Enthalpy Calculations And the specific heat equation Enthalpy




- Slides: 18

Enthalpy Calculations And the specific heat equation

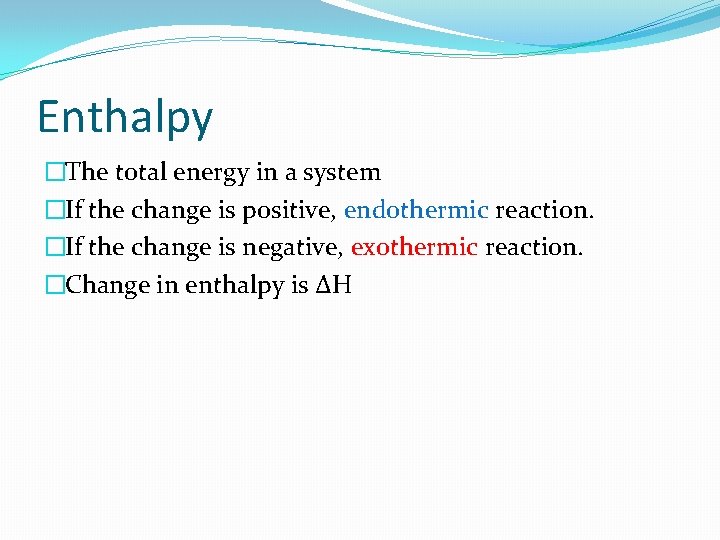
Enthalpy �The total energy in a system �If the change is positive, endothermic reaction. �If the change is negative, exothermic reaction. �Change in enthalpy is ∆H

Enthalpy calculation �In some cases we are give the values and we simply add them up. �In effect, the energy required to break the bonds added to the energy released when the new bonds are formed. �These are the simple ones!

Specific heat capacity �As we do so many reactions in water, we measure the temperature change of the water. �Do you need as much energy to heat 100 g of water by 5 K as would be needed to heat 100 g of air by the same amount? �Why?

Bonding again �Due to differences in bonding, many substances can absorb more energy than others. �This is called the Specific Heat Capacity. �Water has a higher heat capacity than air and therefore needs more energy to raise its temperature. �The specific heat capacity of water is 4. 18 Jg-1

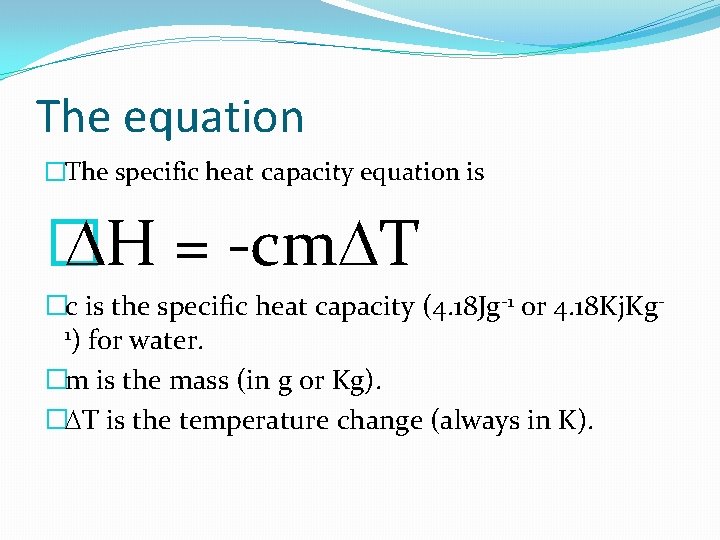
The equation �The specific heat capacity equation is � DH = -cm. DT �c is the specific heat capacity (4. 18 Jg-1 or 4. 18 Kj. Kg 1) for water. �m is the mass (in g or Kg). �DT is the temperature change (always in K).

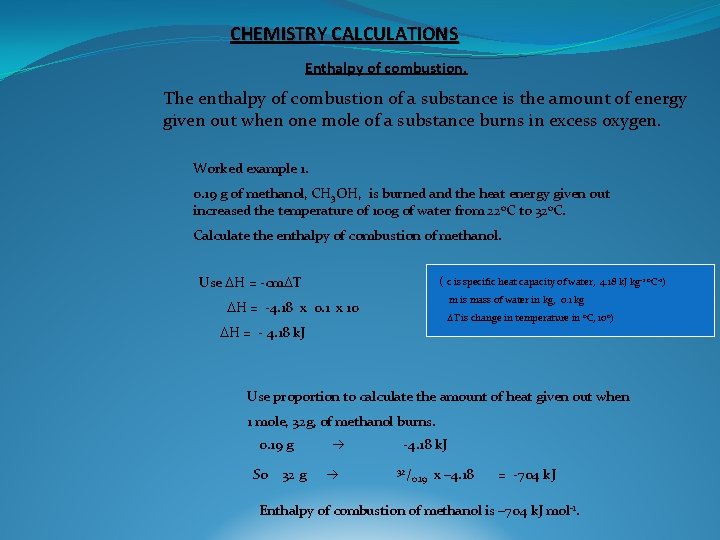
CHEMISTRY CALCULATIONS Enthalpy of combustion. The enthalpy of combustion of a substance is the amount of energy given out when one mole of a substance burns in excess oxygen. Worked example 1. 0. 19 g of methanol, CH 3 OH, is burned and the heat energy given out increased the temperature of 100 g of water from 22 o. C to 32 o. C. Calculate the enthalpy of combustion of methanol. Use DH = -cm. DT ( c is specific heat capacity of water, 4. 18 k. J kg-1 o. C-1) m is mass of water in kg, 0. 1 kg DH = -4. 18 x 0. 1 x 10 DT is change in temperature in o. C, 10 o) DH = - 4. 18 k. J Use proportion to calculate the amount of heat given out when 1 mole, 32 g, of methanol burns. 0. 19 g So 32 g -4. 18 k. J 32/ 0. 19 x – 4. 18 = -704 k. J Enthalpy of combustion of methanol is – 704 k. J mol-1.

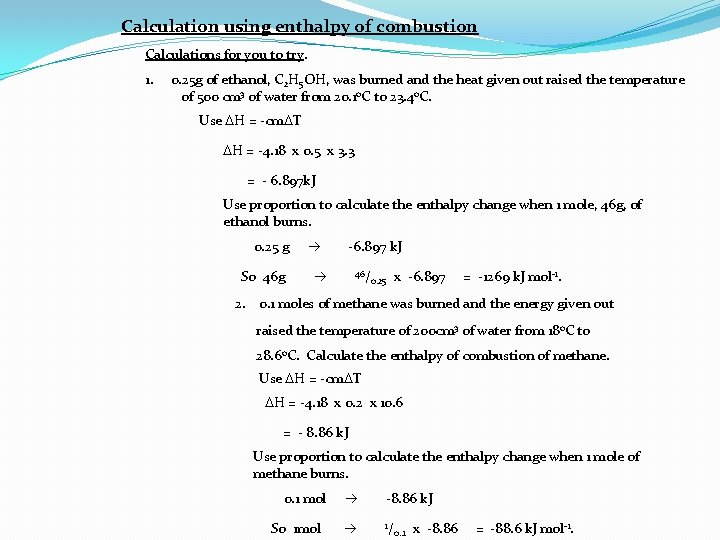
Calculation using enthalpy of combustion Calculations for you to try. 1. 0. 25 g of ethanol, C 2 H 5 OH, was burned and the heat given out raised the temperature of 500 cm 3 of water from 20. 1 o. C to 23. 4 o. C. Use DH = -cm. DT DH = -4. 18 x 0. 5 x 3. 3 = - 6. 897 k. J Use proportion to calculate the enthalpy change when 1 mole, 46 g, of ethanol burns. 0. 25 g So 46 g -6. 897 k. J 46/ 0. 25 x -6. 897 = -1269 k. J mol-1. 2. 0. 1 moles of methane was burned and the energy given out raised the temperature of 200 cm 3 of water from 18 o. C to 28. 6 o. C. Calculate the enthalpy of combustion of methane. Use DH = -cm. DT DH = -4. 18 x 0. 2 x 10. 6 = - 8. 86 k. J Use proportion to calculate the enthalpy change when 1 mole of methane burns. 0. 1 mol So 1 mol -8. 86 k. J 1/ 0. . 1 x -8. 86 = -88. 6 k. J mol-1.

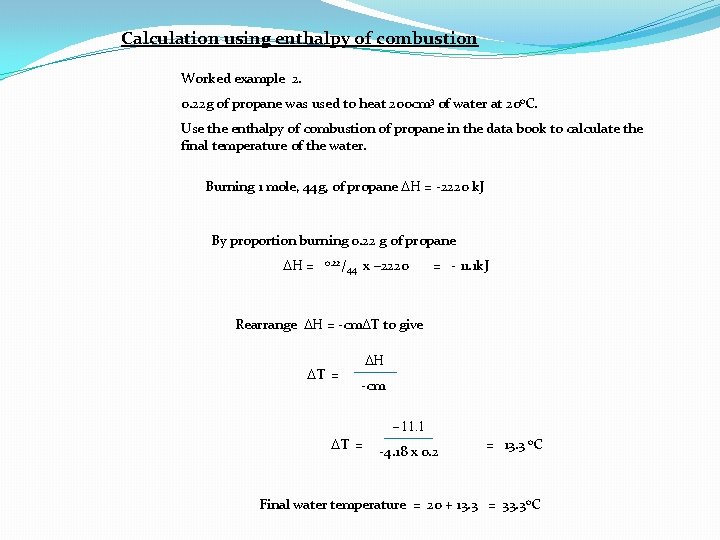
Calculation using enthalpy of combustion Worked example 2. 0. 22 g of propane was used to heat 200 cm 3 of water at 20 o. C. Use the enthalpy of combustion of propane in the data book to calculate the final temperature of the water. Burning 1 mole, 44 g, of propane DH = -2220 k. J By proportion burning 0. 22 g of propane DH = 0. 22/ x – 2220 44 = - 11. 1 k. J Rearrange DH = -cm. DT to give DT = DH -cm -11. 1 DT = -4. 18 x 0. 2 = 13. 3 o. C Final water temperature = 20 + 13. 3 = 33. 3 o. C

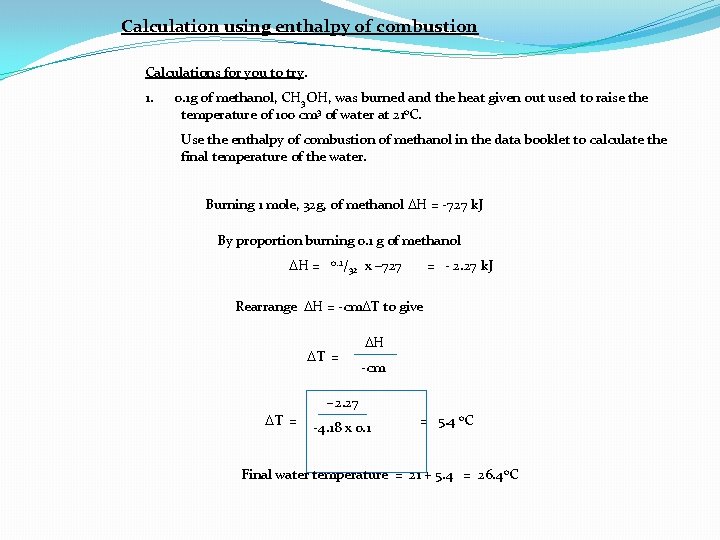
Calculation using enthalpy of combustion Calculations for you to try. 1. 0. 1 g of methanol, CH 3 OH, was burned and the heat given out used to raise the temperature of 100 cm 3 of water at 21 o. C. Use the enthalpy of combustion of methanol in the data booklet to calculate the final temperature of the water. Burning 1 mole, 32 g, of methanol DH = -727 k. J By proportion burning 0. 1 g of methanol DH = 0. . 1/ 32 x – 727 = - 2. 27 k. J Rearrange DH = -cm. DT to give DT = DH -cm -2. 27 DT = -4. 18 x 0. 1 = 5. 4 o. C Final water temperature = 21 + 5. 4 = 26. 4 o. C

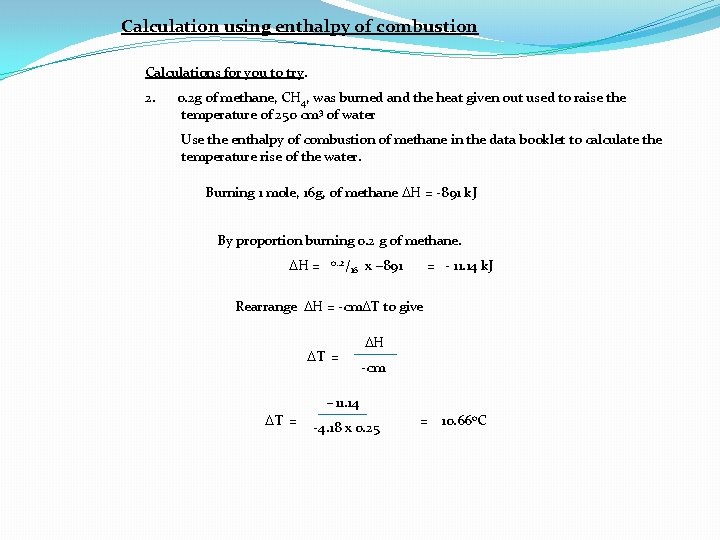
Calculation using enthalpy of combustion Calculations for you to try. 2. 0. 2 g of methane, CH 4, was burned and the heat given out used to raise the temperature of 250 cm 3 of water Use the enthalpy of combustion of methane in the data booklet to calculate the temperature rise of the water. Burning 1 mole, 16 g, of methane DH = -891 k. J By proportion burning 0. 2 g of methane. DH = 0. . 2/ 16 x – 891 = - 11. 14 k. J Rearrange DH = -cm. DT to give DT = DH -cm -11. 14 DT = -4. 18 x 0. 25 = 10. 66 o. C

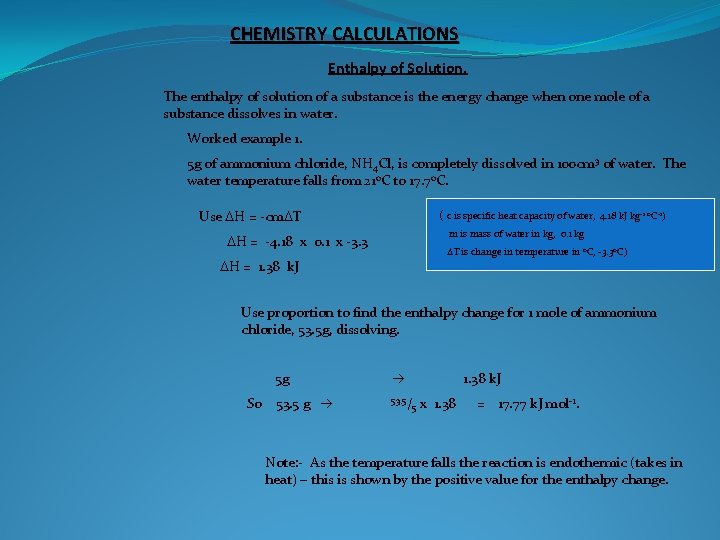
CHEMISTRY CALCULATIONS Enthalpy of Solution. The enthalpy of solution of a substance is the energy change when one mole of a substance dissolves in water. Worked example 1. 5 g of ammonium chloride, NH 4 Cl, is completely dissolved in 100 cm 3 of water. The water temperature falls from 21 o. C to 17. 7 o. C. Use DH = -cm. DT ( c is specific heat capacity of water, 4. 18 k. J kg-1 o. C-1) m is mass of water in kg, 0. 1 kg DH = -4. 18 x 0. 1 x -3. 3 DT is change in temperature in o. C, -3. 3 o. C) DH = 1. 38 k. J Use proportion to find the enthalpy change for 1 mole of ammonium chloride, 53. 5 g, dissolving. So 5 g 53. 5 g 53. 5/ 1. 38 k. J 5 x 1. 38 = 17. 77 k. J mol-1. Note: - As the temperature falls the reaction is endothermic (takes in heat) – this is shown by the positive value for the enthalpy change.

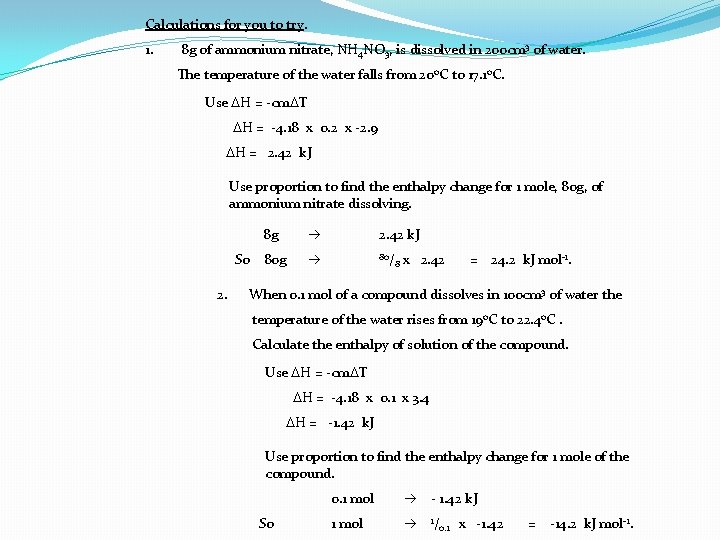
Calculations for you to try. 1. 8 g of ammonium nitrate, NH 4 NO 3, is dissolved in 200 cm 3 of water. The temperature of the water falls from 20 o. C to 17. 1 o. C. Use DH = -cm. DT DH = -4. 18 x 0. 2 x -2. 9 DH = 2. 42 k. J Use proportion to find the enthalpy change for 1 mole, 80 g, of ammonium nitrate dissolving. 8 g So 80 g 2. 2. 42 k. J 80/ 8 x 2. 42 = 24. 2 k. J mol-1. When 0. 1 mol of a compound dissolves in 100 cm 3 of water the temperature of the water rises from 19 o. C to 22. 4 o. C. Calculate the enthalpy of solution of the compound. Use DH = -cm. DT DH = -4. 18 x 0. 1 x 3. 4 DH = -1. 42 k. J Use proportion to find the enthalpy change for 1 mole of the compound. So 0. 1 mol - 1. 42 k. J 1 mol 1/ 0. 1 x -1. 42 = -14. 2 k. J mol-1.

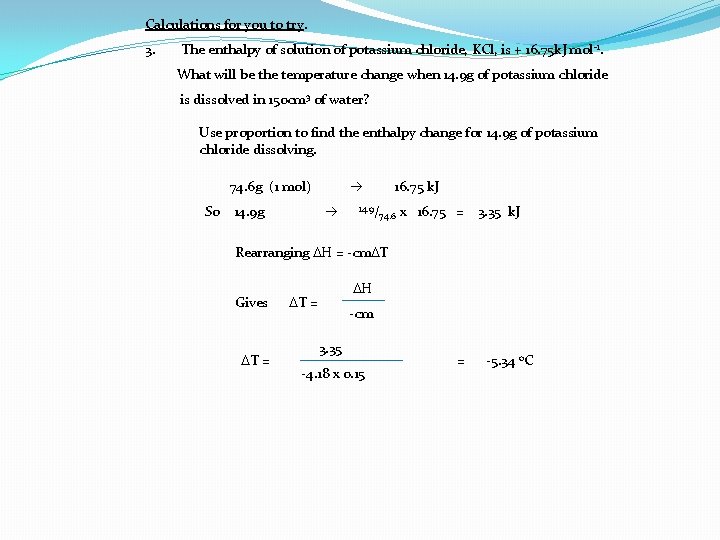
Calculations for you to try. 3. The enthalpy of solution of potassium chloride, KCl, is + 16. 75 k. J mol -1. What will be the temperature change when 14. 9 g of potassium chloride is dissolved in 150 cm 3 of water? Use proportion to find the enthalpy change for 14. 9 g of potassium chloride dissolving. 74. 6 g (1 mol) So 14. 9 g 16. 75 k. J 14. 9/ 74. 6 x 16. 75 = 3. 35 k. J Rearranging DH = -cm. DT Gives DT = DH DT = -cm 3. 35 -4. 18 x 0. 15 = -5. 34 o. C

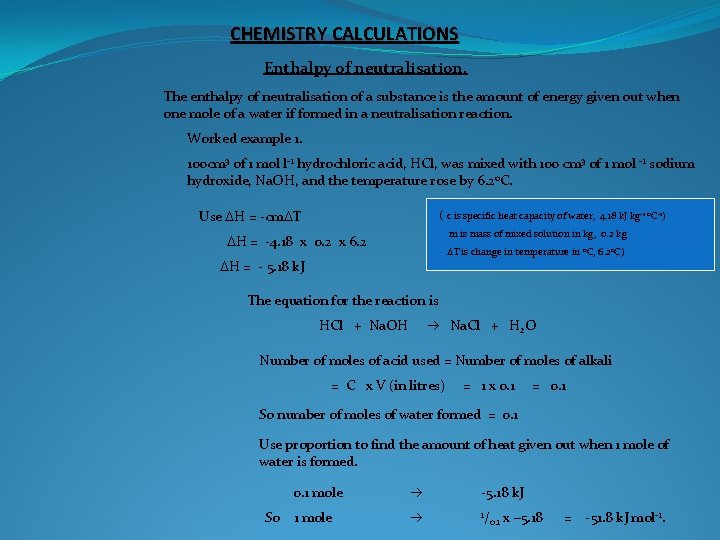
CHEMISTRY CALCULATIONS Enthalpy of neutralisation. The enthalpy of neutralisation of a substance is the amount of energy given out when one mole of a water if formed in a neutralisation reaction. Worked example 1. 100 cm 3 of 1 mol l-1 hydrochloric acid, HCl, was mixed with 100 cm 3 of 1 mol -1 sodium hydroxide, Na. OH, and the temperature rose by 6. 2 o. C. Use DH = -cm. DT ( c is specific heat capacity of water, 4. 18 k. J kg-1 o. C-1) m is mass of mixed solution in kg, 0. 2 kg DH = -4. 18 x 0. 2 x 6. 2 DT is change in temperature in o. C, 6. 2 o. C) DH = - 5. 18 k. J The equation for the reaction is HCl + Na. OH Na. Cl + H 2 O Number of moles of acid used = Number of moles of alkali = C x V (in litres) = 1 x 0. 1 = 0. 1 So number of moles of water formed = 0. 1 Use proportion to find the amount of heat given out when 1 mole of water is formed. So 0. 1 mole -5. 18 k. J 1 mole 1/ 0. 1 x – 5. 18 = -51. 8 k. J mol-1.

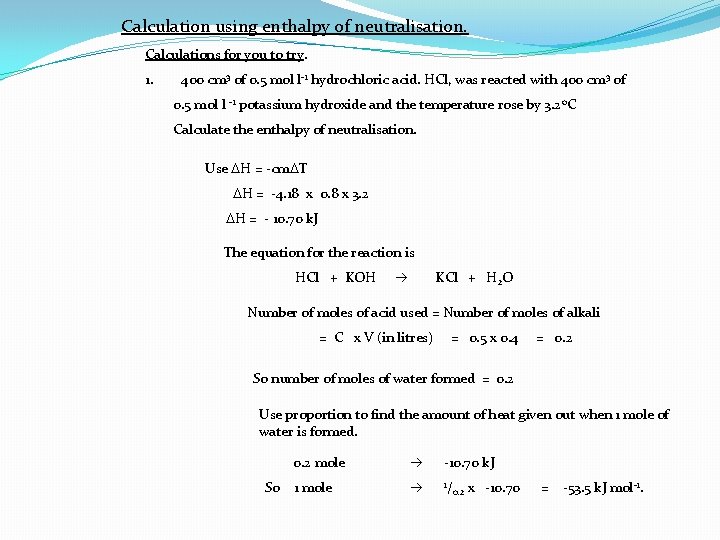
Calculation using enthalpy of neutralisation. Calculations for you to try. 1. 400 cm 3 of 0. 5 mol l-1 hydrochloric acid. HCl, was reacted with 400 cm 3 of 0. 5 mol l -1 potassium hydroxide and the temperature rose by 3. 2 o. C Calculate the enthalpy of neutralisation. Use DH = -cm. DT DH = -4. 18 x 0. 8 x 3. 2 DH = - 10. 70 k. J The equation for the reaction is HCl + KOH KCl + H 2 O Number of moles of acid used = Number of moles of alkali = C x V (in litres) = 0. 5 x 0. 4 = 0. 2 So number of moles of water formed = 0. 2 Use proportion to find the amount of heat given out when 1 mole of water is formed. So 0. 2 mole -10. 70 k. J 1 mole 1/ 0. 2 x -10. 70 = -53. 5 k. J mol-1.

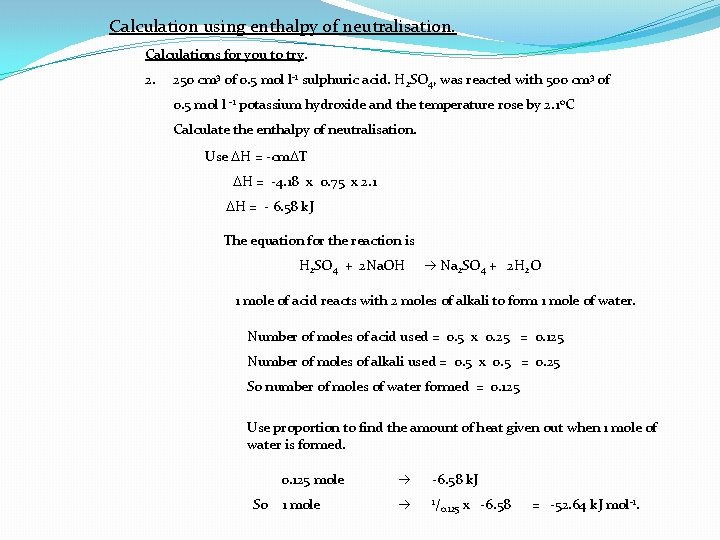
Calculation using enthalpy of neutralisation. Calculations for you to try. 2. 250 cm 3 of 0. 5 mol l-1 sulphuric acid. H 2 SO 4, was reacted with 500 cm 3 of 0. 5 mol l -1 potassium hydroxide and the temperature rose by 2. 1 o. C Calculate the enthalpy of neutralisation. Use DH = -cm. DT DH = -4. 18 x 0. 75 x 2. 1 DH = - 6. 58 k. J The equation for the reaction is H 2 SO 4 + 2 Na. OH Na 2 SO 4 + 2 H 2 O 1 mole of acid reacts with 2 moles of alkali to form 1 mole of water. Number of moles of acid used = 0. 5 x 0. 25 = 0. 125 Number of moles of alkali used = 0. 5 x 0. 5 = 0. 25 So number of moles of water formed = 0. 125 Use proportion to find the amount of heat given out when 1 mole of water is formed. So 0. 125 mole -6. 58 k. J 1 mole 1/ 0. 125 x -6. 58 = -52. 64 k. J mol-1.

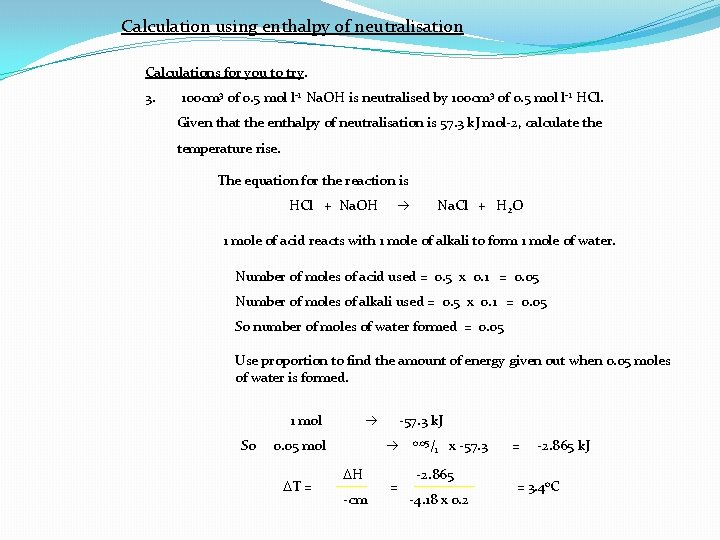
Calculation using enthalpy of neutralisation Calculations for you to try. 3. 100 cm 3 of 0. 5 mol l-1 Na. OH is neutralised by 100 cm 3 of 0. 5 mol l-1 HCl. Given that the enthalpy of neutralisation is 57. 3 k. J mol-2, calculate the temperature rise. The equation for the reaction is HCl + Na. OH Na. Cl + H 2 O 1 mole of acid reacts with 1 mole of alkali to form 1 mole of water. Number of moles of acid used = 0. 5 x 0. 1 = 0. 05 Number of moles of alkali used = 0. 5 x 0. 1 = 0. 05 So number of moles of water formed = 0. 05 Use proportion to find the amount of energy given out when 0. 05 moles of water is formed. 1 mol So 0. 05 mol DT = -57. 3 k. J DH -cm = 0. 05/ 1 x -57. 3 -2. 865 -4. 18 x 0. 2 = -2. 865 k. J = 3. 4 o. C