Quantifying Heat Thermal Capacity Specific Heat Capacity Melting







- Slides: 18

Quantifying Heat Thermal Capacity Specific Heat Capacity Melting & Boiling Condensation & Solidification

Evidences of Heat Transfer Change in temperature Change of state Change in amount

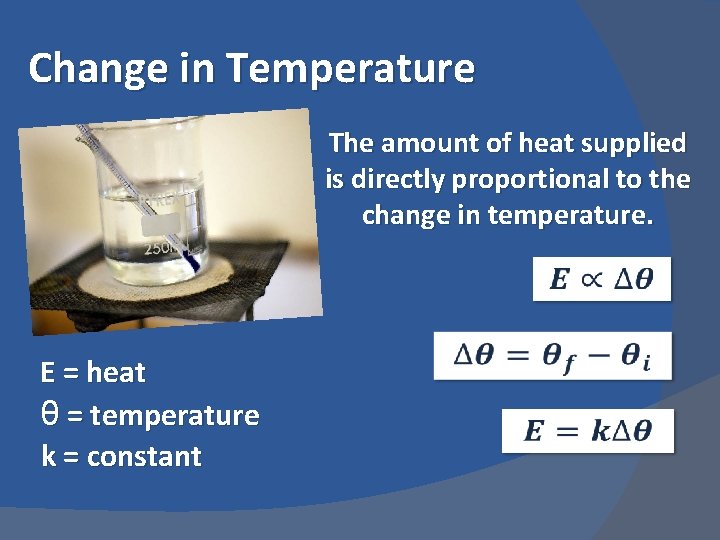
Change in Temperature The amount of heat supplied is directly proportional to the change in temperature. E = heat θ = temperature k = constant

Thermal Capacity The amount of heat required to change the temperature of a substance by 1 C°.

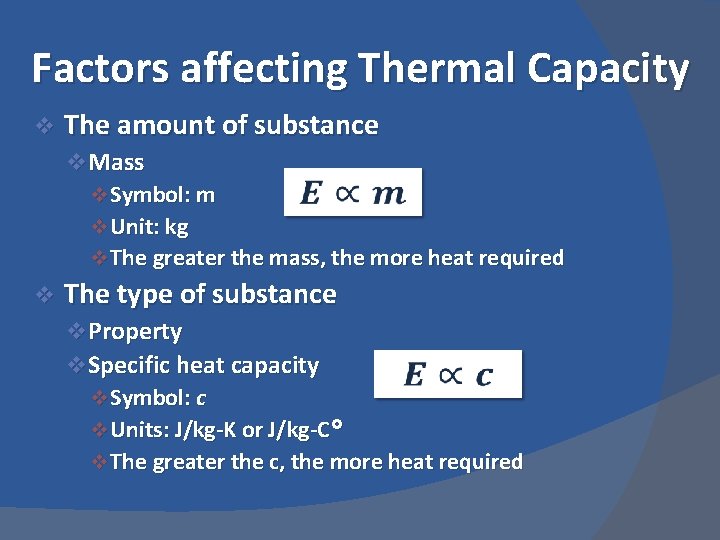
Factors affecting Thermal Capacity v The amount of substance v. Mass v Symbol: m v Unit: kg v The greater the mass, the more heat required v The type of substance v. Property v. Specific heat capacity v Symbol: c v Units: J/kg-K or J/kg-C° v The greater the c, the more heat required

Specific Heat Capacity The amount of heat required to change the temperature of a kilogram of substance by 1 C°. Tells how difficult it is to change the temperature of a substance

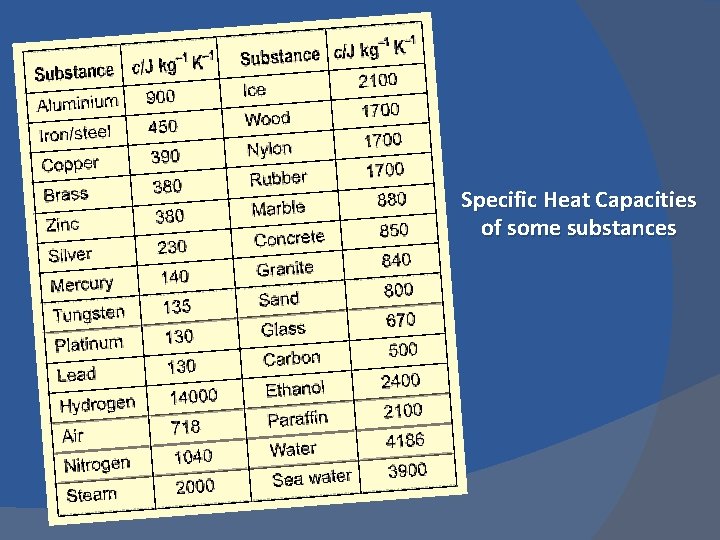
Specific Heat Capacities of some substances

Water is used as heating fluid in radiators. England is green. Water is in a hot compress Pies make you cry.

Quantifying Heat (with change in Temperature) s m e l b o r P e l p m Sa Page 89, SQ Joule meter

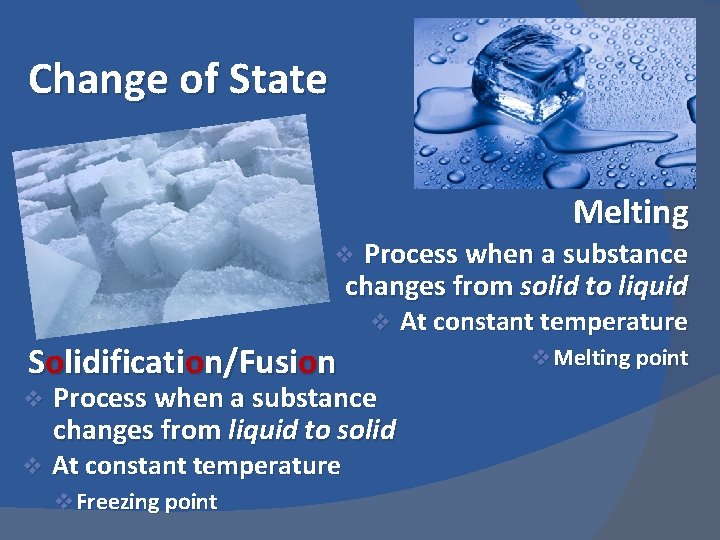
Change of State Melting Process when a substance changes from solid to liquid v v Solidification/Fusion v v Process when a substance changes from liquid to solid At constant temperature v Freezing point At constant temperature v Melting point

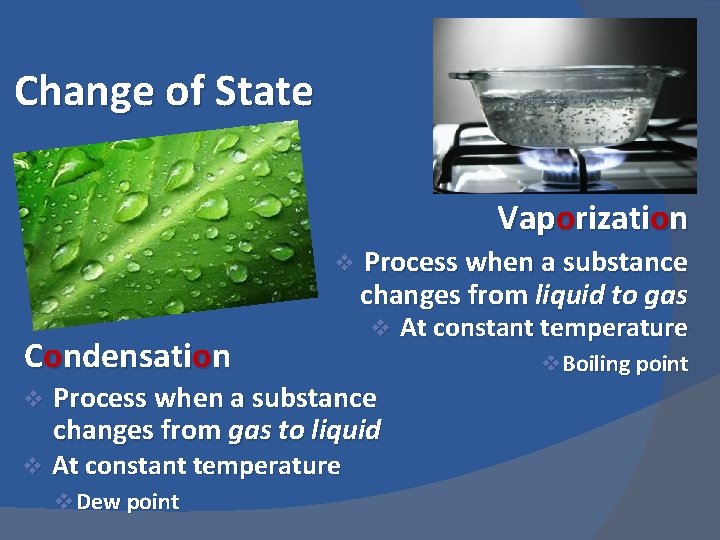
Change of State Vaporization v Condensation v v Process when a substance changes from liquid to gas v Process when a substance changes from gas to liquid At constant temperature v Dew point At constant temperature v Boiling point

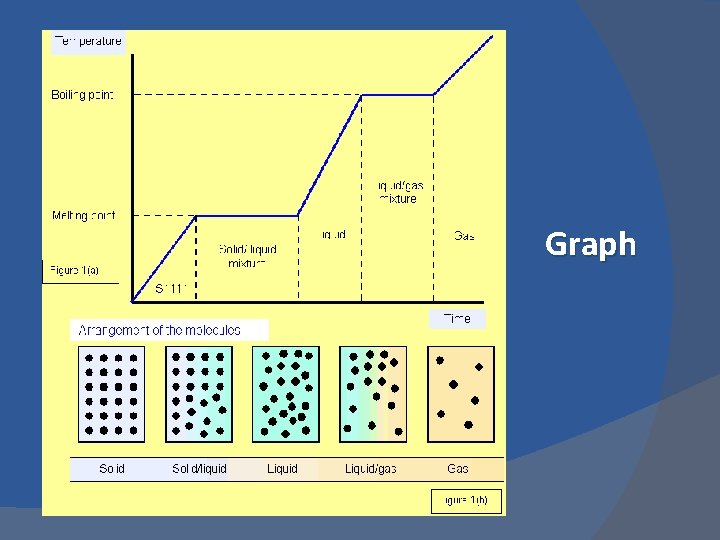
Graph

Boiling versus Evaporation Boiling Evaporation c i f i c e p s a t a s r u Occ temperature Occurs at any temp erature Occurs inside t he liquid e c a f r u s e h t t a Occurs s a g o t id u q li m Change from liquid to gas

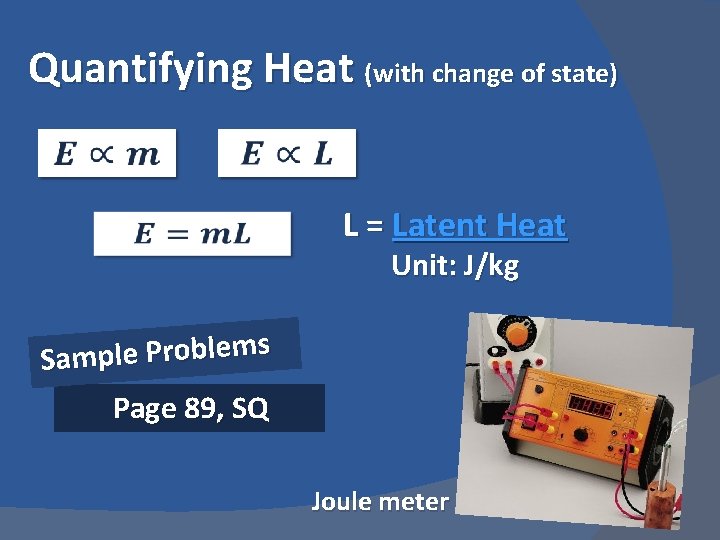
Quantifying Heat (with change of state) L = Latent Heat Unit: J/kg s m e l b o r P e l p m Sa Page 89, SQ Joule meter

p U g n i l e v e L

p U g n i l e v Le A 500 g piece of aluminium is heated with a 500 W heater for 10 minutes. (a) How much energy will be given to the aluminium in this time? (b) If the temperature of the aluminium was 20°C at the beginning, what will its temperature be after 10 minutes?

Latent Heat The amount of heat required to change the state of a kilogram of a substance. Latent heat of fusion for liquid to solid and vice versa Latent heat of vaporization for liquid to gas and vice versa

Latent Heat Used to break molecular bonds Changes potential energy No change in kinetic energy