specific heat Capacity EQ What is specific heat




- Slides: 10

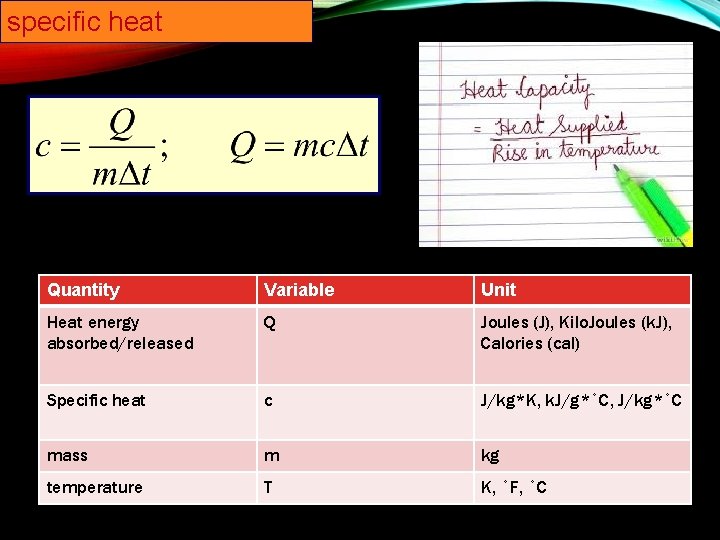
specific heat Capacity EQ: What is specific heat capacity? • We will demonstrate understanding how to measure for specific heat. • I will evaluate the relationship of the variables for the specific heat capacity equation by illustrating the equation

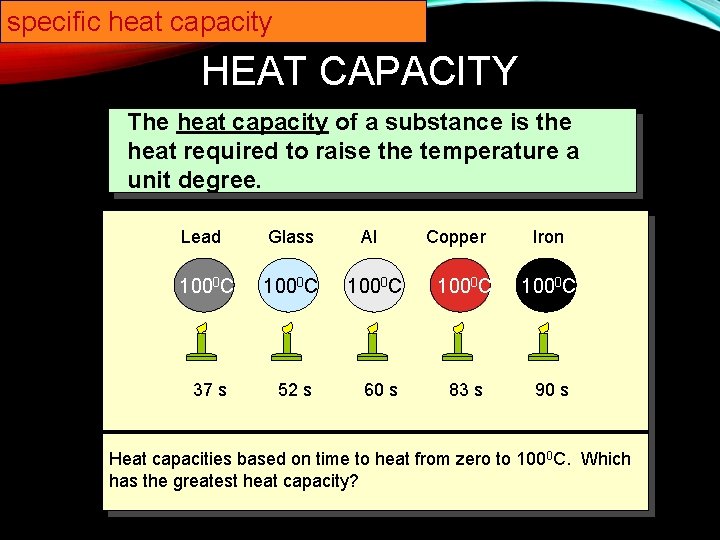
specific heat capacity HEAT CAPACITY The heat capacity of a substance is the heat required to raise the temperature a unit degree. Lead Glass 1000 C 37 s 52 s Al 1000 C 60 s Copper Iron 1000 C 83 s 90 s Heat capacities based on time to heat from zero to 1000 C. Which has the greatest heat capacity?

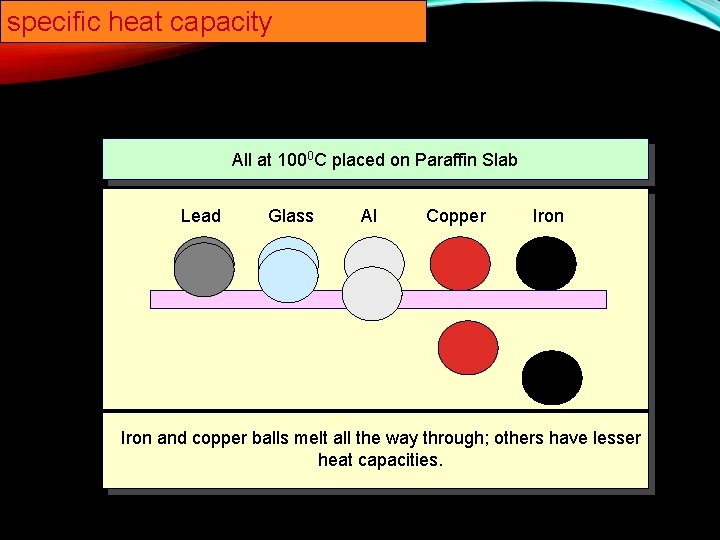
specific heat capacity All at 1000 C placed on Paraffin Slab Lead Glass Al Copper Iron and copper balls melt all the way through; others have lesser heat capacities.

SPECIFIC HEAT • Some objects are easier to heat than others. • When heat flows into an object, its thermal energy and temperature increase. • The amount of the increase in temperature depends on the size of the object, and on the material from which the object is made.

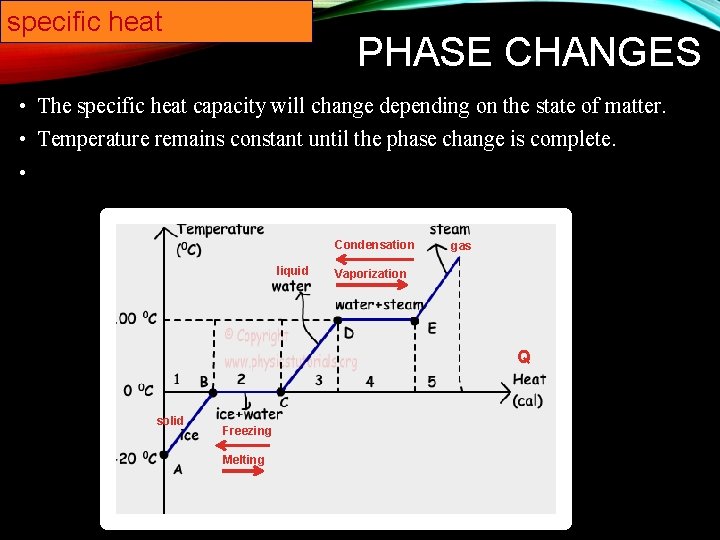
specific heat PHASE CHANGES • The specific heat capacity will change depending on the state of matter. • Temperature remains constant until the phase change is complete. • Condensation liquid gas Vaporization Q solid Freezing Melting

specific heat Quantity Variable Unit Heat energy absorbed/released Q Joules (J), Kilo. Joules (k. J), Calories (cal) Specific heat c J/kg*K, k. J/g*˚C, J/kg*˚C mass m kg temperature T K, ˚F, ˚C

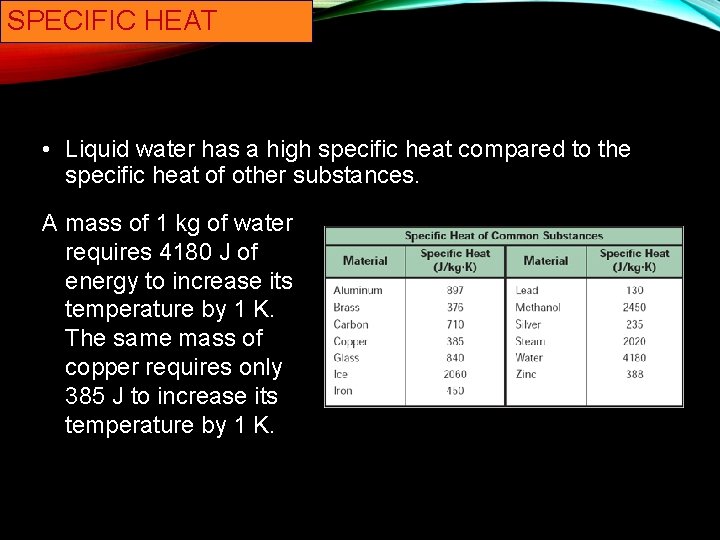
SPECIFIC HEAT • Liquid water has a high specific heat compared to the specific heat of other substances. A mass of 1 kg of water requires 4180 J of energy to increase its temperature by 1 K. The same mass of copper requires only 385 J to increase its temperature by 1 K.

specific heat example WHAT QUANTITY OF HEAT IS REQUIRED TO RAISE THE TEMPERATURE OF 450 GRAMS OF WATER FROM 15°C TO 85°C? THE SPECIFIC HEAT CAPACITY OF WATER IS 4. 180 J/G/°C. The heat required to do this job is: 450 g

specific heat example 1. How much energy must be absorbed by 20. 0 g of water to increase its temperature from 283. 0 ⁰C to 303. 0 ⁰C ? Use the specific heat of water in Joules. 2. If it takes 41. 72 J to heat a piece of gold weighing 18. 69 g from 10. 0 ⁰C to 27. 0 ⁰C. What is the specific heat of gold in J/kg. ⁰C?

CLOSING TASK • You are in charge of developing a tattoo to allow the world to know about “Specific Heat Capacity” • The center of the tattoo must be the equation • The surrounding artwork (minimum of 4 colors) must demonstrate the equation’s concept in a real-life situation. • The artwork must be suitable for all ages and appropriate for viewing in all social situations • A 5 sentence paragraph explainingg how the artwork represents the equation or “Specific Heat Capacity”