Density Specific Gravity and Specific Volume Dr Hayder



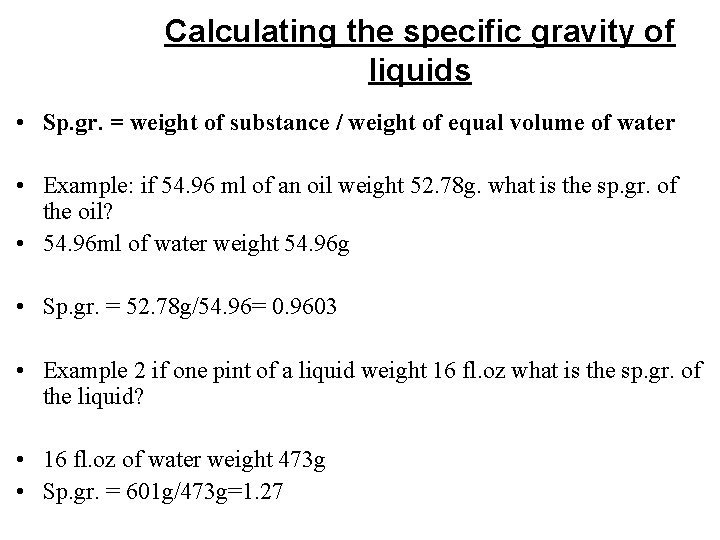



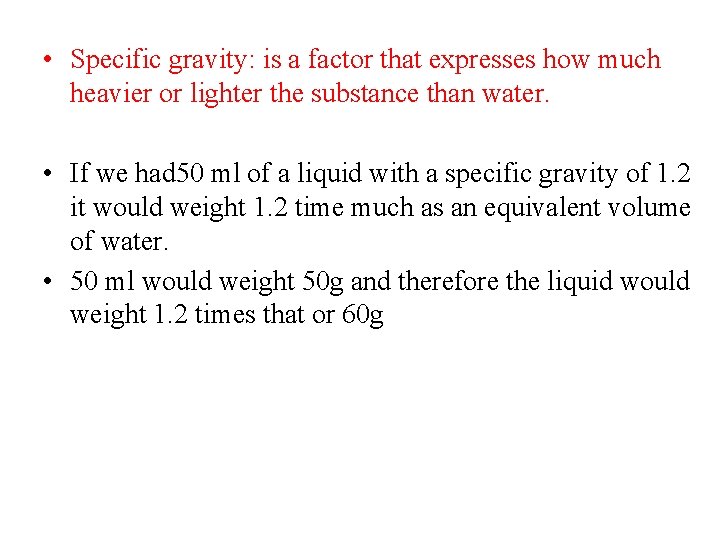





- Slides: 14

Density, Specific Gravity, and Specific Volume Dr. Hayder B Sahib Ph. D. , M. Sc. , DSc. , BSc (Pharmacology)

• Density: is mass /unit volume • It is usually expressed as gram/cubic centimeter (g/cc) • Density of water expressed 1 g/ml • Density= Mass/volume • Example: if 10 ml of sulfuric acid weight 18 g, what is its density? • Density = 18/10= 1. 8 g/ml • Specific Gravity (sp. gr. ): is a ratio expressed decimally of the weight of substance to the weight of an equal volume of substance chosen as a standard, both substances at the same temperature. • Note: water is used as a standard for specific gravity of liquids and solids. The most useful standard for gases is hydrogen.

• Specific Gravity = weight of substance / weight of equal volume of water • sp. gr has no unit • Example : If 10 ml of sulfuric acid weight 18 g and 10 ml of water under similar condition weight 10 g what is the specific gravity of the acid ? • Sp. gr= 18 g/10 ml= 1. 8 • Substances that have sp. gr. less than 1 are lighter than water • Substances that have higher sp. gr. than one are heavier than water
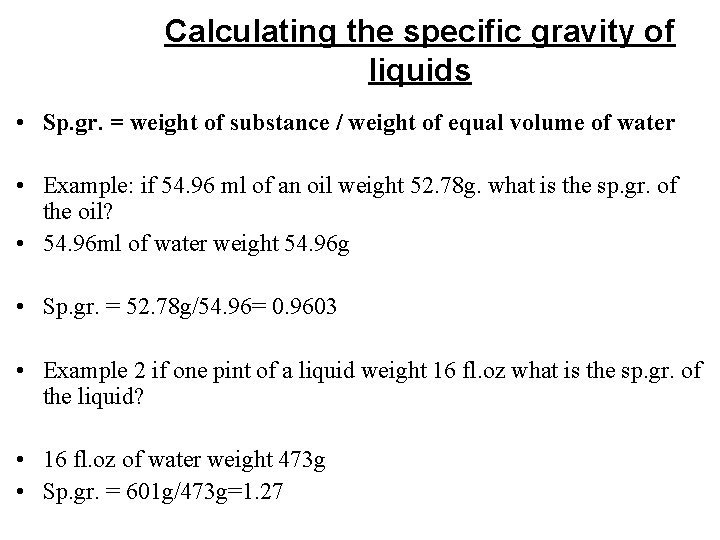
Calculating the specific gravity of liquids • Sp. gr. = weight of substance / weight of equal volume of water • Example: if 54. 96 ml of an oil weight 52. 78 g. what is the sp. gr. of the oil? • 54. 96 ml of water weight 54. 96 g • Sp. gr. = 52. 78 g/54. 96= 0. 9603 • Example 2 if one pint of a liquid weight 16 fl. oz what is the sp. gr. of the liquid? • 16 fl. oz of water weight 473 g • Sp. gr. = 601 g/473 g=1. 27

Pycnometer or specific gravity Bottle • A pycnometer is a special glass bottle used to determine sp. gr it is available in the lab. Its volume ranging from 1 -50 ml. • In using pycnometer, first weight empty bottle, then weigh it again when filled to capacity with water • Example: A 50 ml pycnometer found to weight 120 g when empty. 170 g when filled with water and 160 g when filled with an unknown liquid, calculate the sp. gr of the unknown liquid? • • Sp. gr = wt of substance/ wt of equal volum of water Weight of water = 170 -120=50 g Weight of unknown liquid = 160 -120=40 g Sp. gr. 40/50 = 0. 78

• Example 2 • A sp. gr. bottle weight 23. 66 when filled with water, it weighs 72. 95 g when filled with another liquid it weight 73. 56 g what is the sp. gr. of the liquid ? • 73. 56 -23. 66=49. 90 g • 72. 95 -23. 66=49. 29 g • Sp. gr. = 49. 90/49. 29= 1. 012

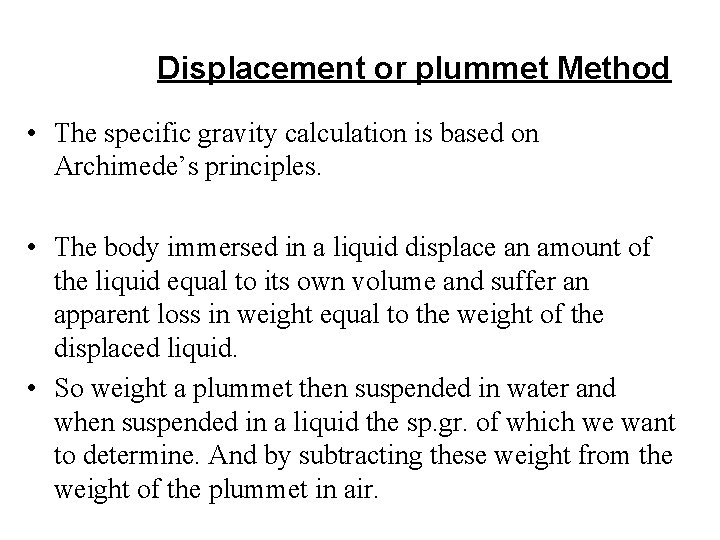
Displacement or plummet Method • The specific gravity calculation is based on Archimede’s principles. • The body immersed in a liquid displace an amount of the liquid equal to its own volume and suffer an apparent loss in weight equal to the weight of the displaced liquid. • So weight a plummet then suspended in water and when suspended in a liquid the sp. gr. of which we want to determine. And by subtracting these weight from the weight of the plummet in air.

• Example • A glass plummet weight 12. 64 g in air, 8. 57 g when immersed water and 9. 12 g when immersed in oil, calculate the sp. gr. of the oil • 12. 64 -9. 12= 3. 52 g • 12. 64 -8. 57 = 4. 07 g displaced water • Sp. gr. = 3. 52 g/4. 07 g = 0. 868 • Note: for the sp. gr of the solid as it has very limited application in the pharmaceutical Sciences so we wont go through it.
• Specific gravity: is a factor that expresses how much heavier or lighter the substance than water. • If we had 50 ml of a liquid with a specific gravity of 1. 2 it would weight 1. 2 time much as an equivalent volume of water. • 50 ml would weight 50 g and therefore the liquid would weight 1. 2 times that or 60 g

Calculate the weight, knowing the volume and specific gravity • Gram = Milliliter X sp. gr • Example: what is the weight in gram of 3620 ml of alcohol having sp. gr. of 0. 820 • 3620 ml of water weight 3620 g • 3620 g X 0. 820 = 2968 • Example: what is the weight in gram of 2 fl. oz of liquid having sp. gr of 1. 118? • 2 X 29. 57 = 59. 14 • 59. 14 ml of water weight 59. 14 g • 59. 14 X 1. 118 = 66. 12 g

Calculating the volume knowing the weight and sp. gr • ml = g/ sp. gr

Pharmaceutical Application of Specific Gravity • • to convert the weight of an ingredient to volume and vice versa in preparation of Parentral Nutrition (TPN) Clinical Application • It is important in urine analysis. In normal adult, the sp. gr of urine is usually within the range 1. 010 to 1. 025 • The specific gravity of urine decrease with age, while in newborn generally within the range of 1. 001 - 1. 020 • The specific gravity is an indicator of both the concentrations of particles in the urine and the patient’s degree of hydration.

• A higher than normal sp. gr indicates that urine is concentrated this may be due to the presence of excess of waste products and electrolyte. • the presence of glucose (glucose urea), protein (protein urea), low fluid intake and excessive water loss. • Low sp. gr indicates that the urine is dilute which may be a result of Diabetes Insipidus, renal disease, increase fluid intake, IV hydration. In modern clinical lab. The sp. gr. of urine is determined by (refractive • index method) but urine analyses preferred by fully automated equipments that determine in seconds urine chemistry, sp. gr, Ph, color, clarity.

• Specific volume: • In Pharmaceutical practice, defined as an abstract number representing the ratio, expressed decimally of the volume of substance to the volume of an equal weight of another substance taken as a standard both having the same temperature, water is the standard • ml = g/ sp. gr