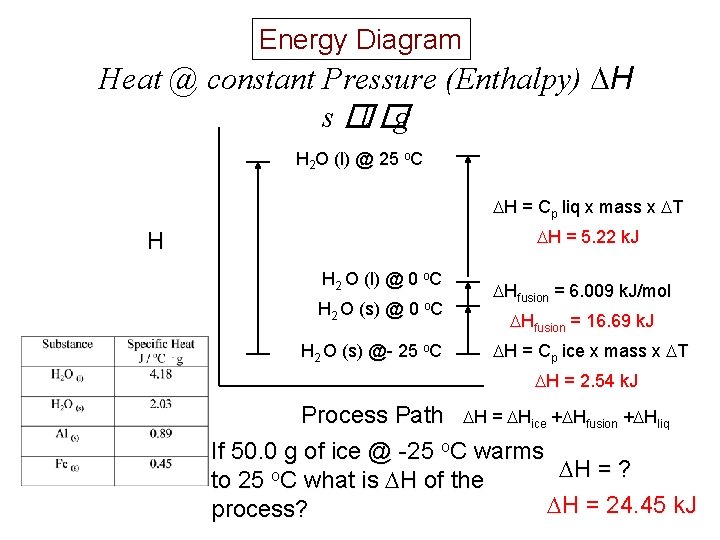
Energy Diagram Heat @ constant Pressure (Enthalpy) H s �l �g H 2 O (l) @ 25 o. C H = Cp liq x mass x T H = 5. 22 k. J H H 2 O (l) @ 0 o. C H 2 O (s) @- 25 o. C Hfusion = 6. 009 k. J/mol Hfusion = 16. 69 k. J H = Cp ice x mass x T H = 2. 54 k. J Process Path H = Hice + Hfusion + Hliq If 50. 0 g of ice @ -25 o. C warms H = ? to 25 o. C what is H of the H = 24. 45 k. J process?

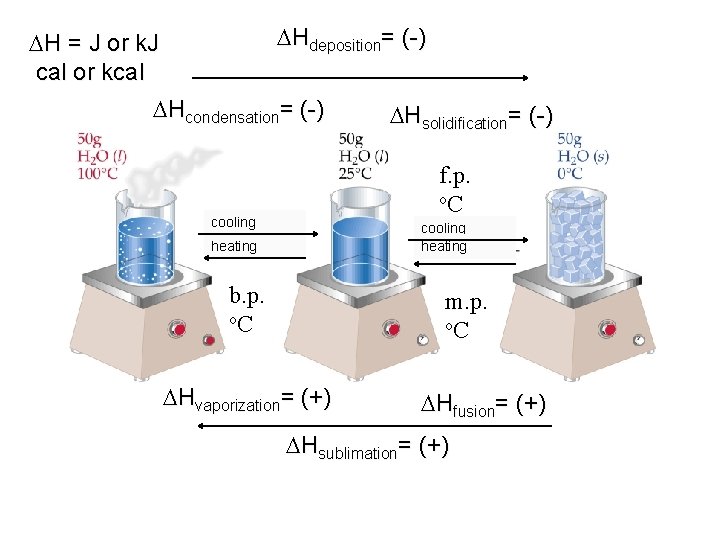
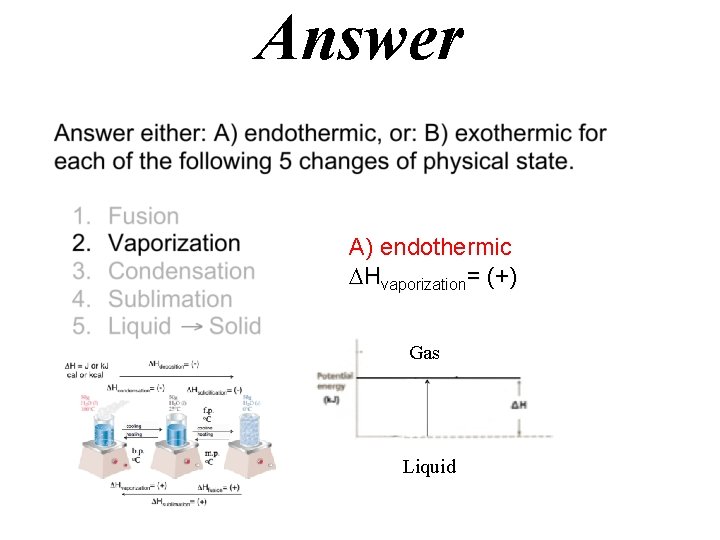
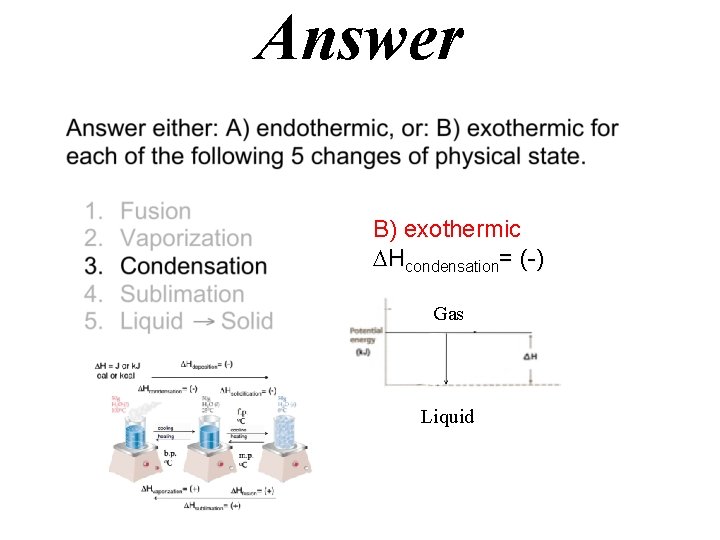
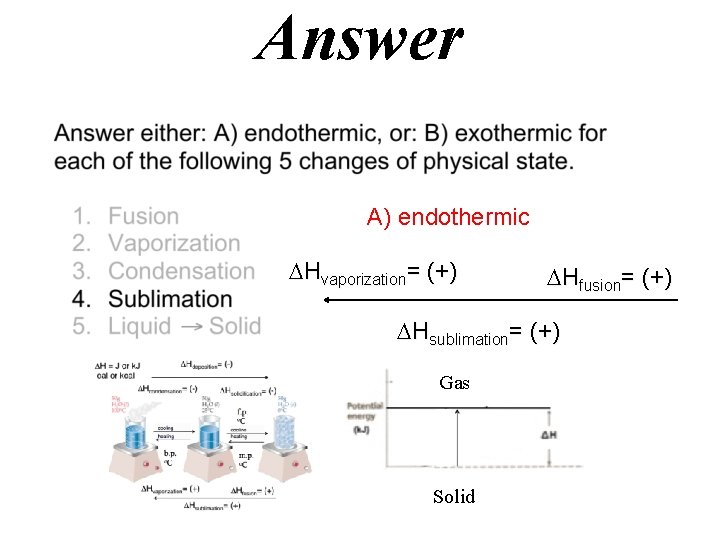
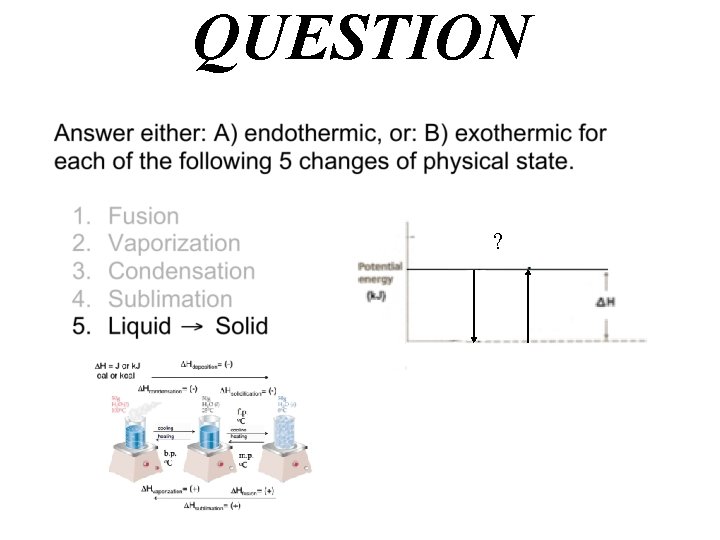
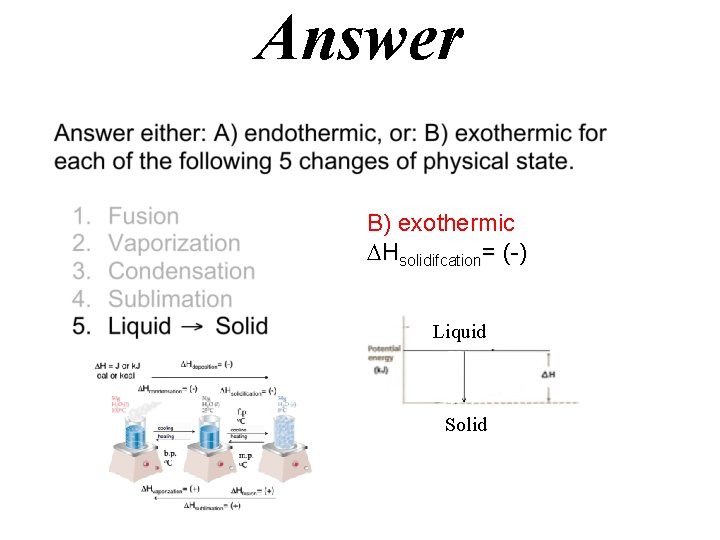
Hdeposition= (-) H = J or k. J cal or kcal Hcondensation= (-) Hsolidification= (-) f. p. o. C cooling heating b. p. o. C m. p. o. C Hvaporization= (+) Hfusion= (+) Hsublimation= (+)

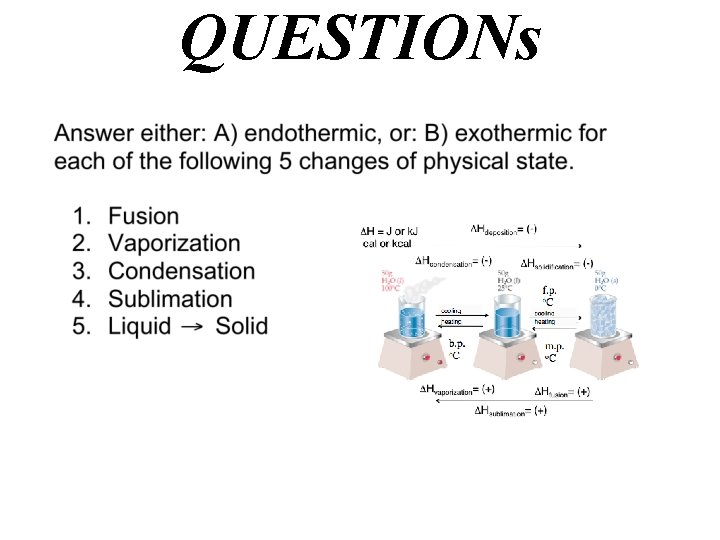
QUESTIONs

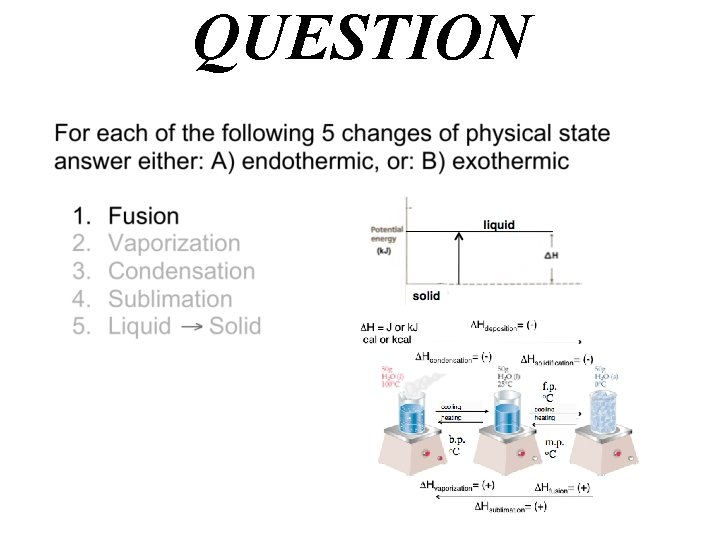
QUESTION

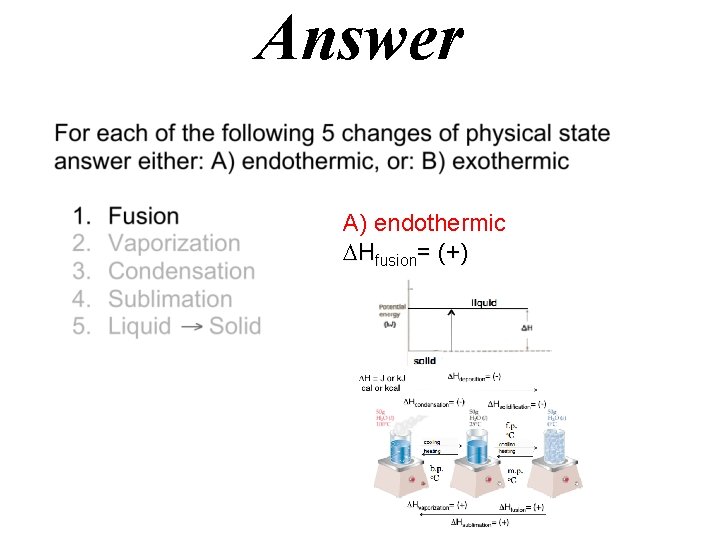
Answer A) endothermic Hfusion= (+)

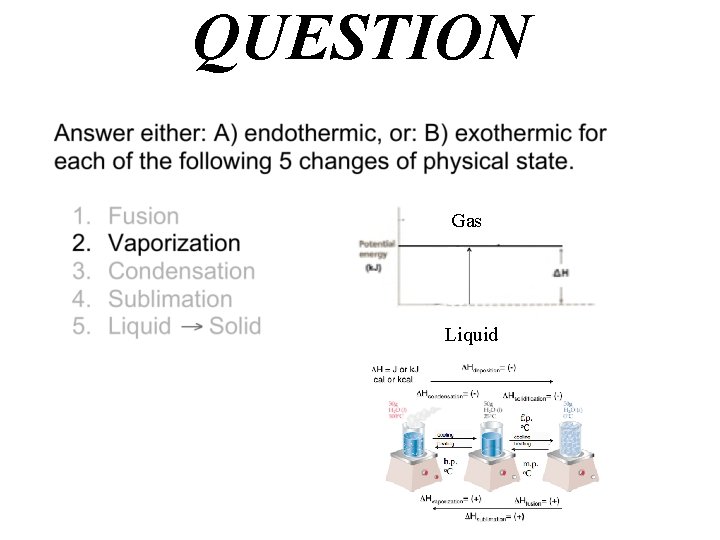
QUESTION Gas Liquid

Answer A) endothermic Hvaporization= (+) Gas Liquid

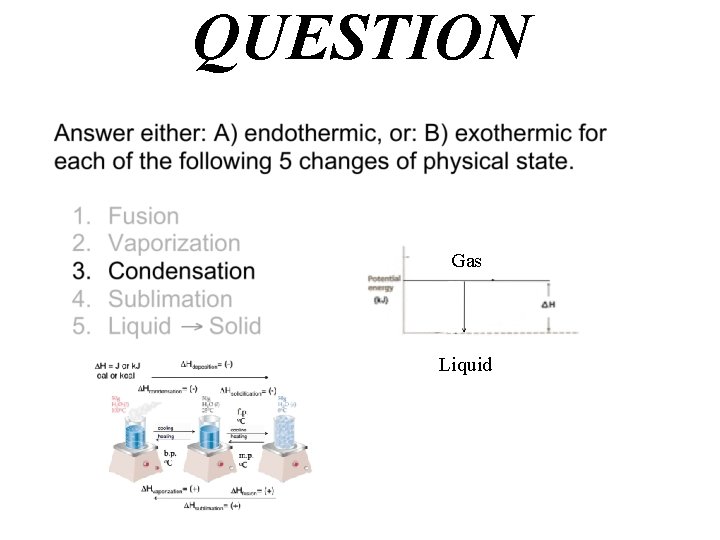
QUESTION Gas Liquid

Answer B) exothermic Hcondensation= (-) Gas Liquid

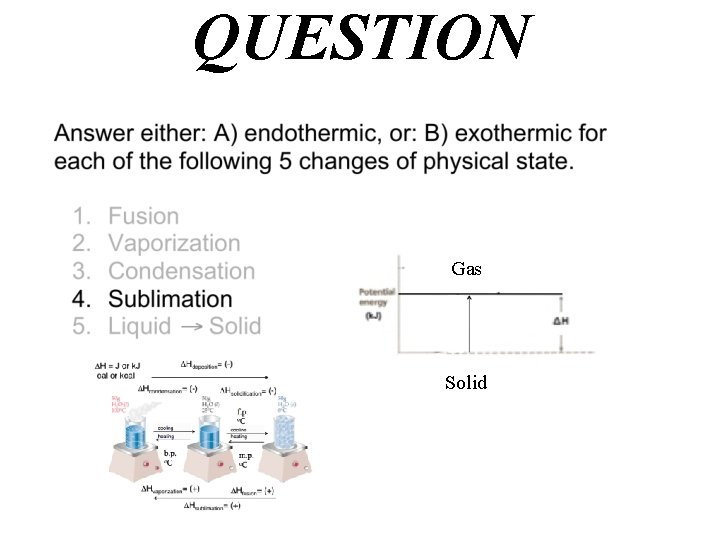
QUESTION Gas Solid

Answer A) endothermic Hvaporization= (+) Hfusion= (+) Hsublimation= (+) Gas Solid

QUESTION ?

Answer B) exothermic Hsolidifcation= (-) Liquid Solid

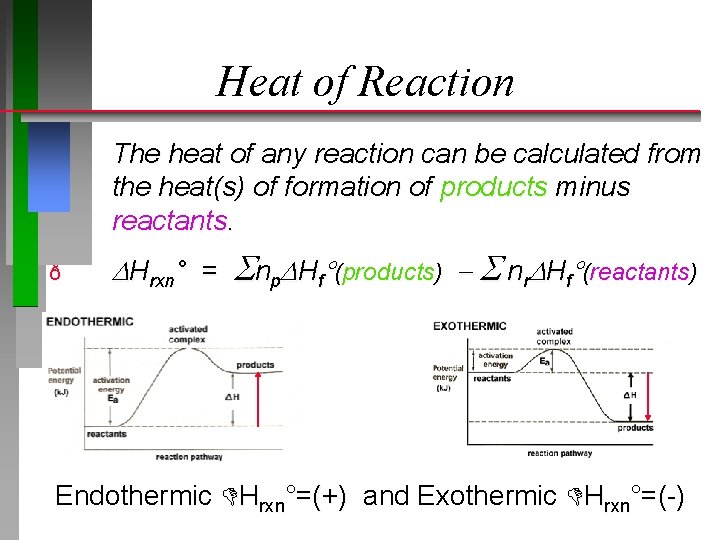
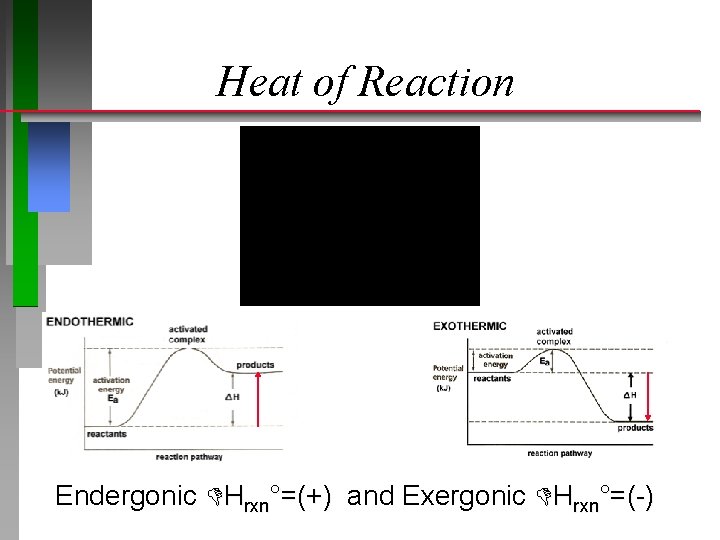
Heat of Reaction The heat of any reaction can be calculated from the heat(s) of formation of products minus reactants. ð Hrxn° = np Hf (products) nr Hf (reactants) Endothermic Hrxn°=(+) and Exothermic Hrxn°=(-)

Heat of Reaction Endergonic Hrxn°=(+) and Exergonic Hrxn°=(-)

QUESTION

Answer

QUESTION

Answer

Endothermic Reactions Photosynthesis 6 CO 2 (g) + 6 H 2 O(l) + energy C 6 H 12 O 6 (s) + 6 O 2(g) https: //www. youtube. com/watch? v=ea. ACs. EXAjh. A Hrxn° = np Hf (products) nr Hf (reactants) Hrxn° = (+) = + 2800 k. J/mol C = (+) = + 2800 k. J/mol 6 H 12 O 6 (glucose or fructose)

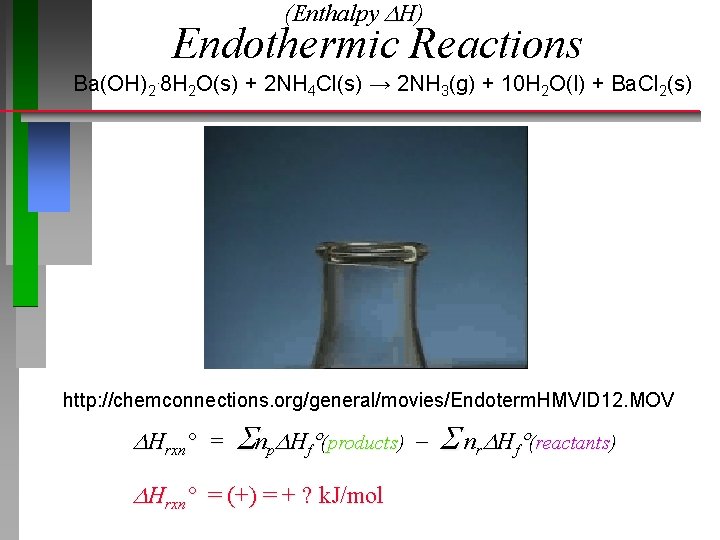
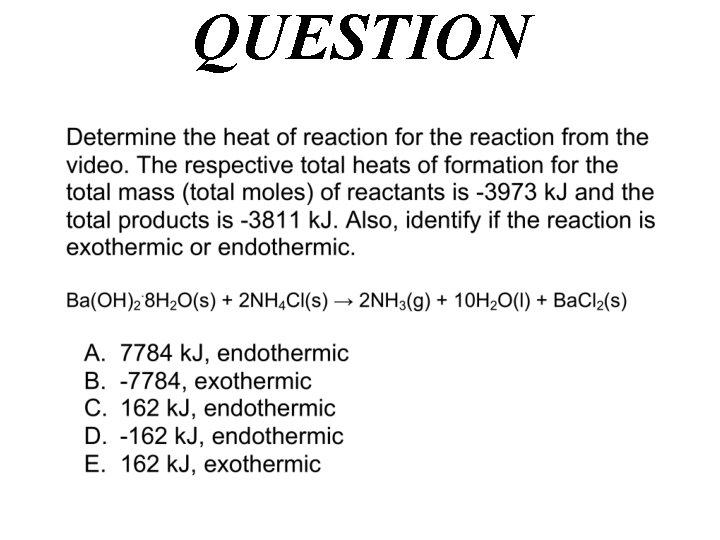
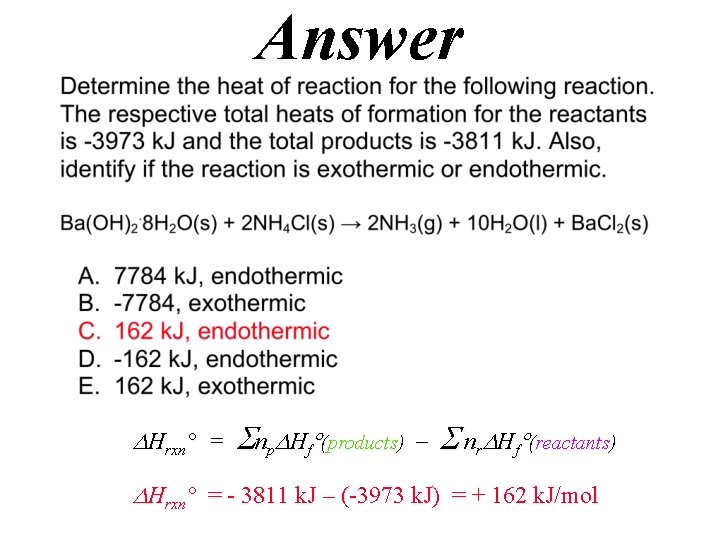
(Enthalpy H) Endothermic Reactions Ba(OH)2. 8 H 2 O(s) + 2 NH 4 Cl(s) → 2 NH 3(g) + 10 H 2 O(l) + Ba. Cl 2(s) http: //chemconnections. org/general/movies/Endoterm. HMVID 12. MOV Hrxn° = np Hf (products) nr Hf (reactants) Hrxn° = (+) = + ? k. J/mol

QUESTION

Answer Hrxn° = np Hf (products) nr Hf (reactants) Hrxn° = - 3811 k. J – (-3973 k. J) = + 162 k. J/mol

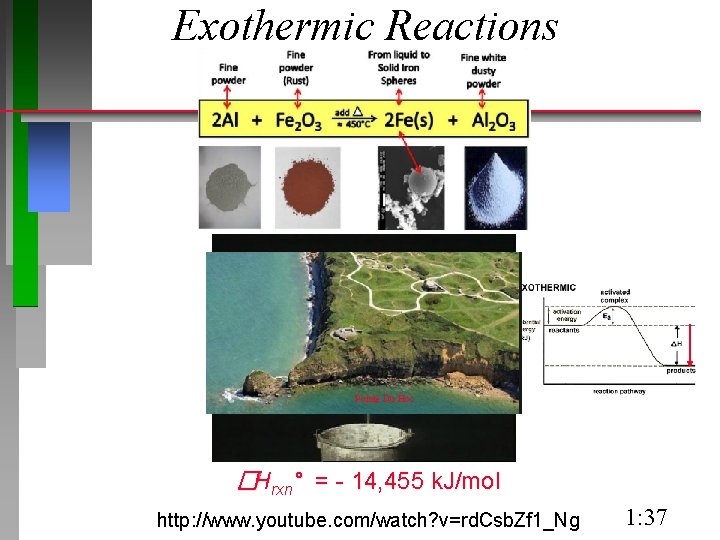
Exothermic Reactions Pointe Du Hoc �Hrxn° = - 14, 455 k. J/mol http: //www. youtube. com/watch? v=rd. Csb. Zf 1_Ng 1: 37

Hot Food: MRE’s Exothermic Reaction Enough heat to raise the temperature of 8 ounces of food to 56 o. C (100 o. F). Mg(s) + 2 H 2 O(l) Mg(OH)2 (aq) + H 2 (g)+ energy:

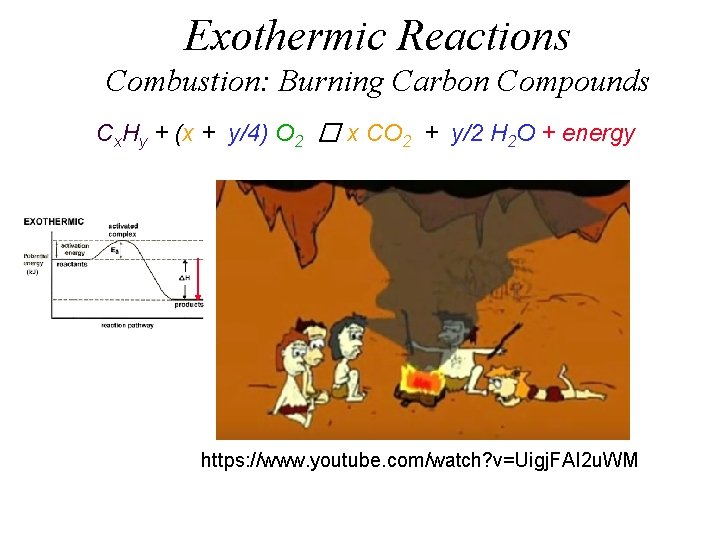
Exothermic Reactions Combustion: Burning Carbon Compounds Cx. Hy + (x + y/4) O 2 � x CO 2 + y/2 H 2 O + energy https: //www. youtube. com/watch? v=Uigj. FAI 2 u. WM

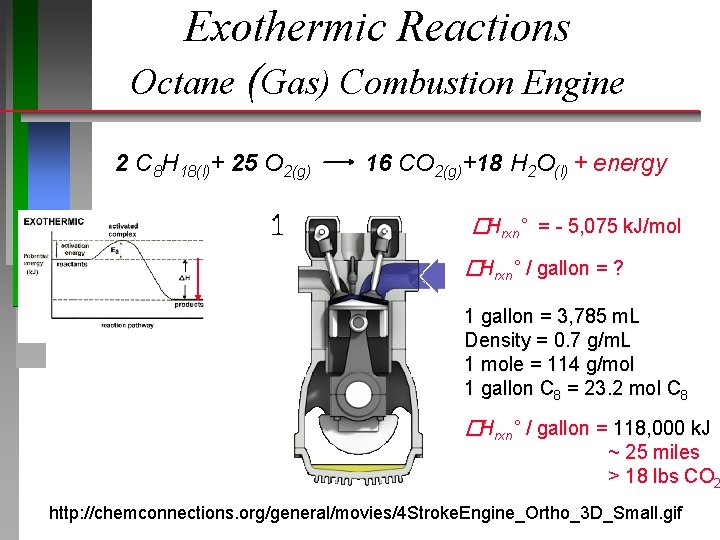
Exothermic Reactions Octane (Gas) Combustion Engine 2 C 8 H 18(l)+ 25 O 2(g) 16 CO 2(g)+18 H 2 O(l) + energy �Hrxn° = - 5, 075 k. J/mol �Hrxn° / gallon = ? 1 gallon = 3, 785 m. L Density = 0. 7 g/m. L 1 mole = 114 g/mol 1 gallon C 8 = 23. 2 mol C 8 �Hrxn° / gallon = 118, 000 k. J ~ 25 miles > 18 lbs CO 2 http: //chemconnections. org/general/movies/4 Stroke. Engine_Ortho_3 D_Small. gif

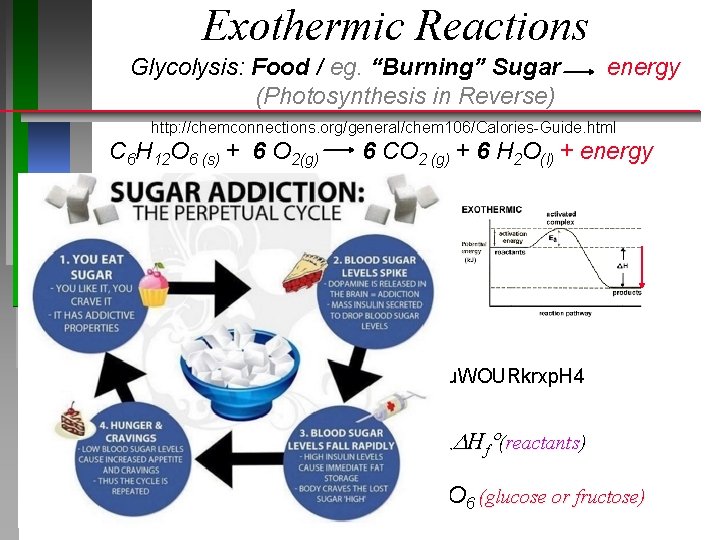
Exothermic Reactions Glycolysis: Food / eg. “Burning” Sugar (Photosynthesis in Reverse) energy http: //chemconnections. org/general/chem 106/Calories-Guide. html C 6 H 12 O 6 (s) + 6 O 2(g) 6 CO 2 (g) + 6 H 2 O(l) + energy https: //www. youtube. com/watch? v=u. WOURkrxp. H 4 Hrxn° = np Hf (products) nr Hf (reactants) Hrxn° = (-) = - 2800 k. J/mol C = (-) = - 2800 k. J/mol 6 H 12 O 6 (glucose or fructose)

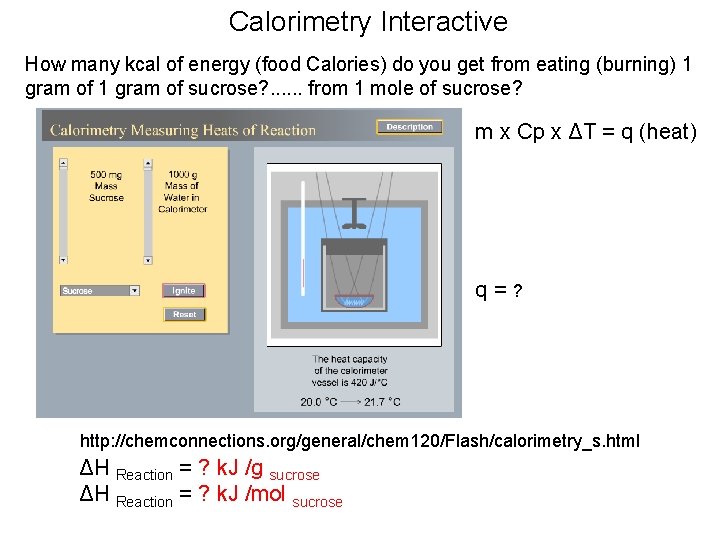
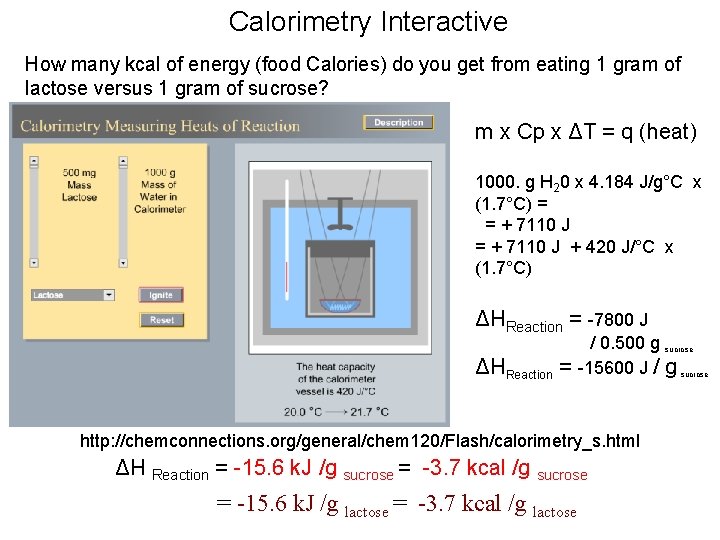
Calorimetry Interactive How many kcal of energy (food Calories) do you get from eating (burning) 1 gram of sucrose? . . . from 1 mole of sucrose? m x Cp x ΔT = q (heat) q=? http: //chemconnections. org/general/chem 120/Flash/calorimetry_s. html ΔH Reaction = ? k. J /g sucrose ΔH Reaction = ? k. J /mol sucrose

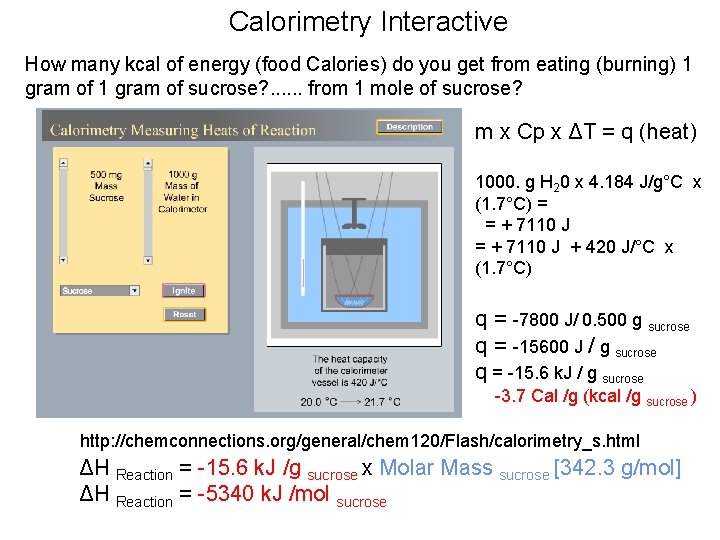
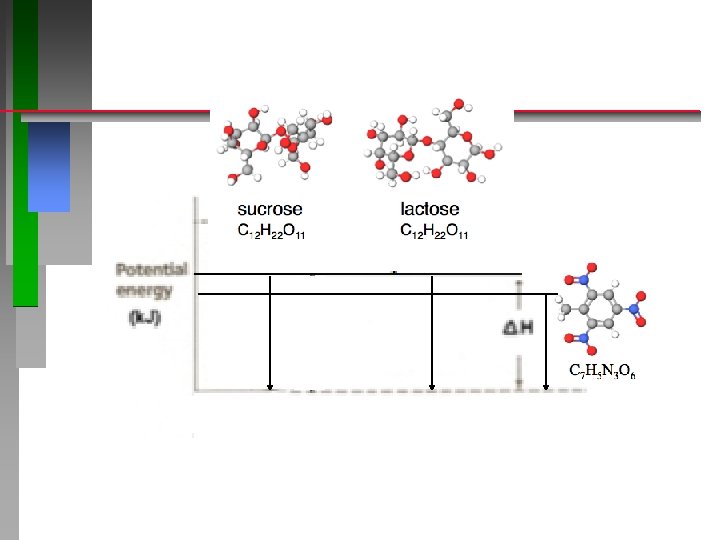
Calorimetry Interactive How many kcal of energy (food Calories) do you get from eating (burning) 1 gram of sucrose? . . . from 1 mole of sucrose? m x Cp x ΔT = q (heat) 1000. g H 20 x 4. 184 J/g°C x (1. 7°C) = = + 7110 J + 420 J/°C x (1. 7°C) q = -7800 J/ 0. 500 g sucrose q = -15600 J / g sucrose q = -15. 6 k. J / g sucrose -3. 7 Cal /g (kcal /g sucrose ) http: //chemconnections. org/general/chem 120/Flash/calorimetry_s. html ΔH Reaction = -15. 6 k. J /g sucrose x Molar Mass sucrose [342. 3 g/mol] ΔH Reaction = -5340 k. J /mol sucrose

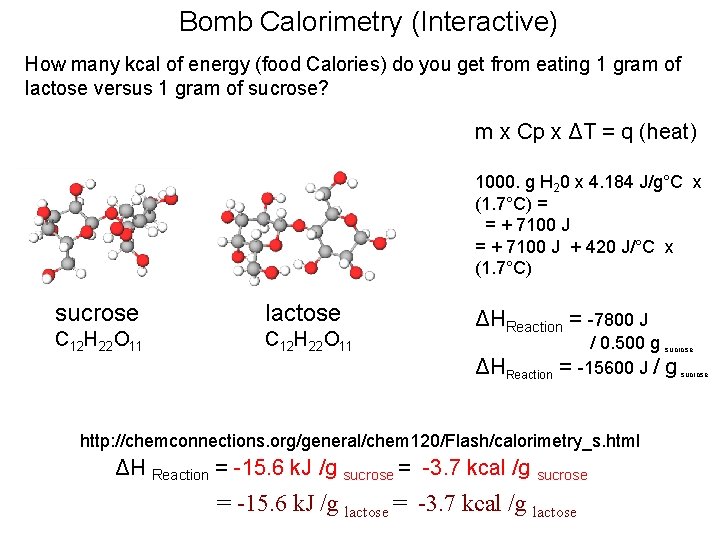
Calorimetry Interactive How many kcal of energy (food Calories) do you get from eating 1 gram of lactose versus 1 gram of sucrose? m x Cp x ΔT = q (heat) 1000. g H 20 x 4. 184 J/g°C x (1. 7°C) = = + 7110 J + 420 J/°C x (1. 7°C) ΔHReaction = -7800 J ΔHReaction = / 0. 500 g sucrose -15600 J / g sucrose http: //chemconnections. org/general/chem 120/Flash/calorimetry_s. html ΔH Reaction = -15. 6 k. J /g sucrose = -3. 7 kcal /g sucrose = -15. 6 k. J /g lactose = -3. 7 kcal /g lactose

Bomb Calorimetry (Interactive) How many kcal of energy (food Calories) do you get from eating 1 gram of lactose versus 1 gram of sucrose? m x Cp x ΔT = q (heat) 1000. g H 20 x 4. 184 J/g°C x (1. 7°C) = = + 7100 J + 420 J/°C x (1. 7°C) sucrose lactose C 12 H 22 O 11 ΔHReaction = -7800 J ΔHReaction = / 0. 500 g sucrose -15600 J / g sucrose http: //chemconnections. org/general/chem 120/Flash/calorimetry_s. html ΔH Reaction = -15. 6 k. J /g sucrose = -3. 7 kcal /g sucrose = -15. 6 k. J /g lactose = -3. 7 kcal /g lactose

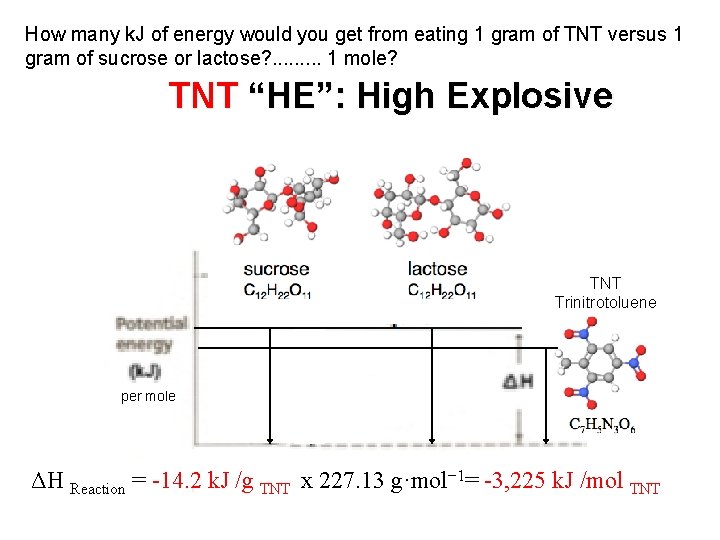
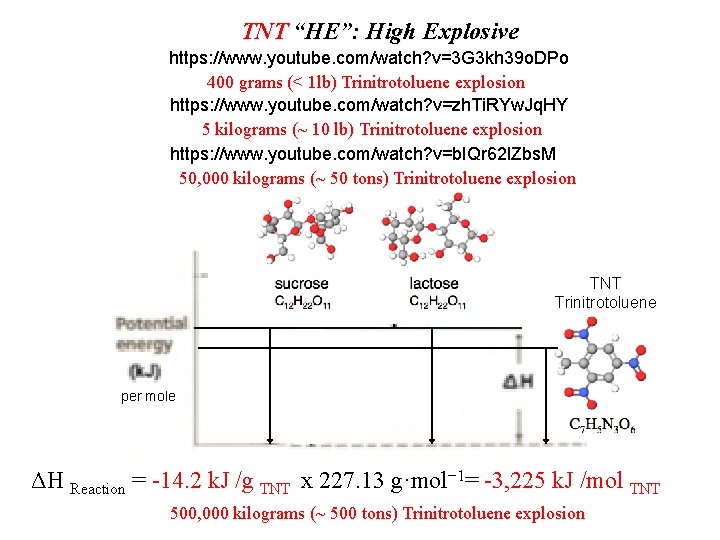
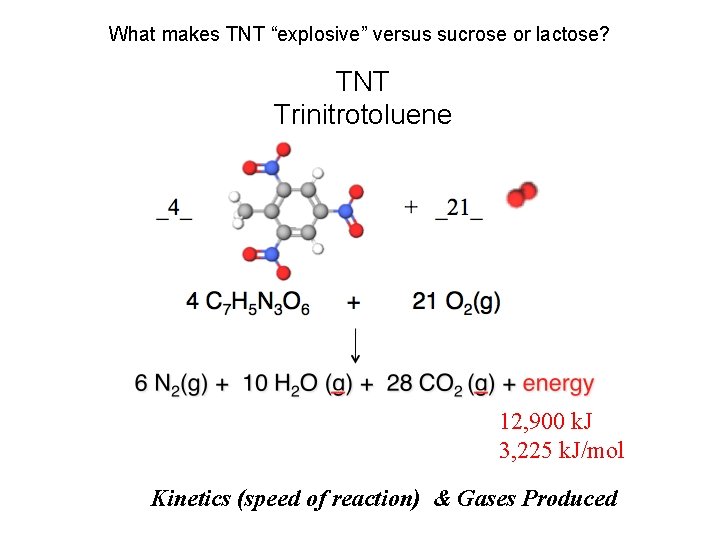
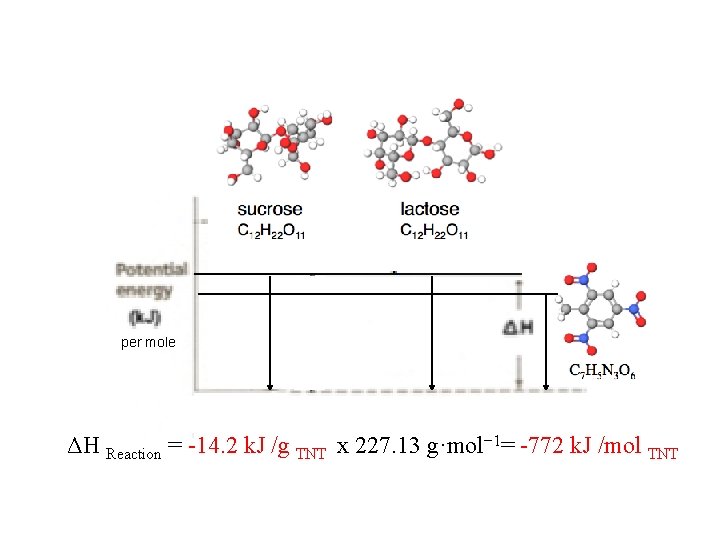
How many k. J of energy would you get from eating 1 gram of TNT versus 1 gram of sucrose or lactose? . . 1 mole? TNT “HE”: High Explosive TNT Trinitrotoluene per mole ΔH Reaction = -14. 2 k. J /g TNT x 227. 13 g·mol− 1= -3, 225 k. J /mol TNT

TNT “HE”: High Explosive https: //www. youtube. com/watch? v=3 G 3 kh 39 o. DPo 400 grams (< 1 lb) Trinitrotoluene explosion https: //www. youtube. com/watch? v=zh. Ti. RYw. Jq. HY 5 kilograms (~ 10 lb) Trinitrotoluene explosion https: //www. youtube. com/watch? v=b. IQr 62 l. Zbs. M 50, 000 kilograms (~ 50 tons) Trinitrotoluene explosion TNT Trinitrotoluene per mole ΔH Reaction = -14. 2 k. J /g TNT x 227. 13 g·mol− 1= -3, 225 k. J /mol TNT 500, 000 kilograms (~ 500 tons) Trinitrotoluene explosion

What makes TNT “explosive” versus sucrose or lactose? TNT Trinitrotoluene 12, 900 k. J 3, 225 k. J/mol Kinetics (speed of reaction) & Gases Produced


per mole ΔH Reaction = -14. 2 k. J /g TNT x 227. 13 g·mol− 1= -772 k. J /mol TNT

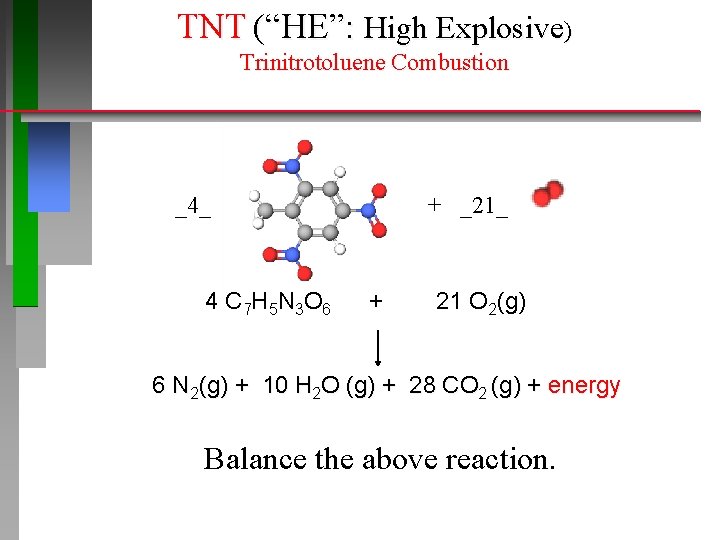
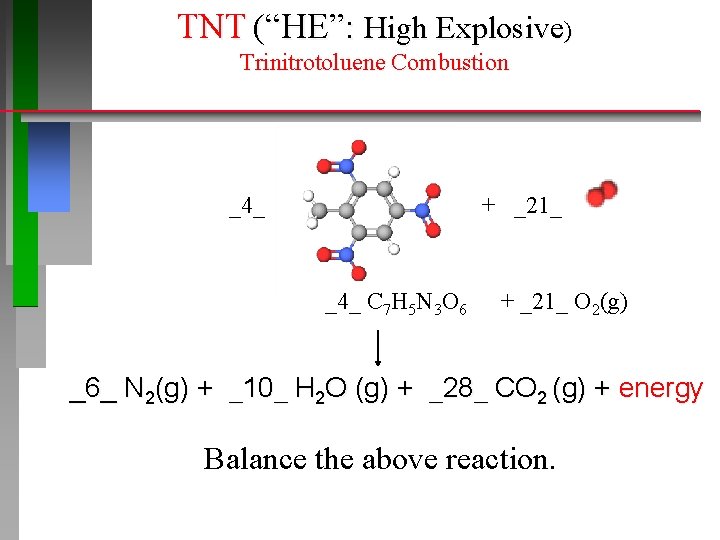
TNT (“HE”: High Explosive) Trinitrotoluene Combustion _4_ 4 C 7 H 5 N 3 O 6 + _21_ + 21 O 2(g) 6 N 2(g) + 10 H 2 O (g) + 28 CO 2 (g) + energy Balance the above reaction.

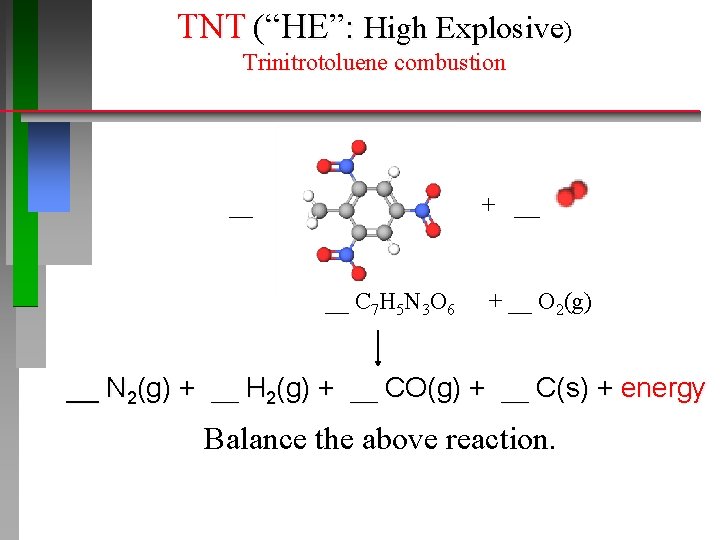
TNT (“HE”: High Explosive) Trinitrotoluene combustion __ + ____ __ C 7 H 5 N 3 O 6 + __ O 2(g) __ N 2(g) + __ H 2(g) + __ CO(g) + __ C(s) + energy Balance the above reaction.

TNT (“HE”: High Explosive) Trinitrotoluene Combustion _4_ + _21_ _4_ C 7 H 5 N 3 O 6 + _21_ O 2(g) _6_ N 2(g) + _10_ H 2 O (g) + _28_ CO 2 (g) + energy Balance the above reaction.

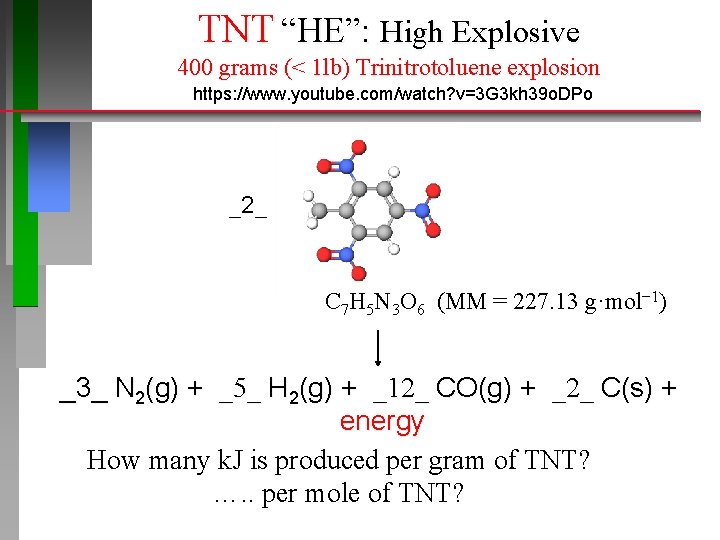
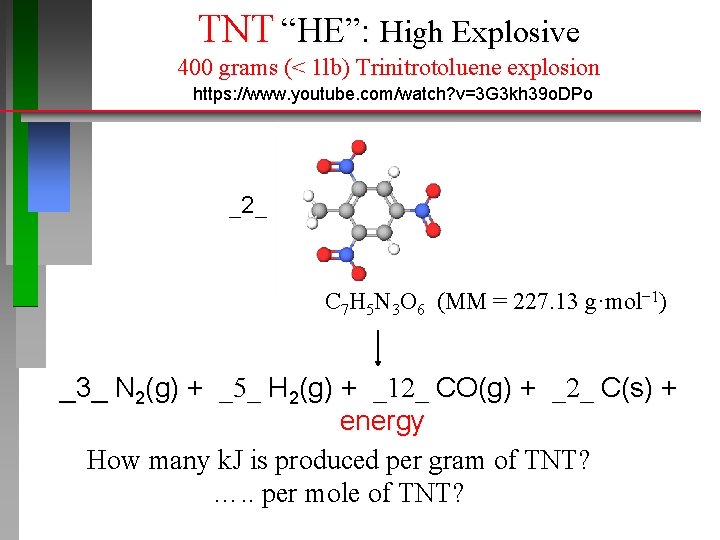
TNT “HE”: High Explosive 400 grams (< 1 lb) Trinitrotoluene explosion https: //www. youtube. com/watch? v=3 G 3 kh 39 o. DPo _2_ C 7 H 5 N 3 O 6 (MM = 227. 13 g·mol− 1) _3_ N 2(g) + _5_ H 2(g) + _12_ CO(g) + _2_ C(s) + energy How many k. J is produced per gram of TNT? …. . per mole of TNT?

TNT “HE”: High Explosive 400 grams (< 1 lb) Trinitrotoluene explosion https: //www. youtube. com/watch? v=3 G 3 kh 39 o. DPo _2_ C 7 H 5 N 3 O 6 (MM = 227. 13 g·mol− 1) _3_ N 2(g) + _5_ H 2(g) + _12_ CO(g) + _2_ C(s) + energy How many k. J is produced per gram of TNT? …. . per mole of TNT?

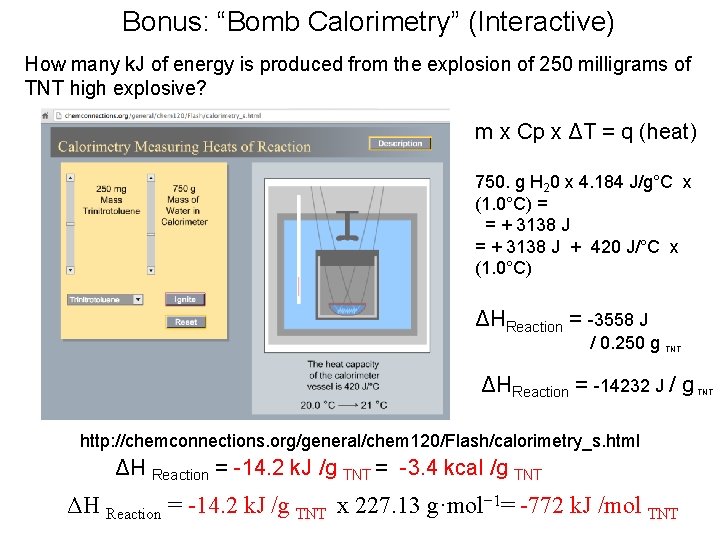
Bonus: “Bomb Calorimetry” (Interactive) How many k. J of energy is produced from the explosion of 250 milligrams of TNT high explosive? m x Cp x ΔT = q (heat) 750. g H 20 x 4. 184 J/g°C x (1. 0°C) = = + 3138 J + 420 J/°C x (1. 0°C) ΔHReaction = -3558 J / 0. 250 g TNT ΔHReaction = -14232 J / g http: //chemconnections. org/general/chem 120/Flash/calorimetry_s. html ΔH Reaction = -14. 2 k. J /g TNT = -3. 4 kcal /g TNT ΔH Reaction = -14. 2 k. J /g TNT x 227. 13 g·mol− 1= -772 k. J /mol TNT




Chemical Energy (Heat) Thermodynamics https: //www. youtube. com/watch? v=Rfz. Yl 7 Ll 5 Ug

https: //www. youtube. com/watch? v =YM-uyk. Vfq_E Enthalpy H (“Heat”) Entropy S (“Disorder”) https: //www. youtube. com/watch? v =8 m 6 Rt. Opqvt. U

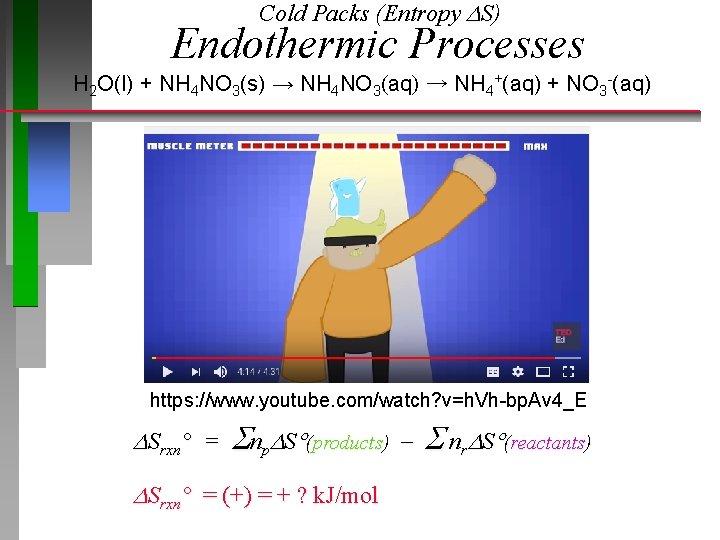
Cold Packs (Entropy S) Endothermic Processes H 2 O(l) + NH 4 NO 3(s) → NH 4 NO 3(aq) → NH 4+(aq) + NO 3 -(aq) https: //www. youtube. com/watch? v=h. Vh-bp. Av 4_E Srxn° = np S (products) nr S (reactants) Srxn° = (+) = + ? k. J/mol


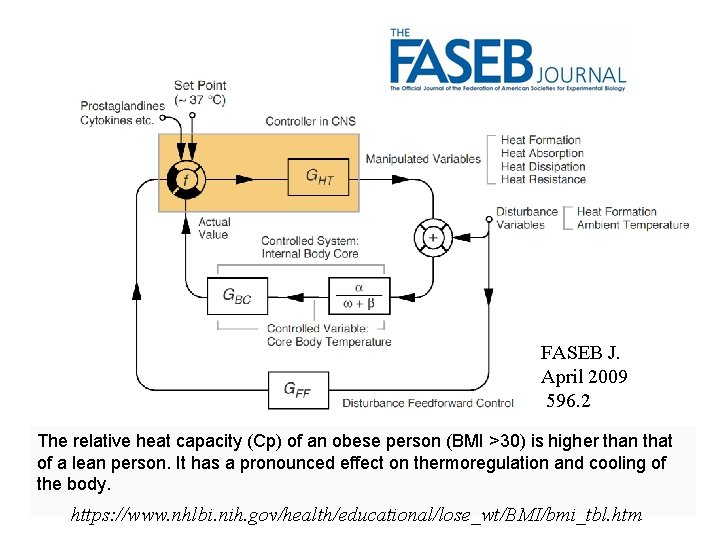
FASEB J. April 2009 596. 2 The relative heat capacity (Cp) of an obese person (BMI >30) is higher than that of a lean person. It has a pronounced effect on thermoregulation and cooling of the body. https: //www. nhlbi. nih. gov/health/educational/lose_wt/BMI/bmi_tbl. htm

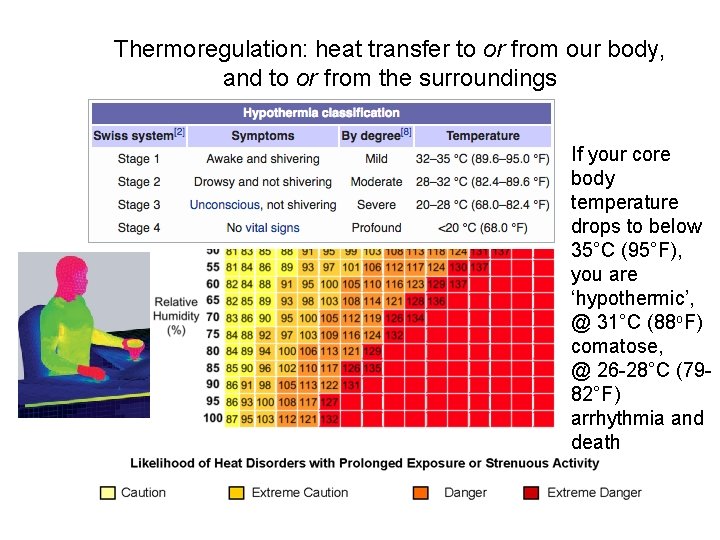
Thermoregulation: heat transfer to or from our body, and to or from the surroundings If your core body temperature drops to below 35°C (95°F), you are ‘hypothermic’, @ 31°C (88 o. F) comatose, @ 26 -28°C (7982°F) arrhythmia and death
 Introduction to chemical engineering thermodynamics
Introduction to chemical engineering thermodynamics Reschual
Reschual Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Chemical engineering thermodynamics 8th solution chapter 4
Chemical engineering thermodynamics 8th solution chapter 4 Thermodynamics
Thermodynamics Chapter 6
Chapter 6 Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Duhem theorem
Duhem theorem Thermal energy vs heat energy
Thermal energy vs heat energy As a roller coaster goes downhill
As a roller coaster goes downhill ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Potential energy examples
Potential energy examples Photosynthesis transforms light energy into chemical energy
Photosynthesis transforms light energy into chemical energy Entropy equation
Entropy equation Energy balance thermodynamics
Energy balance thermodynamics Si unit of energy
Si unit of energy Internal energy in thermodynamics definition
Internal energy in thermodynamics definition Conservation of energy thermodynamics
Conservation of energy thermodynamics Internal energy formula
Internal energy formula Change in heat formula
Change in heat formula Introduction and basic concepts of thermodynamics
Introduction and basic concepts of thermodynamics Thermodynamics introduction and basic concepts
Thermodynamics introduction and basic concepts Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Specific heat capacity
Specific heat capacity Formula for specific latent heat
Formula for specific latent heat Combination cooking techniques
Combination cooking techniques Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Empirical formula pogil
Empirical formula pogil Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Love formula
Love formula Are kc and kp equal
Are kc and kp equal Gh
Gh Properties of sulphuric acid
Properties of sulphuric acid Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties Undesirable change in the physical chemical
Undesirable change in the physical chemical Whats the difference between physical and chemical changes
Whats the difference between physical and chemical changes Is mixing kool aid a chemical change
Is mixing kool aid a chemical change Thermal conductivity in dentistry
Thermal conductivity in dentistry Nitrogen is a colorless gas physical or chemical
Nitrogen is a colorless gas physical or chemical Is chopping wood a chemical reaction
Is chopping wood a chemical reaction Aluminum foil is cut in half physical or chemical change
Aluminum foil is cut in half physical or chemical change Is separating sand from gravel physical or chemical
Is separating sand from gravel physical or chemical Physical change
Physical change Compare and contrast physical and chemical changes
Compare and contrast physical and chemical changes First sign of puberty in males
First sign of puberty in males Physical and chemical changes
Physical and chemical changes Chemical or physical property blue color
Chemical or physical property blue color A bicycle changes color as it rusts physical or chemical
A bicycle changes color as it rusts physical or chemical Physical and chemical properties
Physical and chemical properties Physcial change
Physcial change Physical and chemical phenomena
Physical and chemical phenomena Is sawing wood a chemical change
Is sawing wood a chemical change Wood rotting physical or chemical change
Wood rotting physical or chemical change Examples for physical change
Examples for physical change What is a phisical change
What is a phisical change Physical and chemical changes generation genius
Physical and chemical changes generation genius Is wadding paper a physical change
Is wadding paper a physical change Is rotting garbage a chemical change
Is rotting garbage a chemical change Chemical change and physical change
Chemical change and physical change Physical weathering and chemical weathering venn diagram
Physical weathering and chemical weathering venn diagram Difference between weathering and erosion
Difference between weathering and erosion Is a sparkler a chemical or physical change
Is a sparkler a chemical or physical change Mechanical and chemical weathering
Mechanical and chemical weathering Physical and chemical change grade 10
Physical and chemical change grade 10 Difference between a chemical and physical change
Difference between a chemical and physical change Is dissolving salt in water chemical or physical change
Is dissolving salt in water chemical or physical change Is the stomach a physical or chemical change
Is the stomach a physical or chemical change Is brittleness a physical or chemical property
Is brittleness a physical or chemical property Egg physical change
Egg physical change