Enthalpy Changes Chapter 6 Standards 5 1 5

















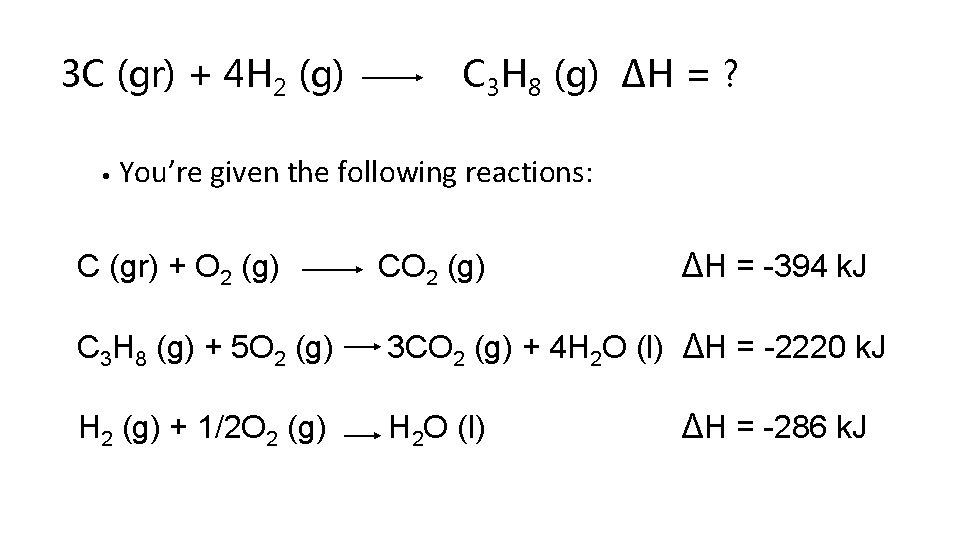
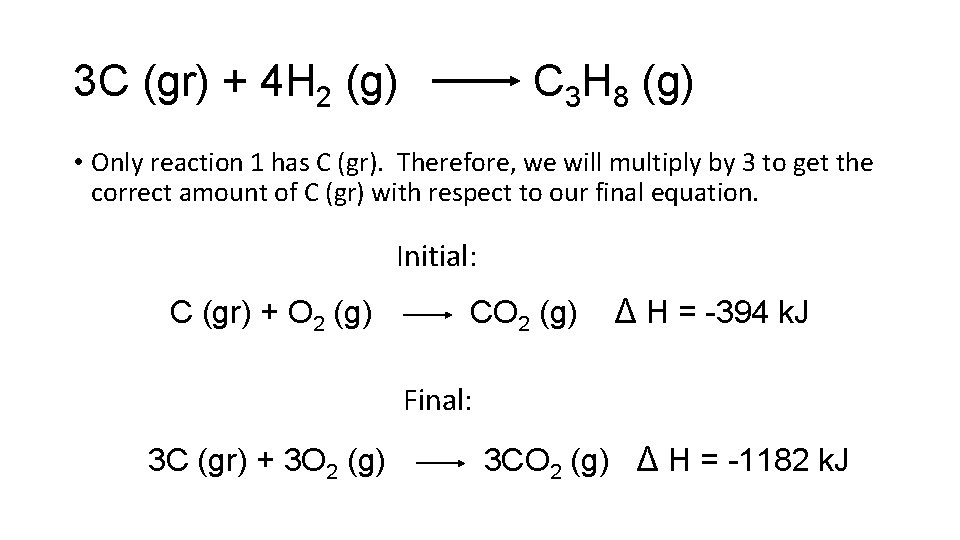
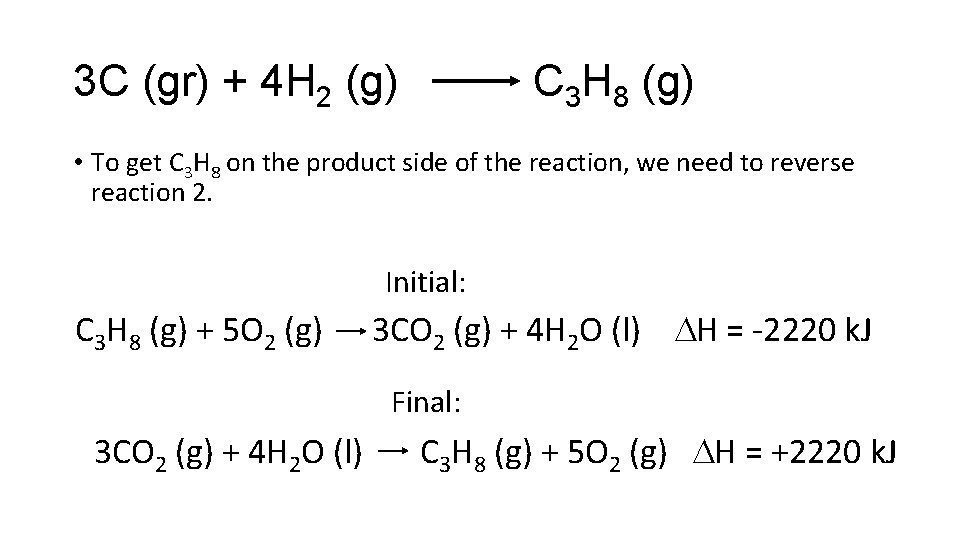
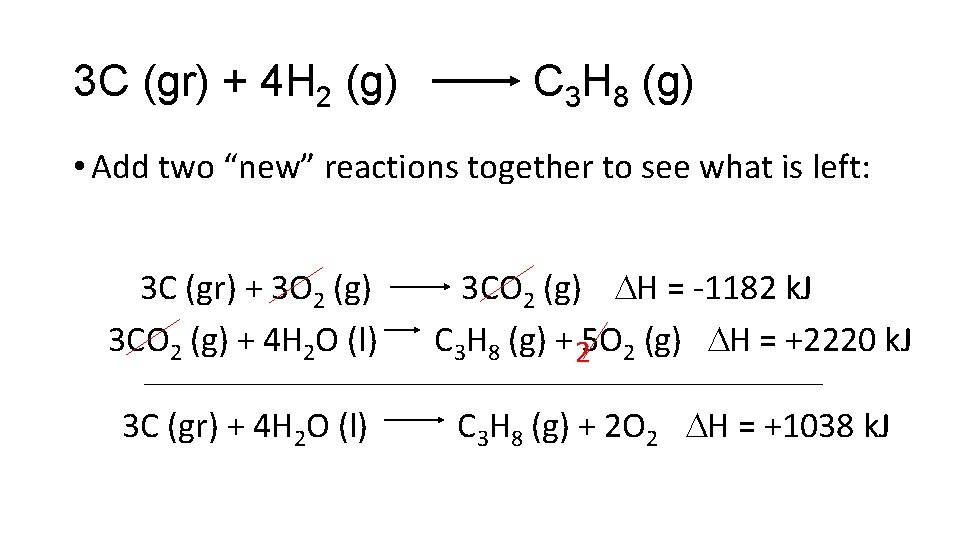
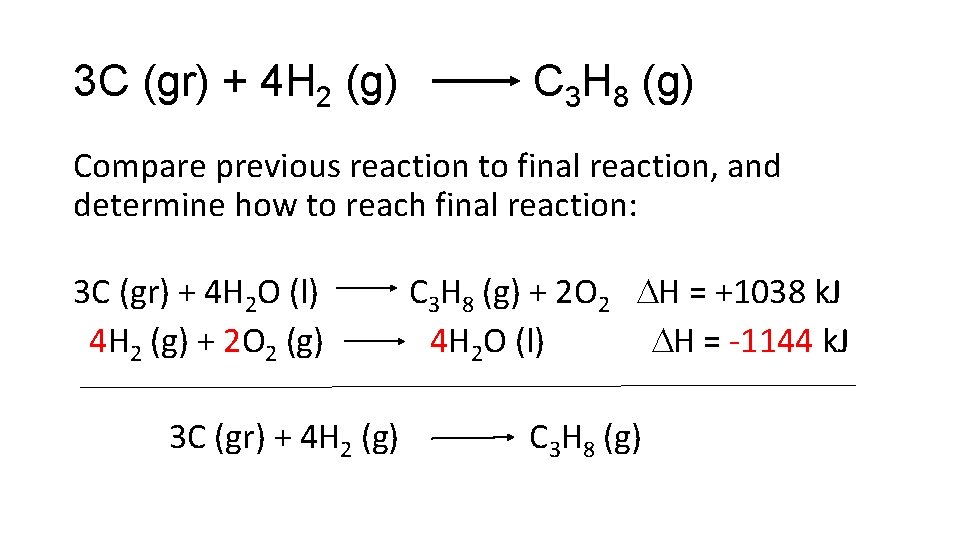
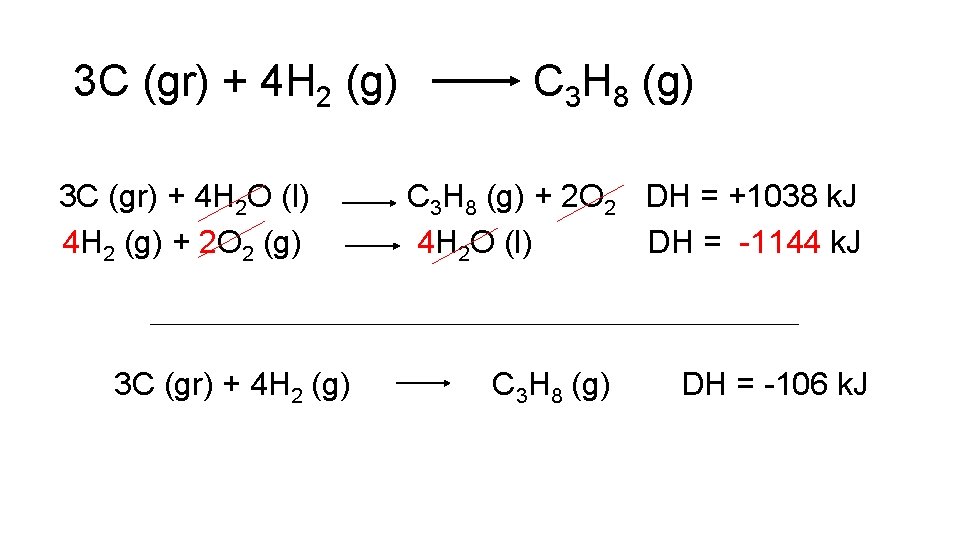
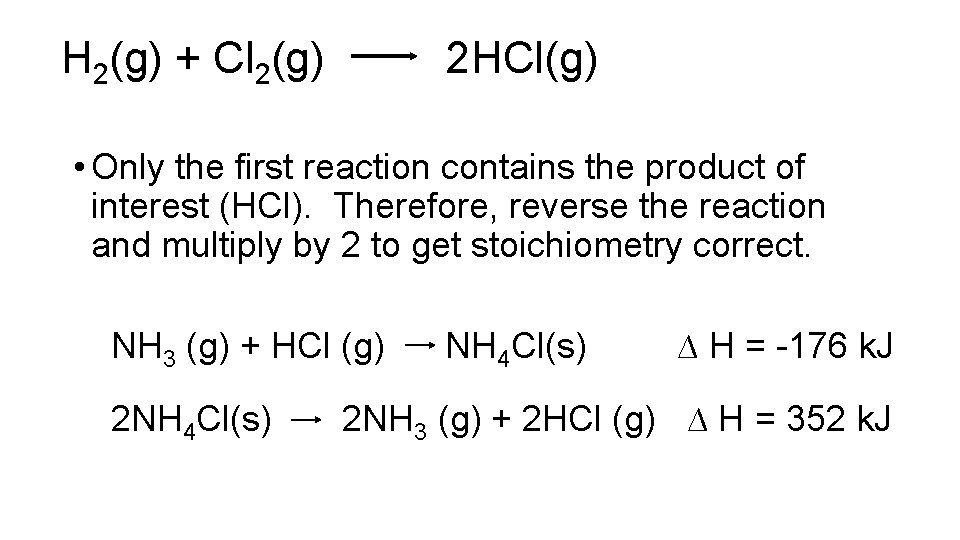
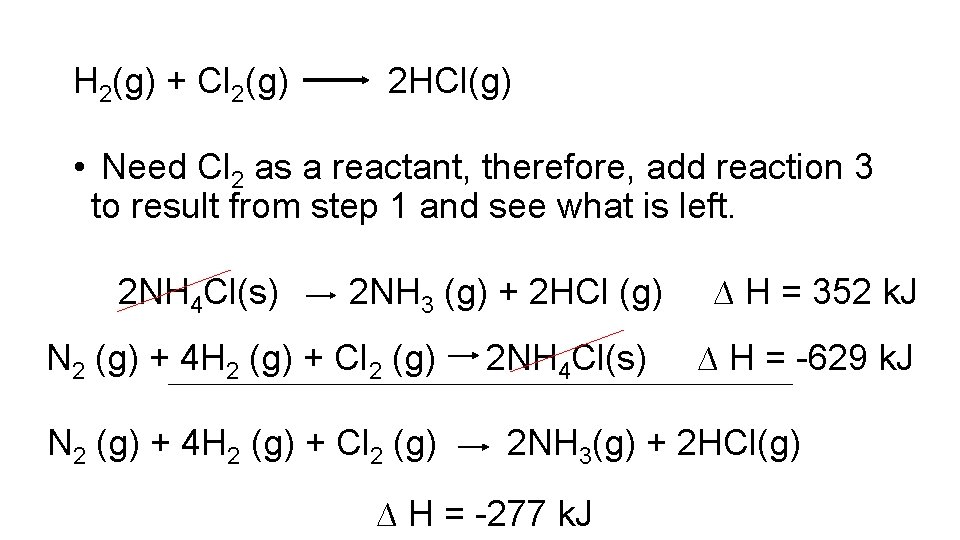








- Slides: 147

Enthalpy Changes Chapter 6 Standards 5. 1, 5. 2, 5. 3 and 5. 4

standards • 5. 1 Enthalpy change ∆H • 5. 2 Hess’ Law, including Born-Haber cycles

Learning outcomes • 5. 1. a explain that chemical reactions are accompanied by energy changes, principally in the form of heat energy; the energy changes can be exothermic (∆H is negative) or endothermic (∆ H is positive) • 5. 1. b explain and use the terms: • Enthalpy change of reaction and standard conditions, with particular reference to: formation, combustion, hydration, solution, neutralization, atomization • Bond energy (∆ H positive, ie. Bond breaking) • Lattice energy (∆ H negative, ie gaseous ions to solid lattice) • 5. 1. c calculate enthalpy changes from appropriate experimental results, including the use of the relationship: enthalpy change, ∆H= -mc ∆T • 5. 2. a apply Hess’ Law to construct simple energy cycles, and carry out calculations involving such cycles and relevant energy terms, with particular reference to: • Determining enthalpy changes that cannot be found by direct experiment: enthalpy change combustion • Average bond energies • 5. 2. b construct and interpret a reaction pathway diagram, in terms of the enthalpy change of the reaction and of the activation energy

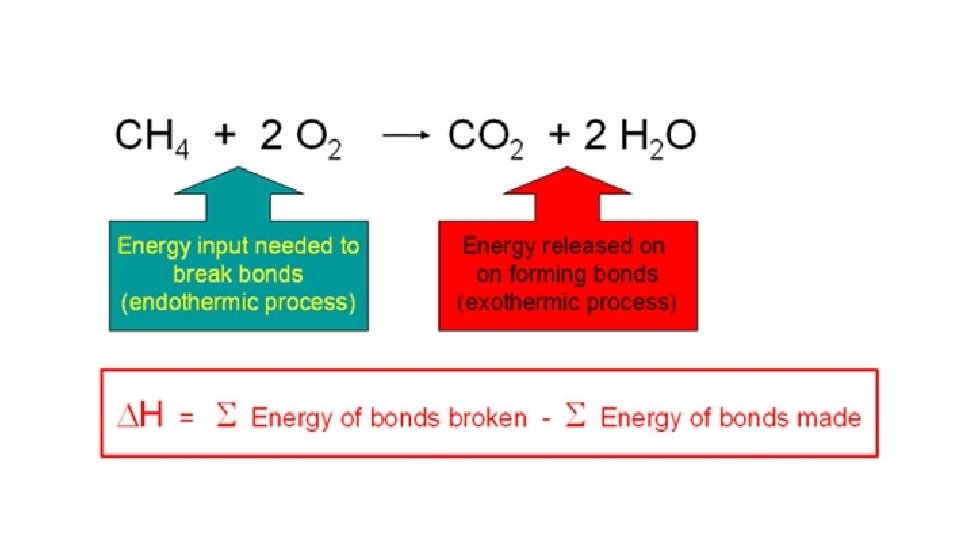
Energy changes during chemical reactions • During a chemical reactions bonds are broken and/or made. When this occurs we can gain or lose chemical potential energy. If we lose chemical potential energy then it must go somewhere because energy is never created or destroyed, it just changes form. In the case of chemical potential energy it transforms into heat energy. • The chemical potential energy of a chemical can also be called Enthalpy. • The enthalpy of a chemical is measured in kilojoules per mole (k. Jmol-1). • Any chemical reaction will involve breaking some bonds and making new ones. Energy is needed to break bonds, and is given out when the new bonds are formed. It is very unlikely that these two processes will involve exactly the same amount of energy - and so some energy will either be absorbed or released during a reaction

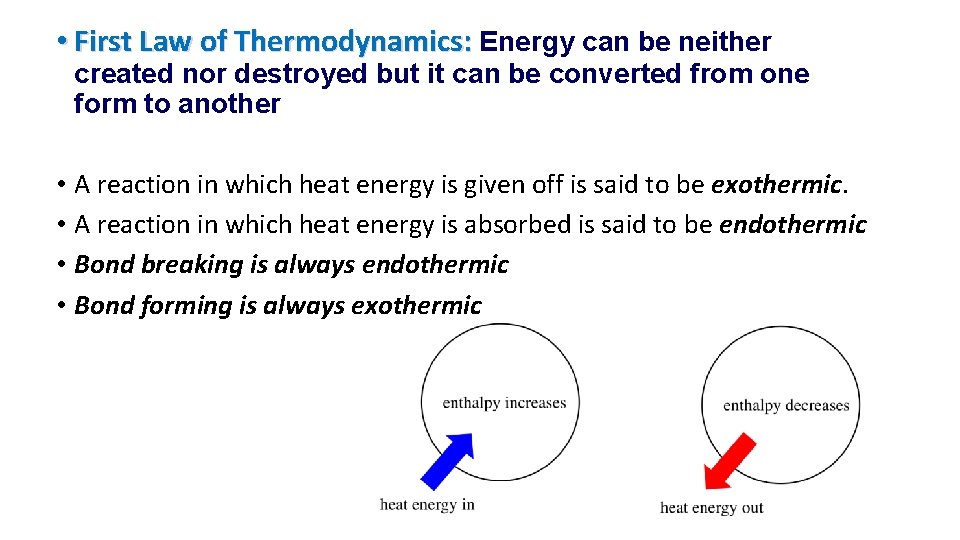
• First Law of Thermodynamics: Energy can be neither created nor destroyed but it can be converted from one form to another • A reaction in which heat energy is given off is said to be exothermic. • A reaction in which heat energy is absorbed is said to be endothermic • Bond breaking is always endothermic • Bond forming is always exothermic

exothermic • Reaction that releases energy into the surroundings • In other words exothermic reactions are reactions where heat is made. For example a fire is an exothermic reaction. • Exothermic reactions show that the reactants have lost chemical potential energy (enthalpy). This can also be expressed by saying they have a negative change in enthalpy (- ΔH). • Examples of exothermic reactions: • Combustion of fuel • Oxidation of carbohydrates in the bodies of animals and plants (respiration) • Reaction of water with quicklime (calcium oxide)

endothermic • Reactions that absorb energy from the surrounding • Temperature of surroundings decreases. The reaction causes the chemicals to get colder • An endothermic reaction causes and increase in enthalpy because the heat energy has been transformed into chemical potential energy. This can also be stated by saying that there has been a positive change in enthalpy (+ ΔH). • Bond breaking reactions are endothermic. • Examples • Decomposition of calcium carbonate (limestone) • Photosynthesis • Dissolving certain ammonium salts in water

Enthalpy • How much energy is in a molecule • Unfortunately its next to impossible to know that number • What we can measure is the change • We can measure how much energy has moved into or out or a system during a chemical reaction • The change in energy is called enthalpy

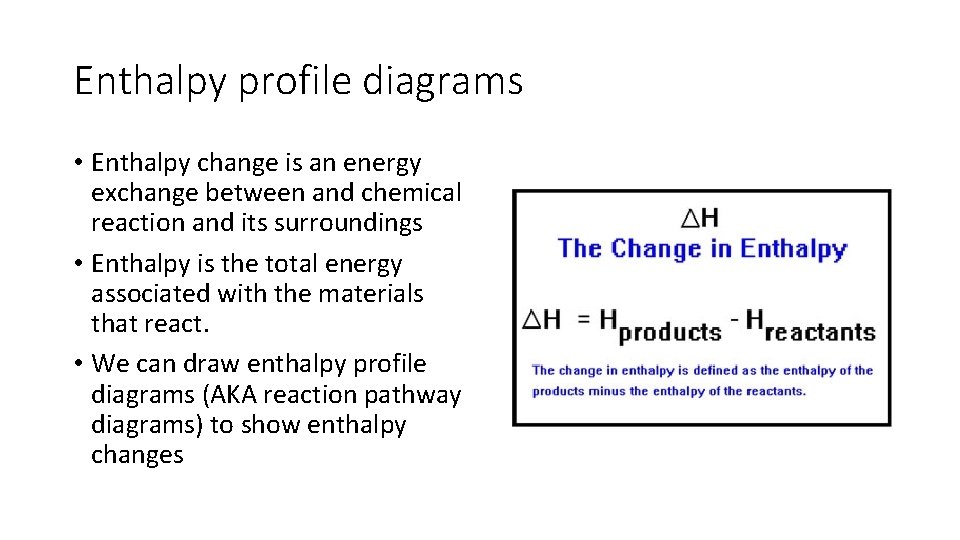
Enthalpy profile diagrams • Enthalpy change is an energy exchange between and chemical reaction and its surroundings • Enthalpy is the total energy associated with the materials that react. • We can draw enthalpy profile diagrams (AKA reaction pathway diagrams) to show enthalpy changes

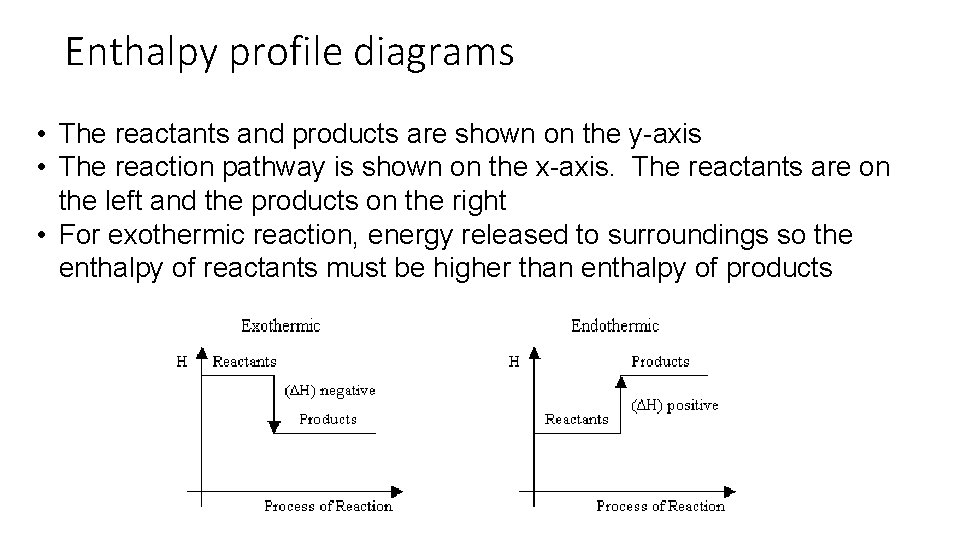
Enthalpy profile diagrams • The reactants and products are shown on the y-axis • The reaction pathway is shown on the x-axis. The reactants are on the left and the products on the right • For exothermic reaction, energy released to surroundings so the enthalpy of reactants must be higher than enthalpy of products

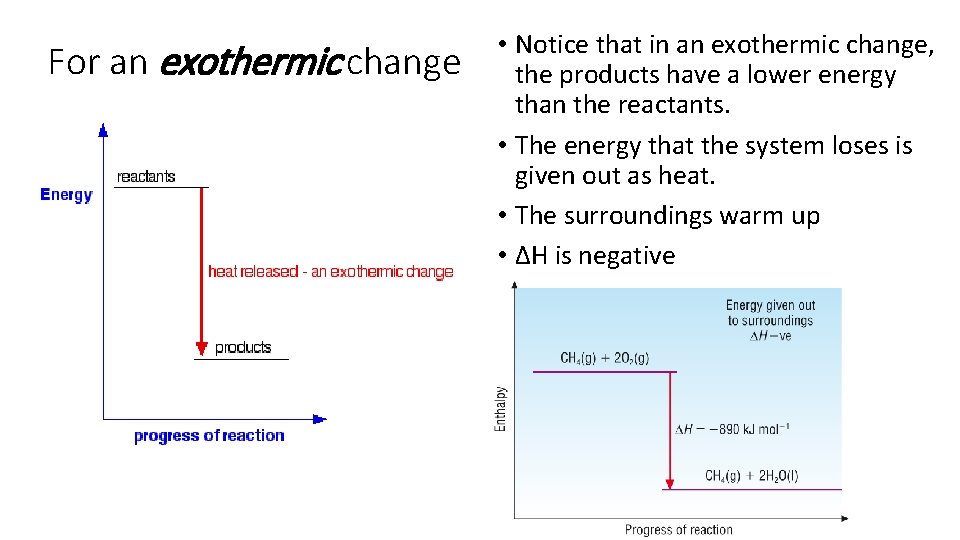
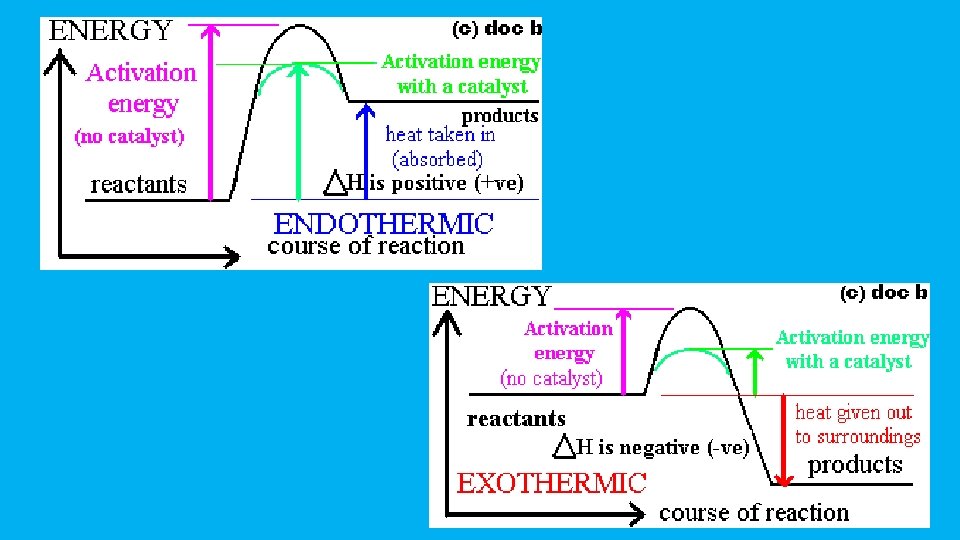
For an exothermic change • Notice that in an exothermic change, the products have a lower energy than the reactants. • The energy that the system loses is given out as heat. • The surroundings warm up • ∆H is negative

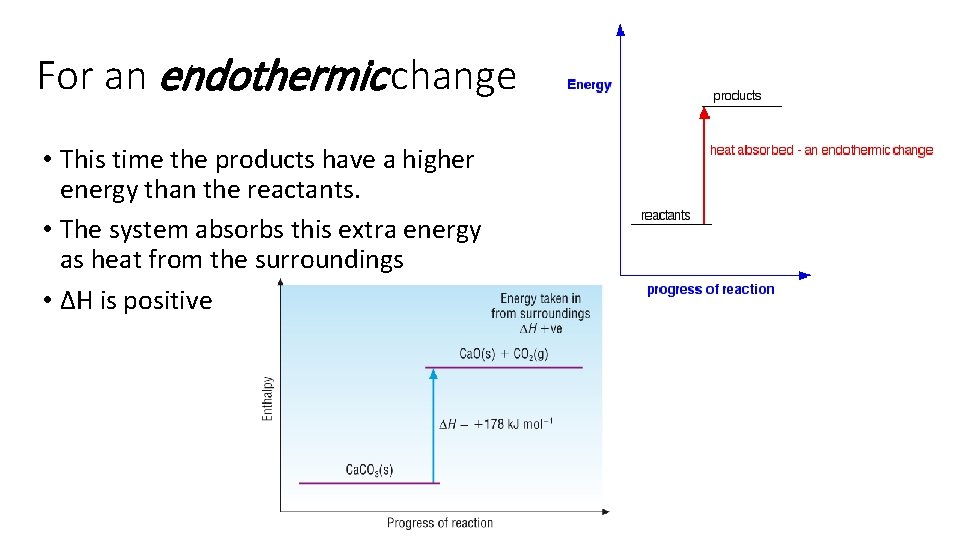
For an endothermic change • This time the products have a higher energy than the reactants. • The system absorbs this extra energy as heat from the surroundings • ∆H is positive

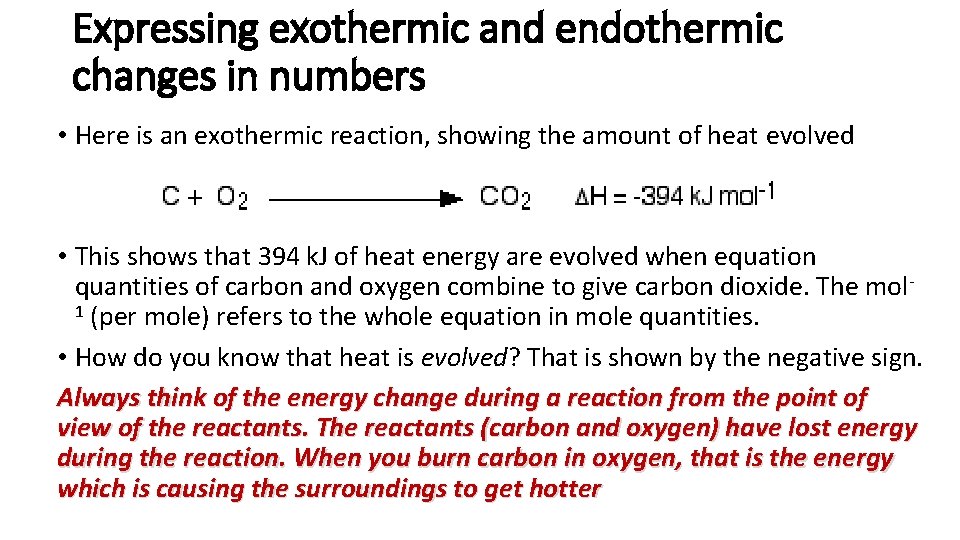
Expressing exothermic and endothermic changes in numbers • Here is an exothermic reaction, showing the amount of heat evolved • This shows that 394 k. J of heat energy are evolved when equation quantities of carbon and oxygen combine to give carbon dioxide. The mol 1 (per mole) refers to the whole equation in mole quantities. • How do you know that heat is evolved? That is shown by the negative sign. Always think of the energy change during a reaction from the point of view of the reactants. The reactants (carbon and oxygen) have lost energy during the reaction. When you burn carbon in oxygen, that is the energy which is causing the surroundings to get hotter

Expressing exothermic and endothermic changes in numbers • Here is an endothermic change • In this case, 178 k. J of heat are absorbed when 1 mole of calcium carbonate reacts to give 1 mole of calcium oxide and 1 mole of carbon dioxide. • You can tell that energy is being absorbed because of the plus sign.

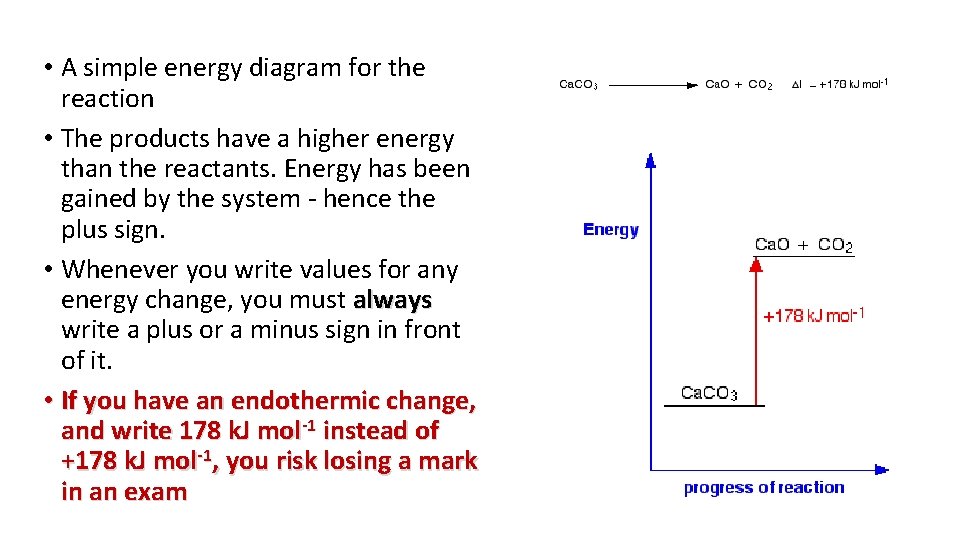
• A simple energy diagram for the reaction • The products have a higher energy than the reactants. Energy has been gained by the system - hence the plus sign. • Whenever you write values for any energy change, you must always write a plus or a minus sign in front of it. • If you have an endothermic change, and write 178 k. J mol-1 instead of +178 k. J mol-1, you risk losing a mark in an exam

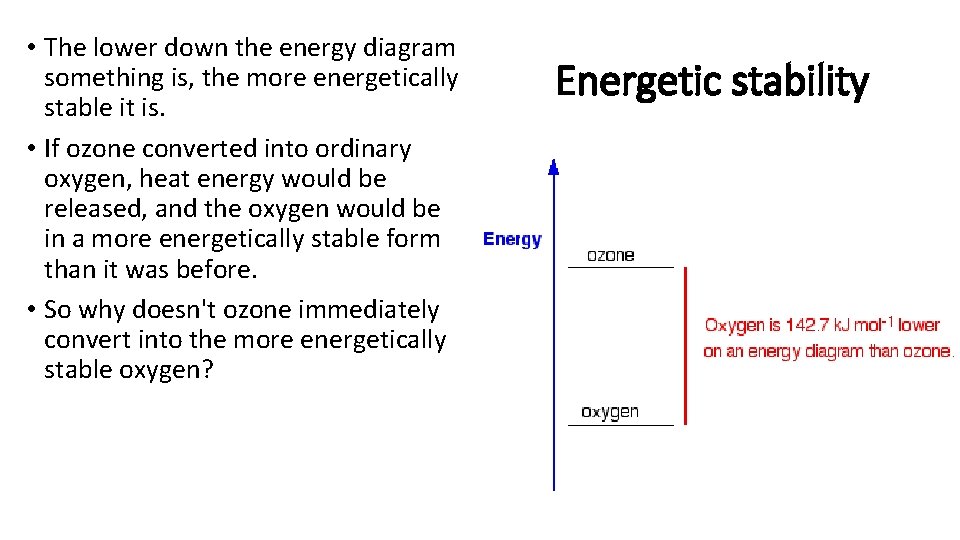
• The lower down the energy diagram something is, the more energetically stable it is. • If ozone converted into ordinary oxygen, heat energy would be released, and the oxygen would be in a more energetically stable form than it was before. • So why doesn't ozone immediately convert into the more energetically stable oxygen? Energetic stability

Activation Energy • Similarly, if you mix gasoline and air at ordinary temperatures (when you are filling up a car, for example), why doesn't it immediately convert into carbon dioxide and water? It would be much more energetically stable if it turned into carbon dioxide and water - you can tell that, because lots of heat is given out when gas burns in air. But there is no reaction when you mix the two. • For any reaction to happen, bonds have to be broken, and new ones made. Breaking bonds takes energy. There is a minimum amount of energy needed before a reaction can start -activation energy. • If the molecules don't hit each other with enough energy, then nothing happens. We say that the mixture is kinetically stable, even though it may be energetically unstable with respect to its possible products

• So a gasoline and air mixture at ordinary temperatures doesn't react, even though a lot of energy would be released if the reaction took place. Gas and air are energetically unstable with respect to carbon dioxide and water - they are much higher up the energy diagram. But a petrol and air mixture is kinetically stable at ordinary temperatures, because the activation energy barrier is too high • If you expose the mixture to a flame or a spark, then you get a major fire or explosion. The initial flame supplies activation energy. The heat given out by the molecules that react first is more than enough to supply the activation energy for the next molecules to react - and so on • The moral of all this is that you should be very careful using the word "stable" in chemistry! And yes, Ms. Strauss is chemically stable, mentally stable is different question

Enthalpy changes • Enthalpy change is the name given to the amount of heat evolved or absorbed in a reaction carried out at constant pressure. It is given the symbol ΔH, read as "delta H“ • Standard enthalpy changes refer to reactions done under standard conditions, and with everything present in their standard states. Standard states are sometimes referred to as "reference states“ • Standard conditions are: 101 k. Pa 275 K or 0 o. C

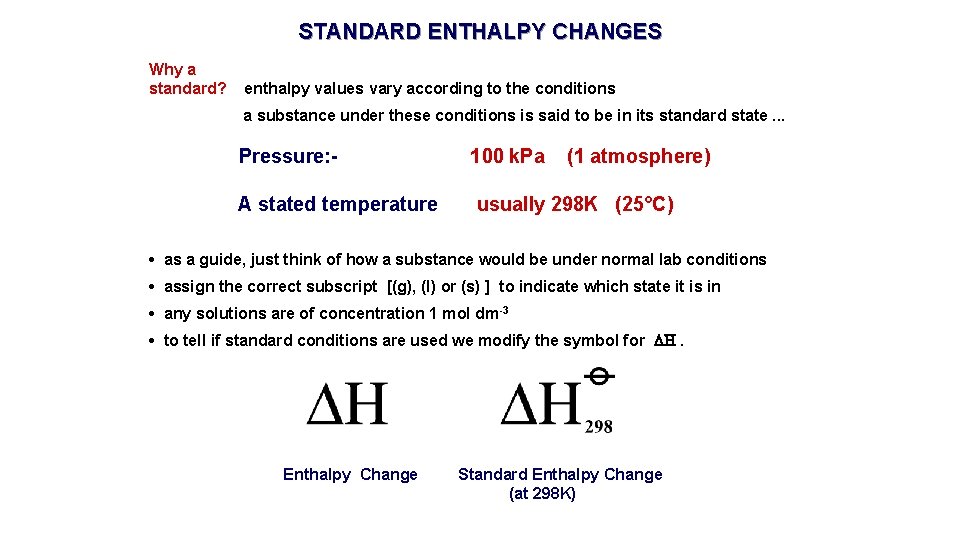
STANDARD ENTHALPY CHANGES Why a standard? enthalpy values vary according to the conditions a substance under these conditions is said to be in its standard state. . . Pressure: A stated temperature 100 k. Pa (1 atmosphere) usually 298 K (25°C) • as a guide, just think of how a substance would be under normal lab conditions • assign the correct subscript [(g), (l) or (s) ] to indicate which state it is in • any solutions are of concentration 1 mol dm-3 • to tell if standard conditions are used we modify the symbol for H. Enthalpy Change Standard Enthalpy Change (at 298 K)

Standard states • For a standard enthalpy change everything has to be present in its standard state. That is the physical and chemical state that you would expect to find it in under standard conditions • That means that the standard state for water, for example, is liquid water, H 2 O(l) - not steam or water vapor or ice • Oxygen's standard state is the gas, O 2(g) - not liquid oxygen or oxygen atoms • For elements which have allotropes (two different forms of the element in the same physical state), the standard state is the most energetically stable of the allotropes • carbon exists in the solid state as both diamond and graphite. Graphite is energetically slightly more stable than diamond, and so graphite is taken as the standard state of carbon. • oxygen can exist as O 2 or as O 3 (called ozone - an allotrope of oxygen). The O 2 form is far more energetically stable than O 3, so the standard state for oxygen is the common O 2(g)

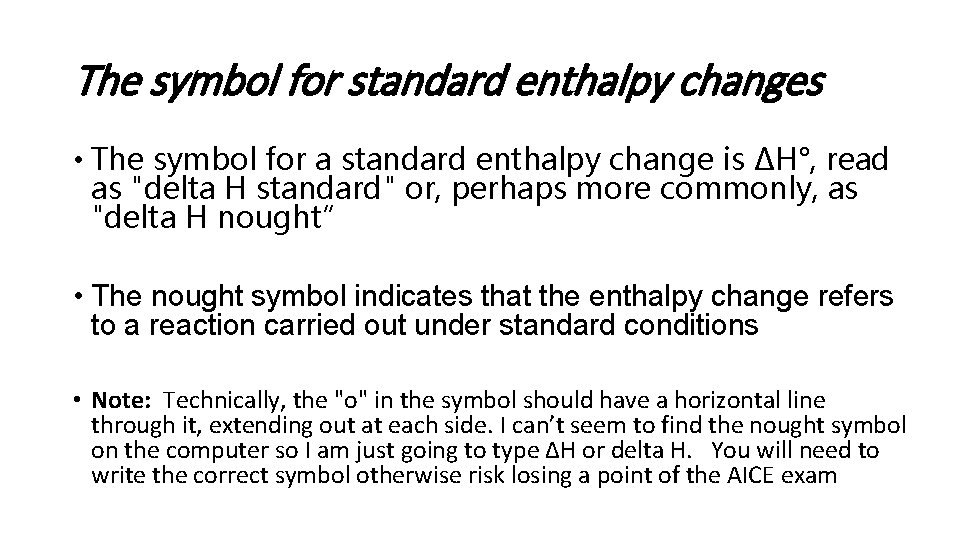
The symbol for standard enthalpy changes • The symbol for a standard enthalpy change is ΔH°, read as "delta H standard" or, perhaps more commonly, as "delta H nought” • The nought symbol indicates that the enthalpy change refers to a reaction carried out under standard conditions • Note: Technically, the "o" in the symbol should have a horizontal line through it, extending out at each side. I can’t seem to find the nought symbol on the computer so I am just going to type ∆H or delta H. You will need to write the correct symbol otherwise risk losing a point of the AICE exam

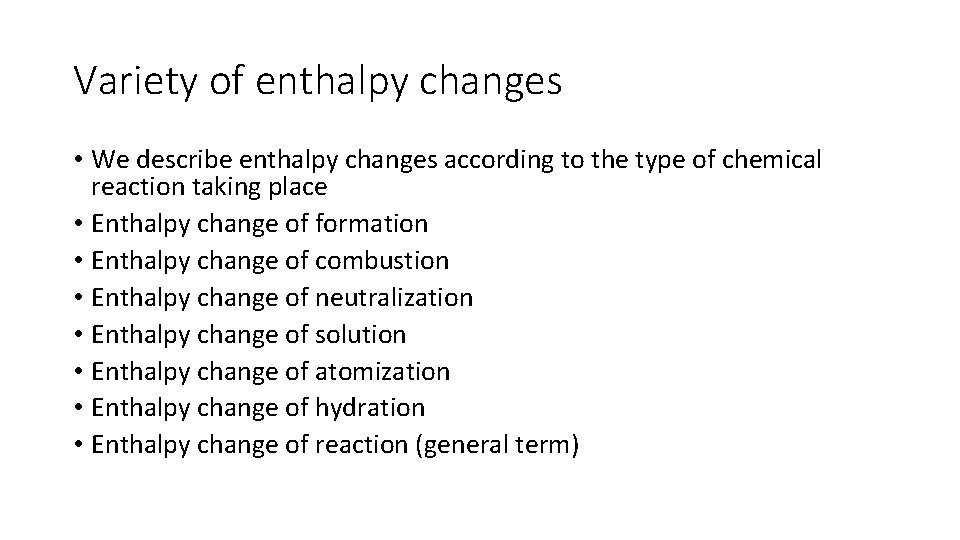
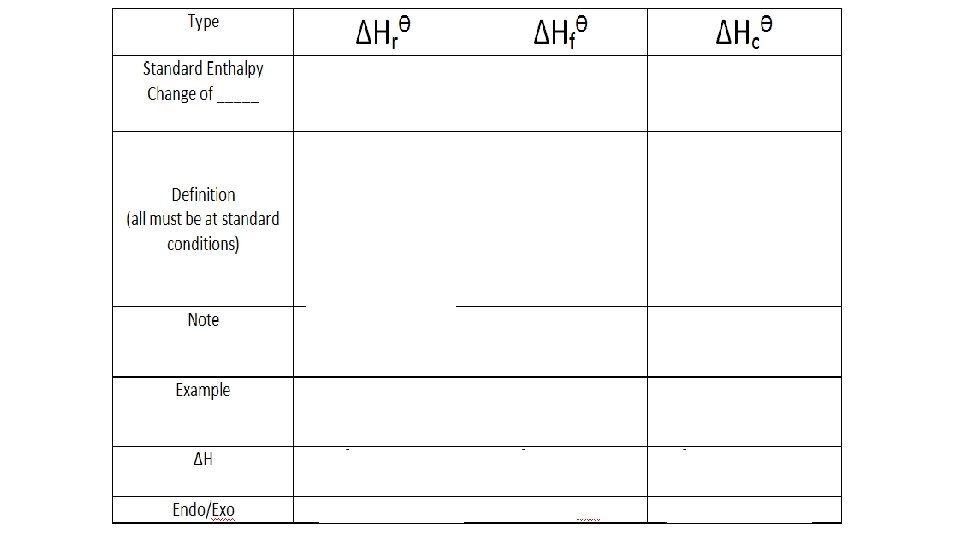
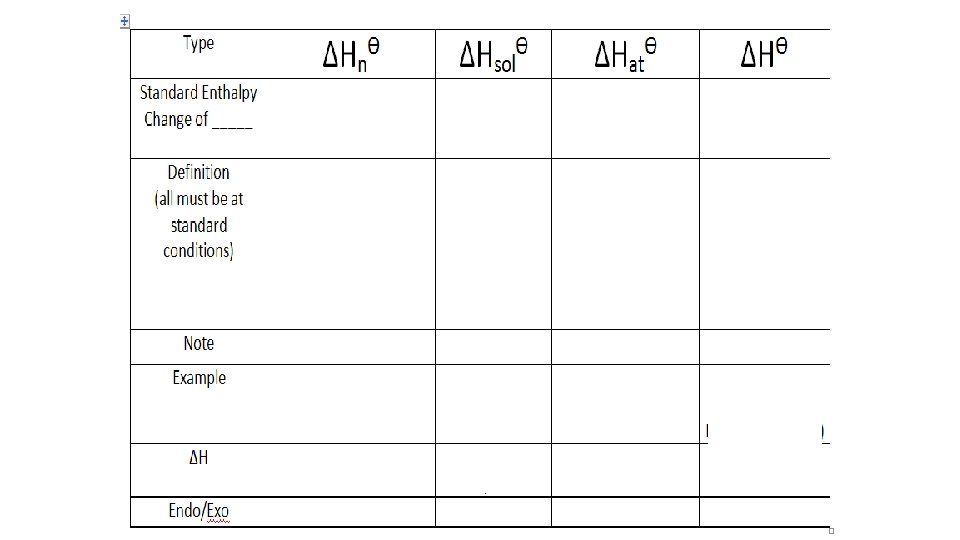
Variety of enthalpy changes • We describe enthalpy changes according to the type of chemical reaction taking place • Enthalpy change of formation • Enthalpy change of combustion • Enthalpy change of neutralization • Enthalpy change of solution • Enthalpy change of atomization • Enthalpy change of hydration • Enthalpy change of reaction (general term)

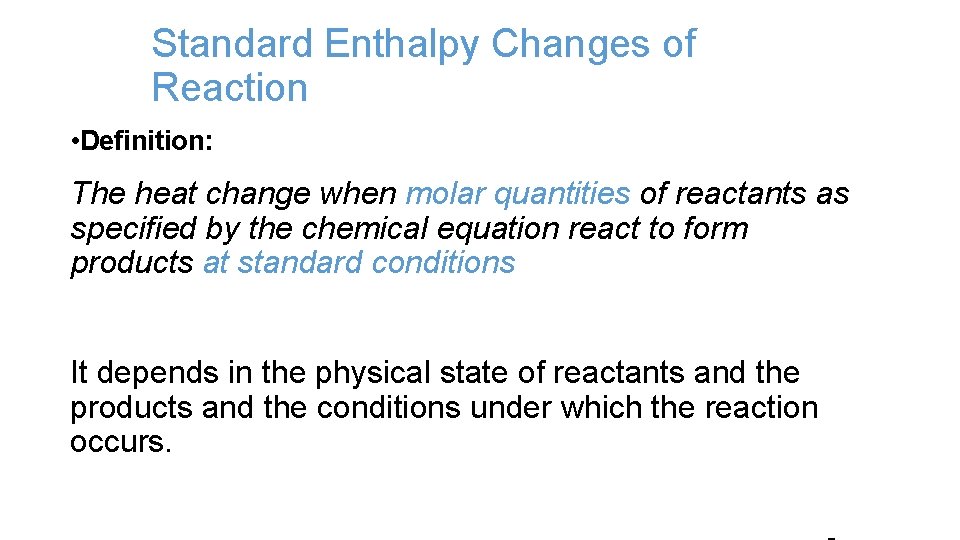
Standard Enthalpy Changes of Reaction • Definition: The heat change when molar quantities of reactants as specified by the chemical equation react to form products at standard conditions It depends in the physical state of reactants and the products and the conditions under which the reaction occurs.

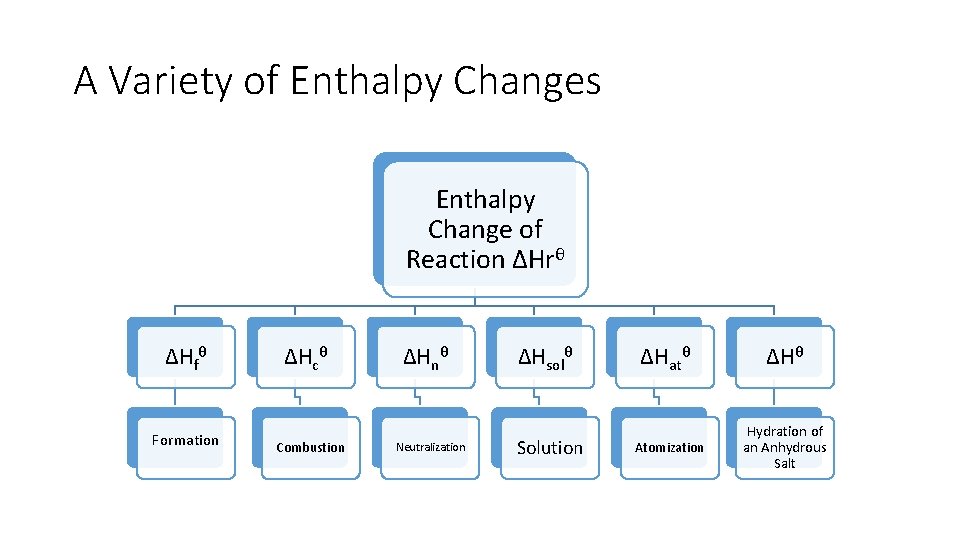
A Variety of Enthalpy Changes Enthalpy Change of Reaction ΔHrθ ΔHfθ Formation ΔHcθ Combustion ΔHnθ Neutralization ΔHsolθ Solution ΔHatθ Atomization ΔHθ Hydration of an Anhydrous Salt

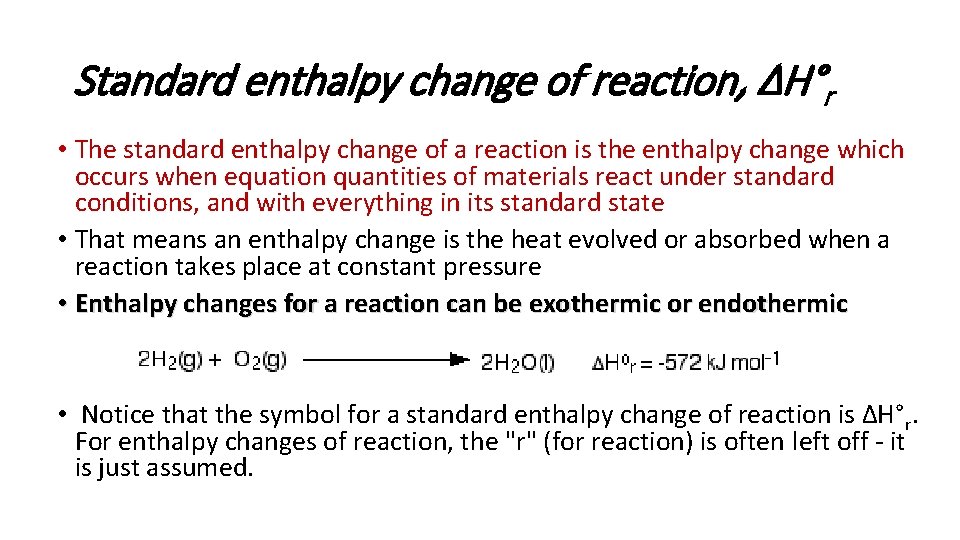
Standard enthalpy change of reaction, ΔH°r • The standard enthalpy change of a reaction is the enthalpy change which occurs when equation quantities of materials react under standard conditions, and with everything in its standard state • That means an enthalpy change is the heat evolved or absorbed when a reaction takes place at constant pressure • Enthalpy changes for a reaction can be exothermic or endothermic • Notice that the symbol for a standard enthalpy change of reaction is ΔH°r. For enthalpy changes of reaction, the "r" (for reaction) is often left off - it is just assumed.

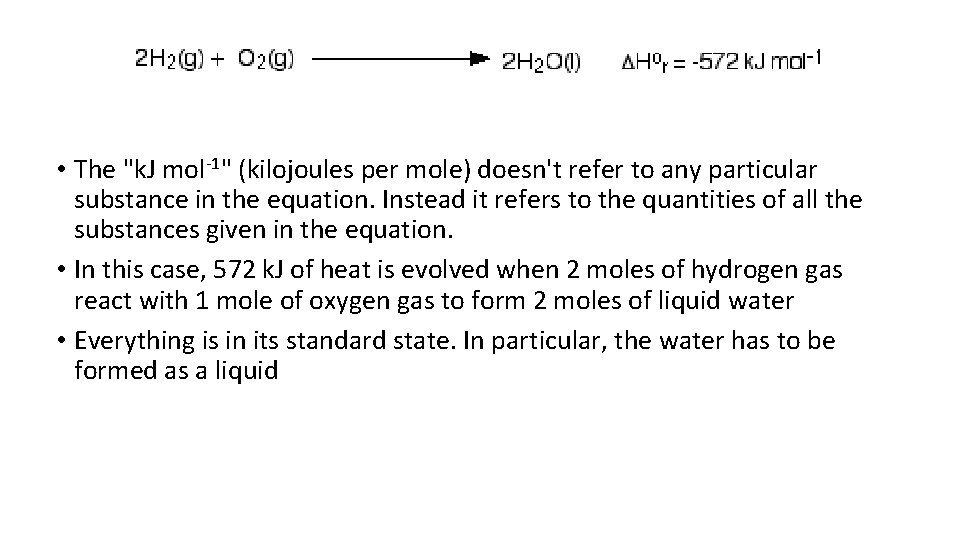
• The "k. J mol-1" (kilojoules per mole) doesn't refer to any particular substance in the equation. Instead it refers to the quantities of all the substances given in the equation. • In this case, 572 k. J of heat is evolved when 2 moles of hydrogen gas react with 1 mole of oxygen gas to form 2 moles of liquid water • Everything is in its standard state. In particular, the water has to be formed as a liquid

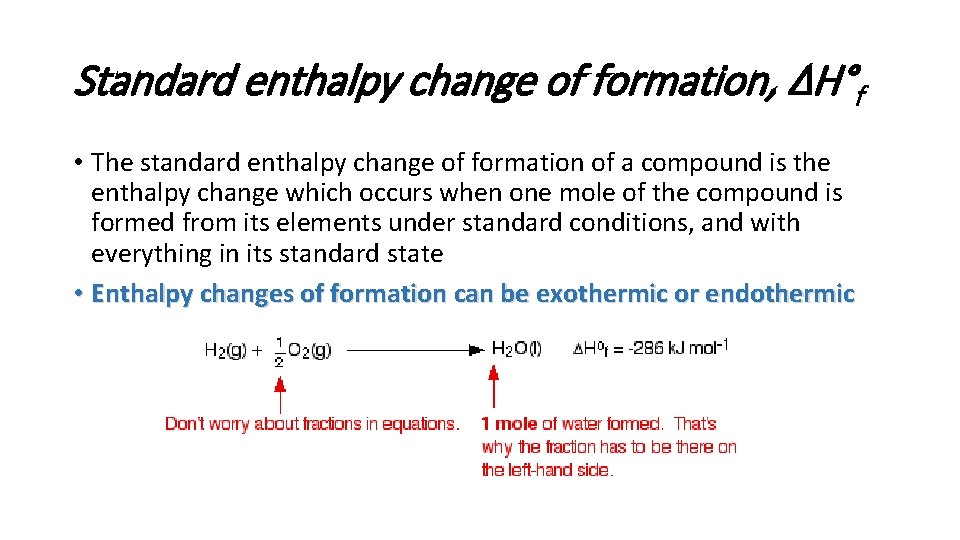
Standard enthalpy change of formation, ΔH°f • The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with everything in its standard state • Enthalpy changes of formation can be exothermic or endothermic

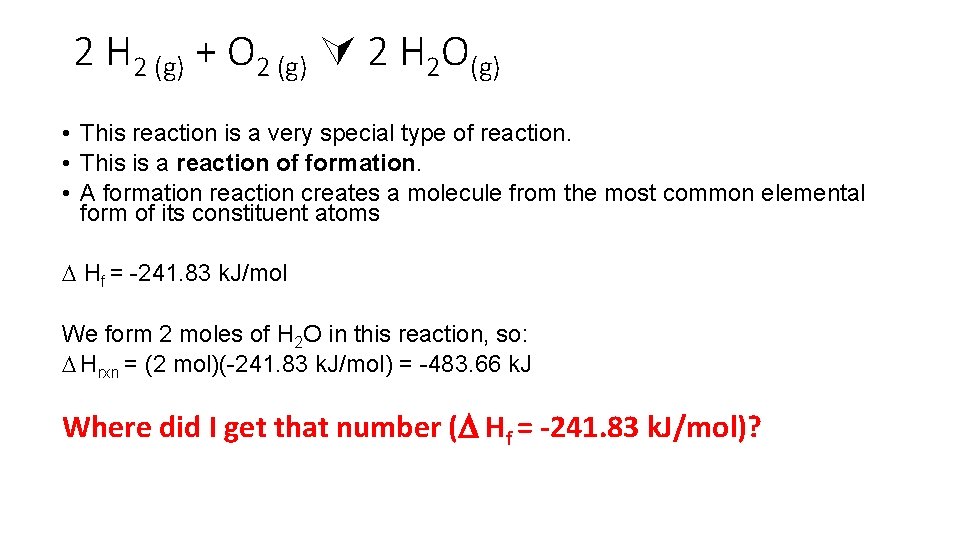
2 H 2 (g) + O 2 (g) 2 H 2 O(g) • This reaction is a very special type of reaction. • This is a reaction of formation. • A formation reaction creates a molecule from the most common elemental form of its constituent atoms Hf = -241. 83 k. J/mol We form 2 moles of H 2 O in this reaction, so: Hrxn = (2 mol)(-241. 83 k. J/mol) = -483. 66 k. J Where did I get that number ( Hf = -241. 83 k. J/mol)?

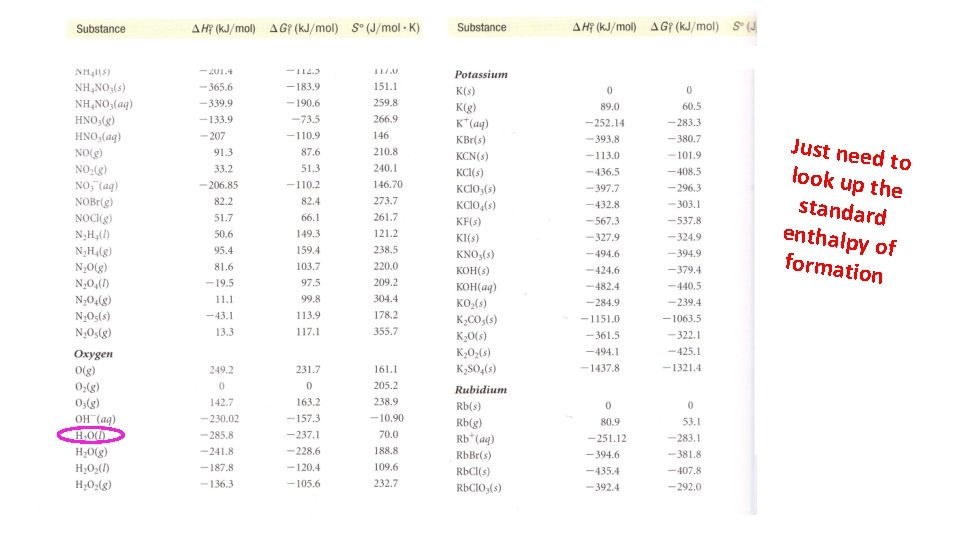
Just nee d to look up t he standard enthalpy of formatio n

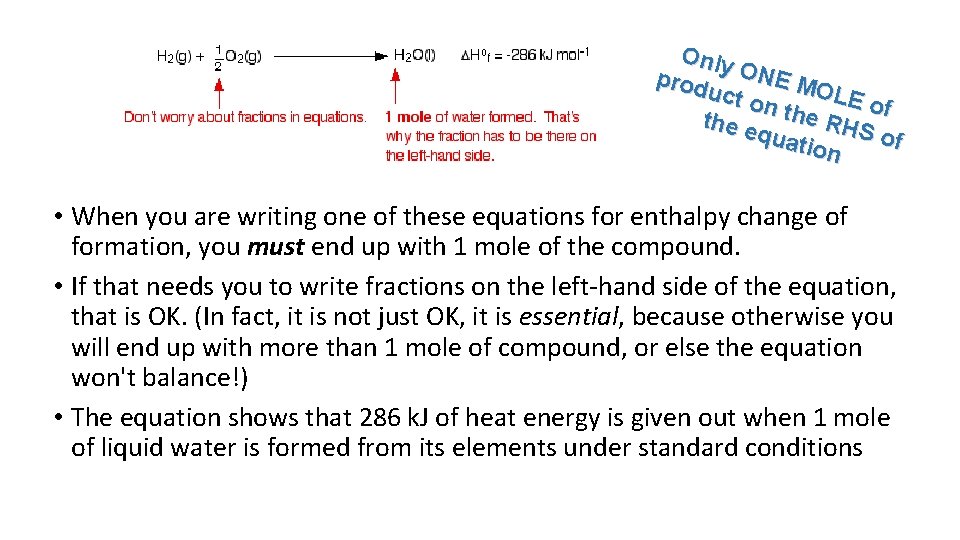
Only prod ONE M OLE uct o of n the e quat RHS of ion • When you are writing one of these equations for enthalpy change of formation, you must end up with 1 mole of the compound. • If that needs you to write fractions on the left-hand side of the equation, that is OK. (In fact, it is not just OK, it is essential, because otherwise you will end up with more than 1 mole of compound, or else the equation won't balance!) • The equation shows that 286 k. J of heat energy is given out when 1 mole of liquid water is formed from its elements under standard conditions

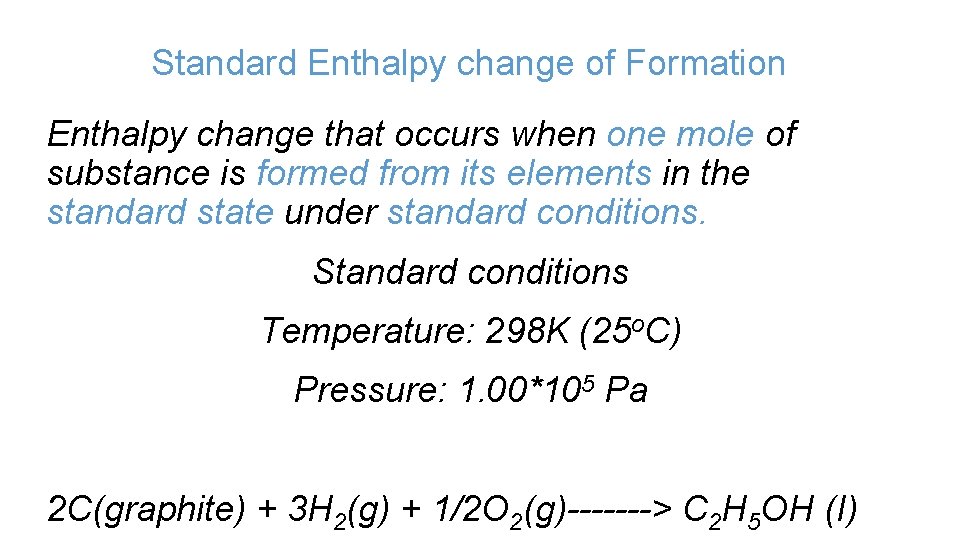
Standard Enthalpy change of Formation Enthalpy change that occurs when one mole of substance is formed from its elements in the standard state under standard conditions. Standard conditions Temperature: 298 K (25 o. C) Pressure: 1. 00*105 Pa 2 C(graphite) + 3 H 2(g) + 1/2 O 2(g)-------> C 2 H 5 OH (I)

• All elements in their standard states (oxygen gas, solid carbon in the form of graphite, etc. ) have a standard enthalpy of formation of zero, as there is no change involved. Eg: O(g) + O(g) ---->O 2(g)

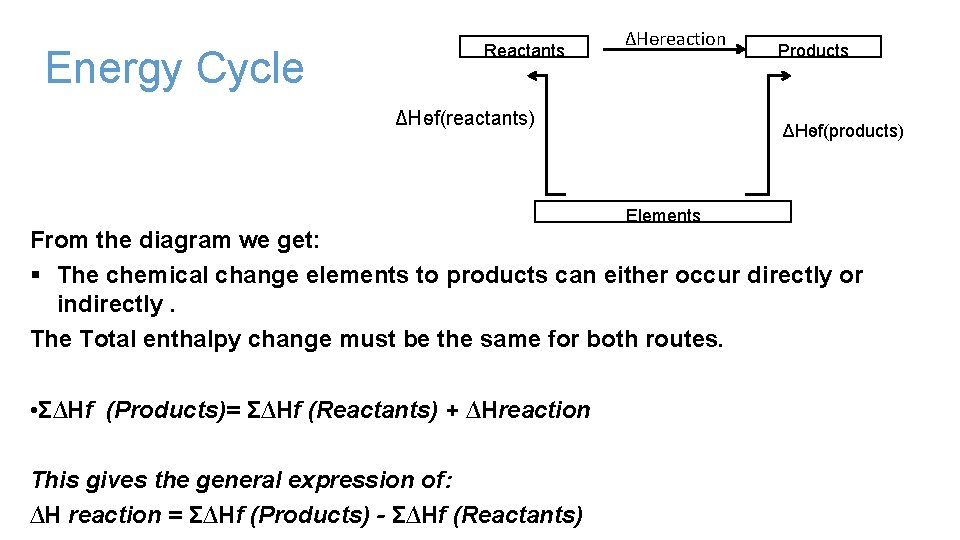
Energy Cycle Reactants ΔHѳreaction ΔHѳf(reactants) Products ΔHѳf(products) Elements From the diagram we get: § The chemical change elements to products can either occur directly or indirectly. The Total enthalpy change must be the same for both routes. • Σ∆Hf (Products)= Σ∆Hf (Reactants) + ∆Hreaction This gives the general expression of: ∆H reaction = Σ∆Hf (Products) - Σ∆Hf (Reactants)

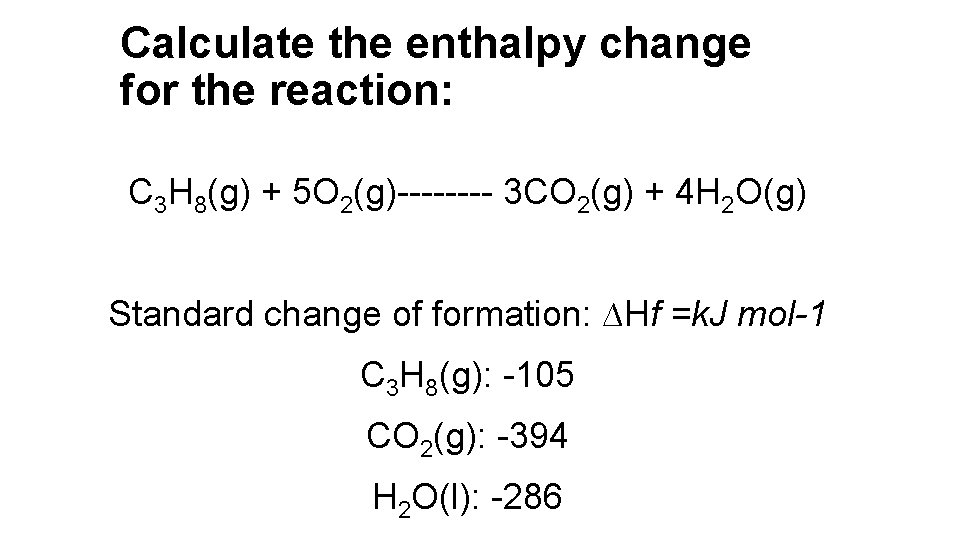
Calculate the enthalpy change for the reaction: C 3 H 8(g) + 5 O 2(g)---- 3 CO 2(g) + 4 H 2 O(g) Standard change of formation: ∆Hf =k. J mol-1 C 3 H 8(g): -105 CO 2(g): -394 H 2 O(l): -286

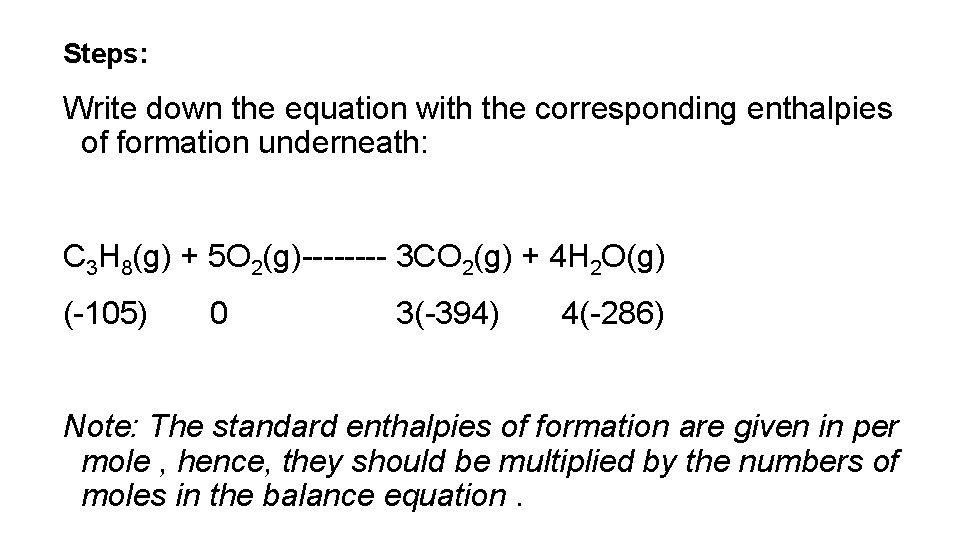
Steps: Write down the equation with the corresponding enthalpies of formation underneath: C 3 H 8(g) + 5 O 2(g)---- 3 CO 2(g) + 4 H 2 O(g) (-105) 0 3(-394) 4(-286) Note: The standard enthalpies of formation are given in per mole , hence, they should be multiplied by the numbers of moles in the balance equation.

∆Hreaction = Σ∆Hf (Products) - Σ∆Hf (Reactants) ∆Hreaction = 3(-394) + 4(-286) -(-105) = -2221 KJ mol-1

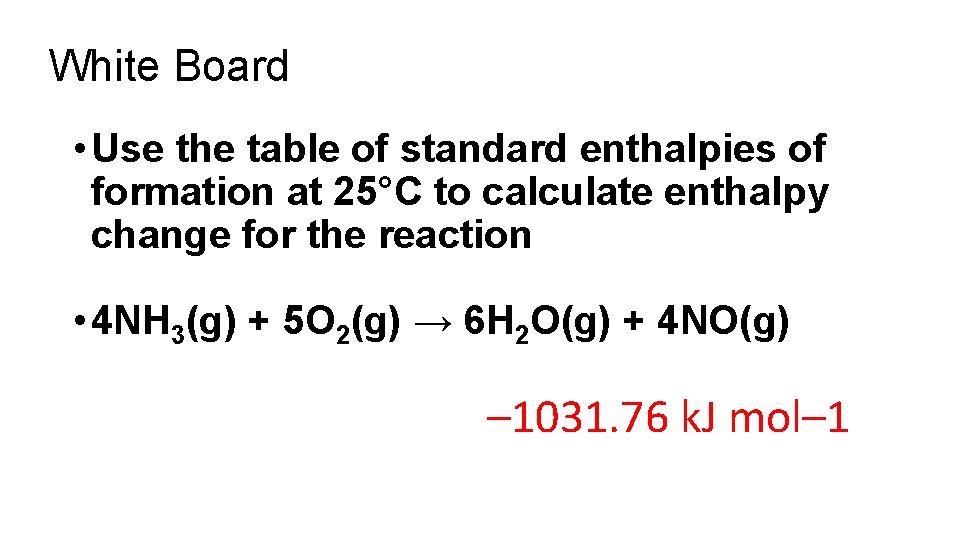
White Board • Use the table of standard enthalpies of formation at 25°C to calculate enthalpy change for the reaction • 4 NH 3(g) + 5 O 2(g) → 6 H 2 O(g) + 4 NO(g) – 1031. 76 k. J mol– 1

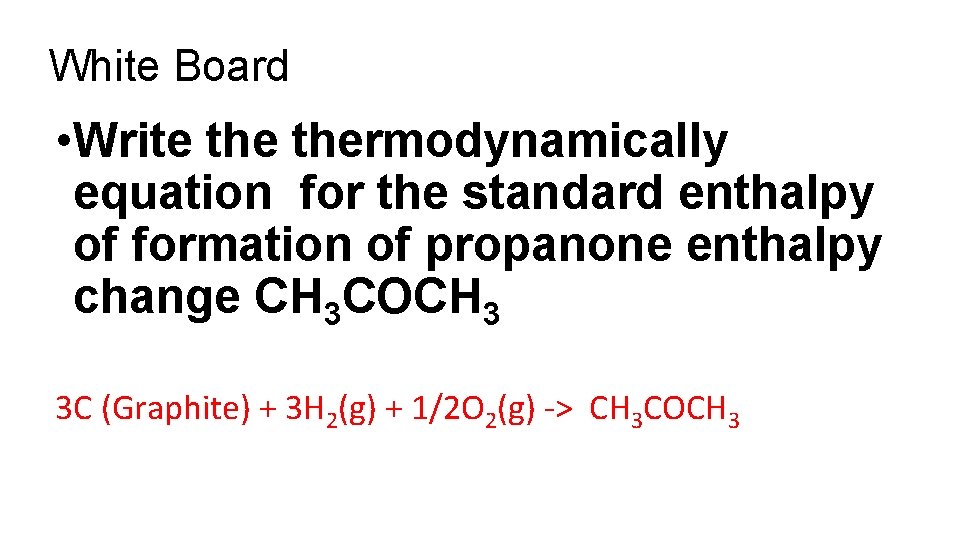
White Board • Write thermodynamically equation for the standard enthalpy of formation of propanone enthalpy change CH 3 COCH 3 3 C (Graphite) + 3 H 2(g) + 1/2 O 2(g) -> CH 3 COCH 3

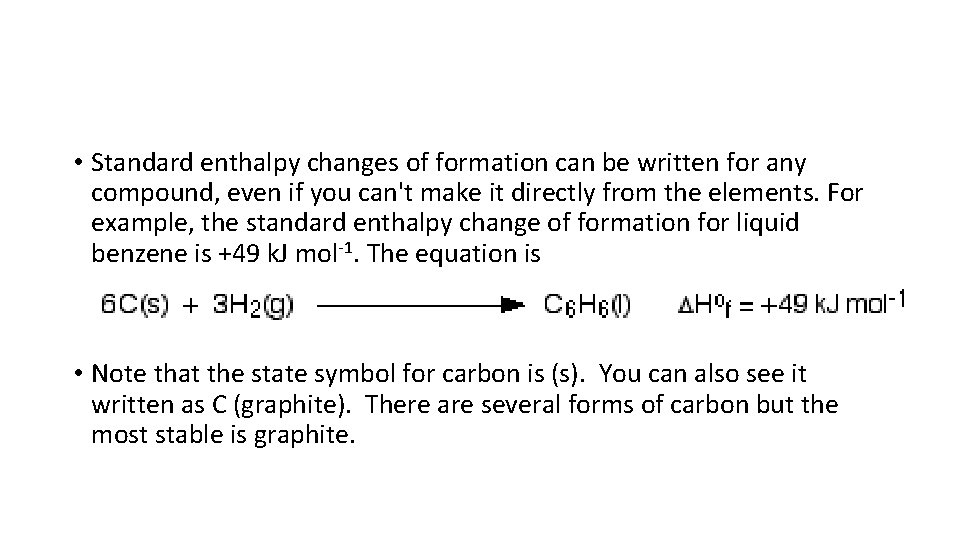
• Standard enthalpy changes of formation can be written for any compound, even if you can't make it directly from the elements. For example, the standard enthalpy change of formation for liquid benzene is +49 k. J mol-1. The equation is • Note that the state symbol for carbon is (s). You can also see it written as C (graphite). There are several forms of carbon but the most stable is graphite.

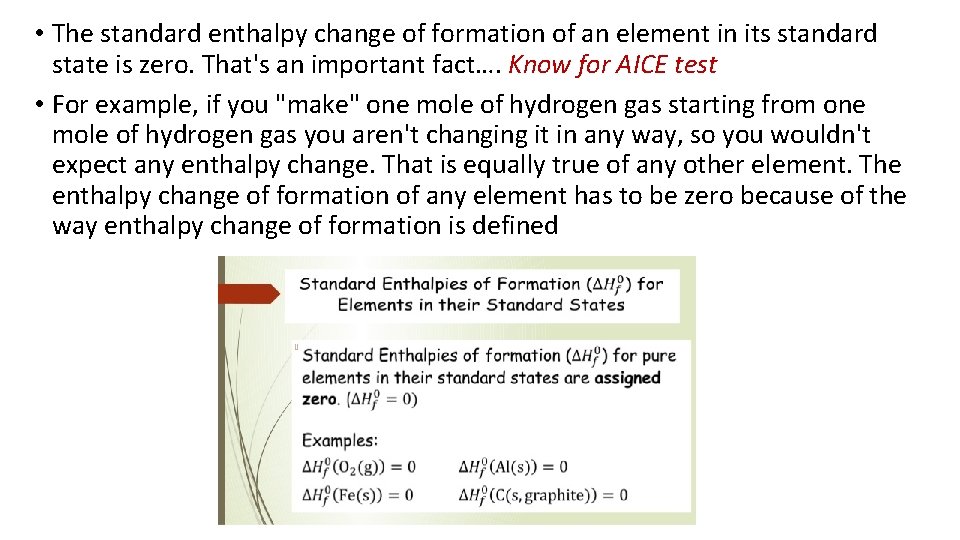
• The standard enthalpy change of formation of an element in its standard state is zero. That's an important fact…. Know for AICE test • For example, if you "make" one mole of hydrogen gas starting from one mole of hydrogen gas you aren't changing it in any way, so you wouldn't expect any enthalpy change. That is equally true of any other element. The enthalpy change of formation of any element has to be zero because of the way enthalpy change of formation is defined

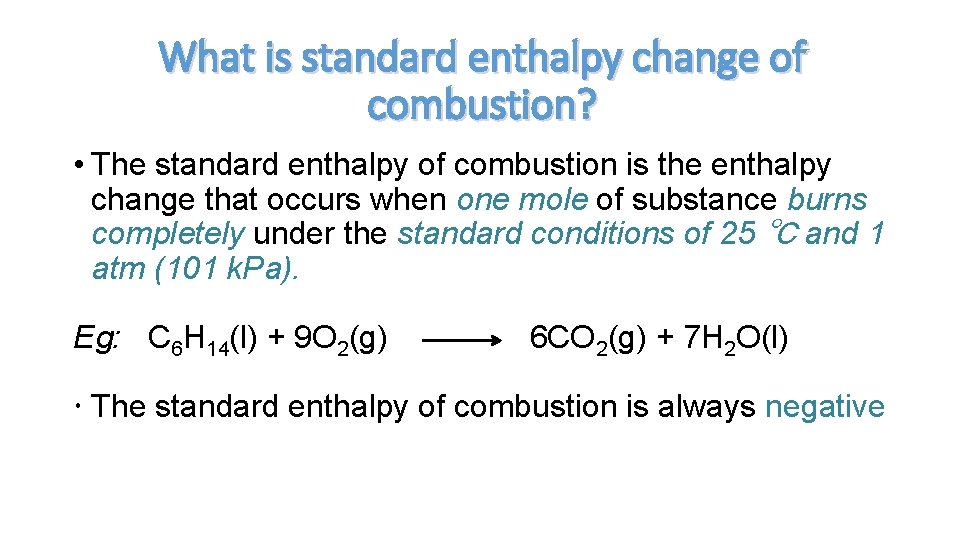
What is standard enthalpy change of combustion? • The standard enthalpy of combustion is the enthalpy change that occurs when one mole of substance burns completely under the standard conditions of 25 ℃ and 1 atm (101 k. Pa). Eg: C 6 H 14(l) + 9 O 2(g) 6 CO 2(g) + 7 H 2 O(l) The standard enthalpy of combustion is always negative

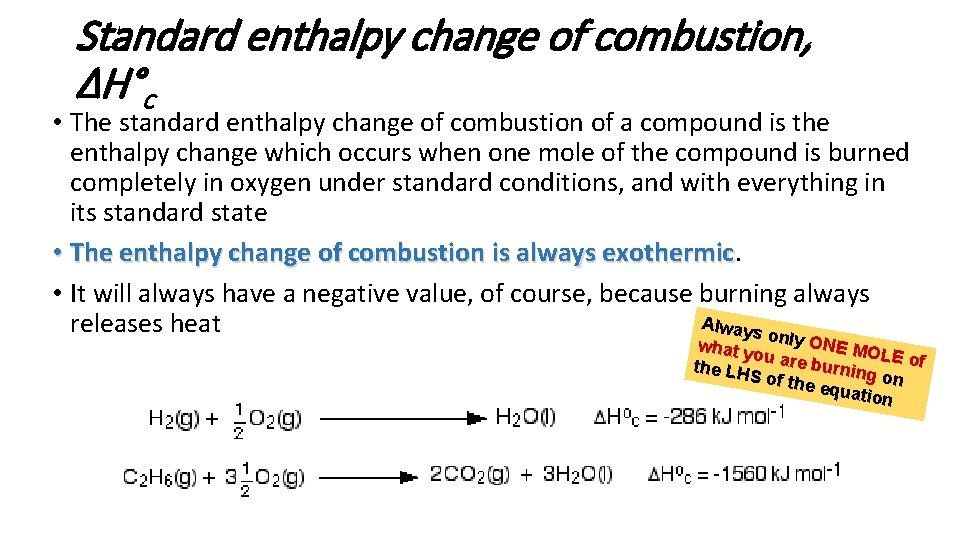
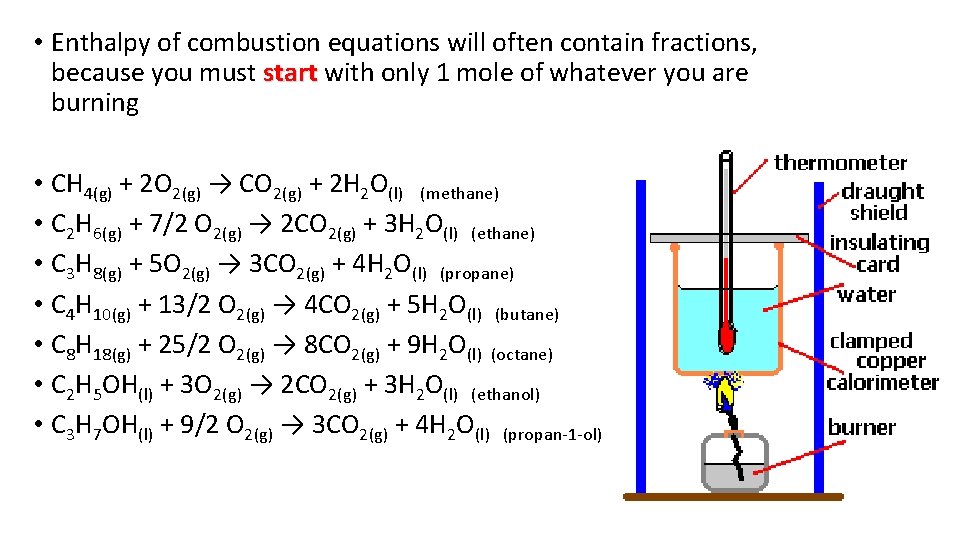
Standard enthalpy change of combustion, ΔH°c • The standard enthalpy change of combustion of a compound is the enthalpy change which occurs when one mole of the compound is burned completely in oxygen under standard conditions, and with everything in its standard state • The enthalpy change of combustion is always exothermic • It will always have a negative value, of course, because burning always Always releases heat only ONE M what y OL ou are burnin E of the LH g S of th e equa on tion


• Enthalpy of combustion equations will often contain fractions, because you must start with only 1 mole of whatever you are start burning • CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) (methane) • C 2 H 6(g) + 7/2 O 2(g) → 2 CO 2(g) + 3 H 2 O(l) (ethane) • C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) (propane) • C 4 H 10(g) + 13/2 O 2(g) → 4 CO 2(g) + 5 H 2 O(l) (butane) • C 8 H 18(g) + 25/2 O 2(g) → 8 CO 2(g) + 9 H 2 O(l) (octane) • C 2 H 5 OH(l) + 3 O 2(g) → 2 CO 2(g) + 3 H 2 O(l) (ethanol) • C 3 H 7 OH(l) + 9/2 O 2(g) → 3 CO 2(g) + 4 H 2 O(l) (propan-1 -ol)

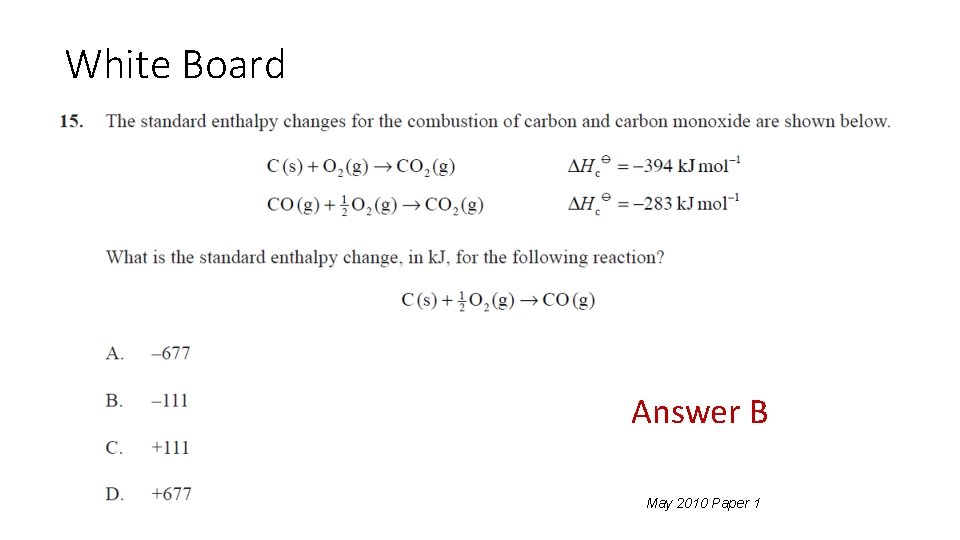
**** White Board May 2010 Paper 1 Answer: B

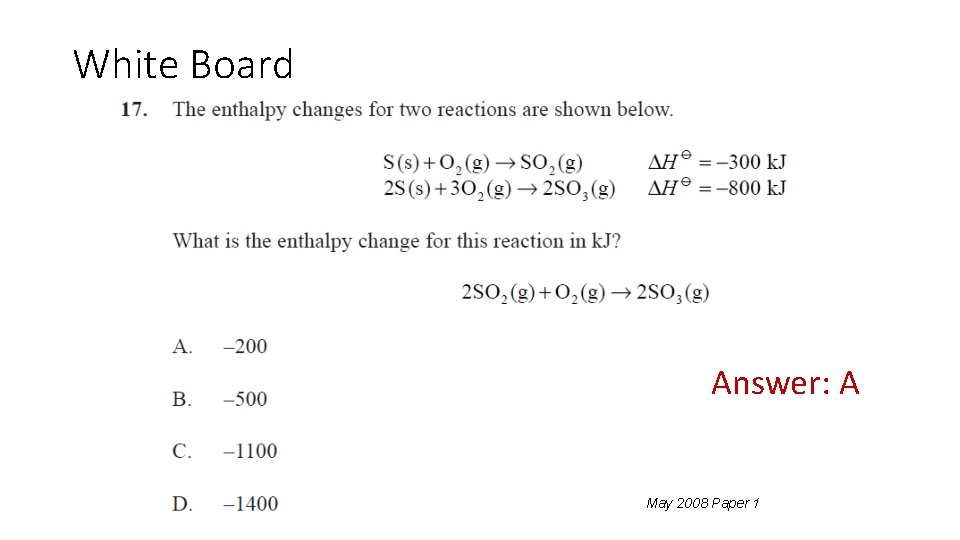
**White Board Answer A May 2008 Paper 1

**White Board Answer D Nov 2007 Paper 1

Enthalpy change of neutralization • The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid an alkali react together under standard conditions to produce 1 mole of water • Notice that enthalpy change of neutralisation is always measured per mole of water formed • Enthalpy changes of neutralisation are always exothermic. They will always exothermic have a negative value - heat is given out when an acid an alkali react.

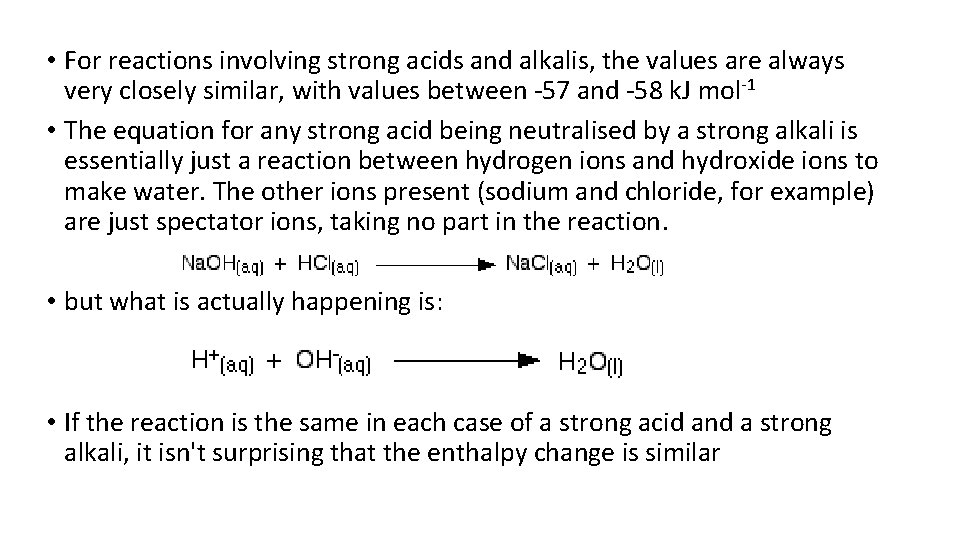
• For reactions involving strong acids and alkalis, the values are always very closely similar, with values between -57 and -58 k. J mol-1 • The equation for any strong acid being neutralised by a strong alkali is essentially just a reaction between hydrogen ions and hydroxide ions to make water. The other ions present (sodium and chloride, for example) are just spectator ions, taking no part in the reaction. • but what is actually happening is: • If the reaction is the same in each case of a strong acid and a strong alkali, it isn't surprising that the enthalpy change is similar

Enthalpy change of solution • The enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution • Enthalpies of solution may be either positive or negative - in other words, some ionic substances dissolved endothermically (for example, Na. Cl); others dissolve exothermically (for example Na. OH) • An infinitely dilute solution is one where there is a sufficiently large excess of water that adding any more doesn't cause any further heat to be absorbed or evolved

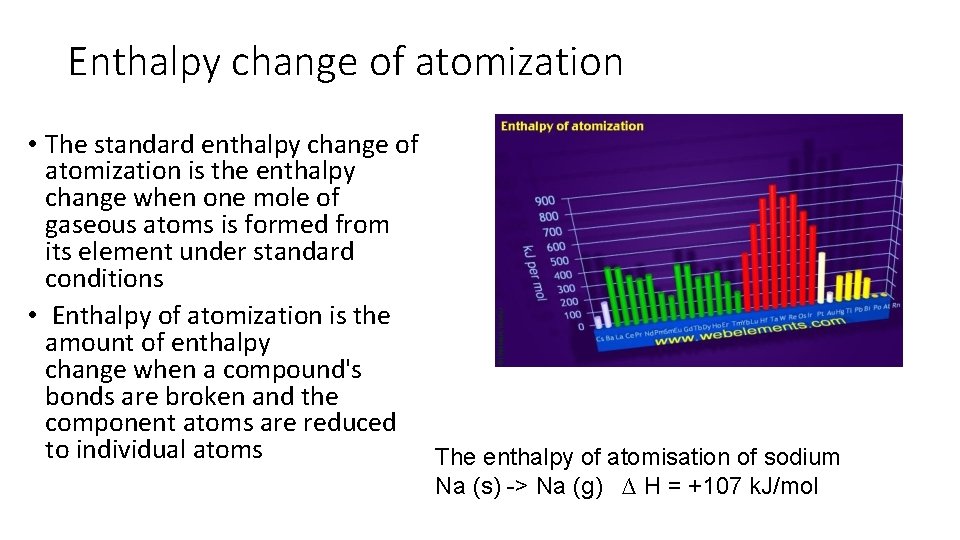
Enthalpy change of atomization • The standard enthalpy change of atomization is the enthalpy change when one mole of gaseous atoms is formed from its element under standard conditions • Enthalpy of atomization is the amount of enthalpy change when a compound's bonds are broken and the component atoms are reduced to individual atoms The enthalpy of atomisation of sodium Na (s) -> Na (g) ∆ H = +107 k. J/mol

v v v

v v

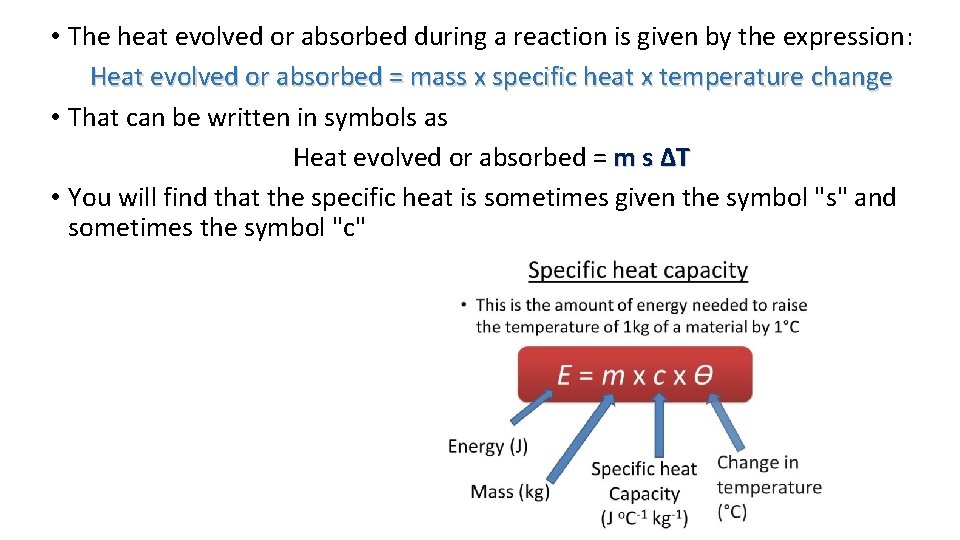
• The heat evolved or absorbed during a reaction is given by the expression: Heat evolved or absorbed = mass x specific heat x temperature change • That can be written in symbols as Heat evolved or absorbed = m s ΔT • You will find that the specific heat is sometimes given the symbol "s" and sometimes the symbol "c"

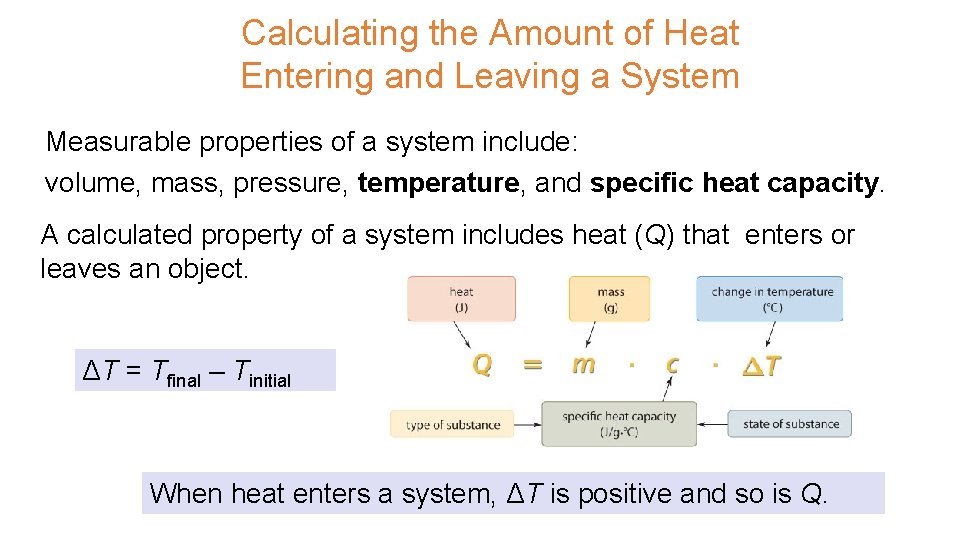
UNIT 3 Chapter 5: Energy Changes Section 5. 1 Calculating the Amount of Heat Entering and Leaving a System Measurable properties of a system include: volume, mass, pressure, temperature, and specific heat capacity. A calculated property of a system includes heat (Q) that enters or leaves an object. ΔT = Tfinal – Tinitial When heat enters a system, ΔT is positive and so is Q.

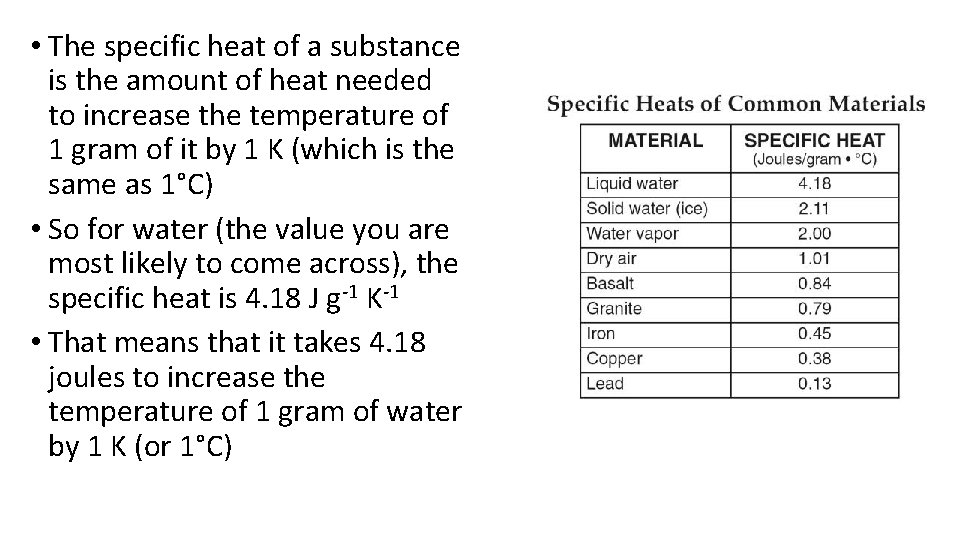
• The specific heat of a substance is the amount of heat needed to increase the temperature of 1 gram of it by 1 K (which is the same as 1°C) • So for water (the value you are most likely to come across), the specific heat is 4. 18 J g-1 K-1 • That means that it takes 4. 18 joules to increase the temperature of 1 gram of water by 1 K (or 1°C)

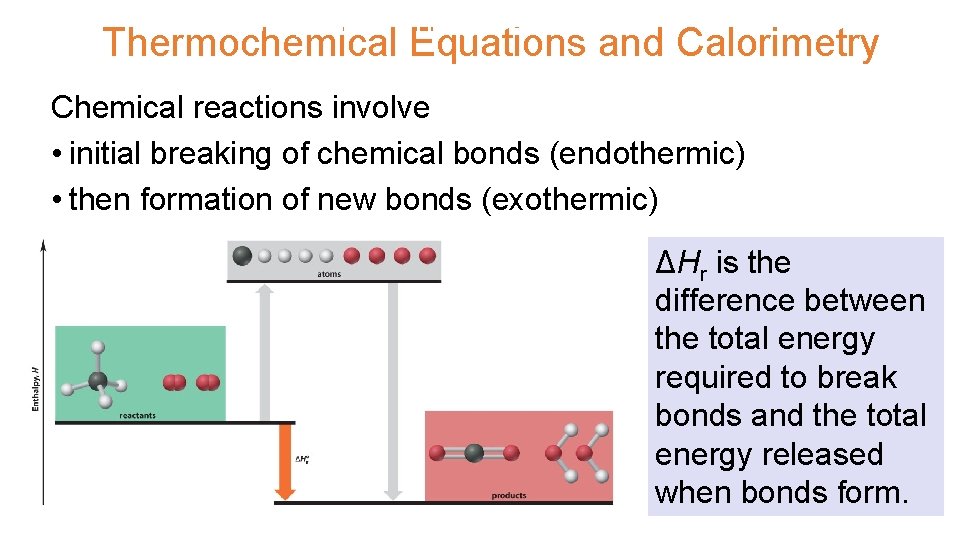
UNIT 3 Chapter 5: Energy Changes Thermochemical Equations and Section Calorimetry 5. 2 Chemical reactions involve • initial breaking of chemical bonds (endothermic) • then formation of new bonds (exothermic) ΔHr is the difference between the total energy required to break bonds and the total energy released when bonds form.

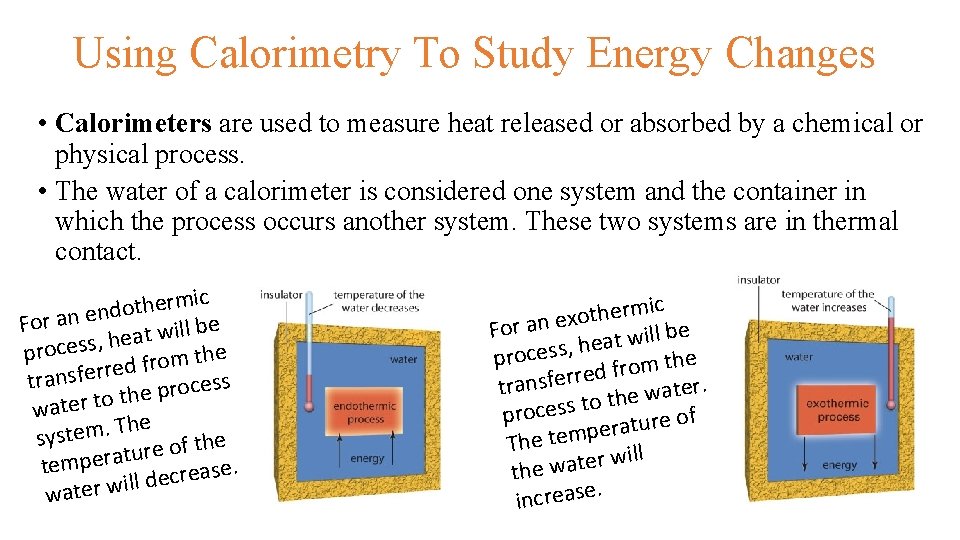
Using Calorimetry To Study Energy Changes • Calorimeters are used to measure heat released or absorbed by a chemical or physical process. • The water of a calorimeter is considered one system and the container in which the process occurs another system. These two systems are in thermal contact. c i m r e h t do n e n a r o F e b l l i w t a he , s s e c o r e p h t m o r f ed r r e f s n a r s t s e c o r p the o t r e t a w he T . m e t s sy e h t f o e r tu tempera ecrease. d l l i w r e t wa c i m r e h t xo For an e at will be e h , s s e c o e pr h t m o r d f e r r e f s n r. tra e t a w e th o t s s e c pro of e r u t a r pe The tem ill w r e t a the w. increase

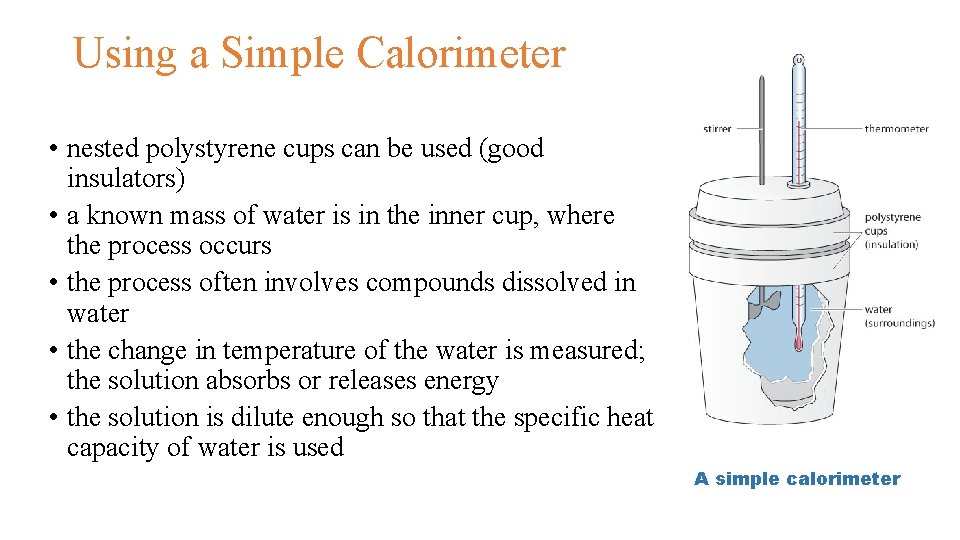
Using a Simple Calorimeter • nested polystyrene cups can be used (good insulators) • a known mass of water is in the inner cup, where the process occurs • the process often involves compounds dissolved in water • the change in temperature of the water is measured; the solution absorbs or releases energy • the solution is dilute enough so that the specific heat capacity of water is used A simple calorimeter

UNIT 3 Chapter 5: Energy Changes Section 5. 2 Using a Simple Calorimeter If the “system” is the process being studied and the “surroundings” is the water in the calorimeter: The change in thermal energy caused by the process can be calculated: Q = mcΔT m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature of the water

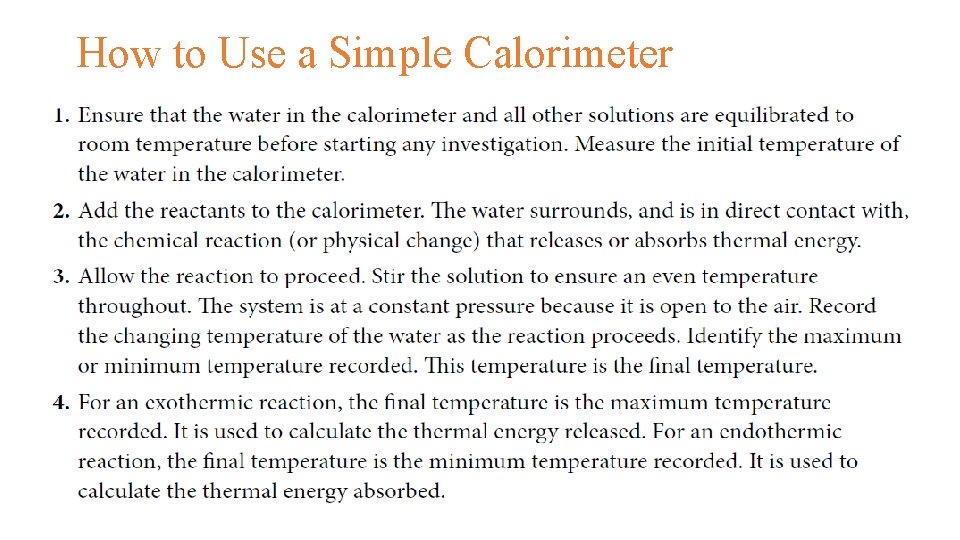
How to Use a Simple Calorimeter

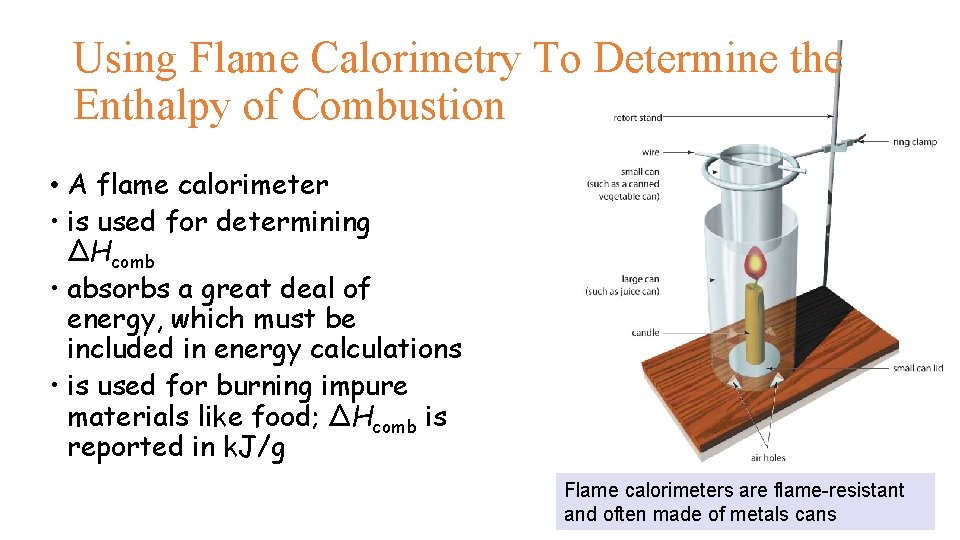
Using Flame Calorimetry To Determine the Enthalpy of Combustion • A flame calorimeter • is used for determining ΔHcomb • absorbs a great deal of energy, which must be included in energy calculations • is used for burning impure materials like food; ΔHcomb is reported in k. J/g Flame calorimeters are flame-resistant and often made of metals cans

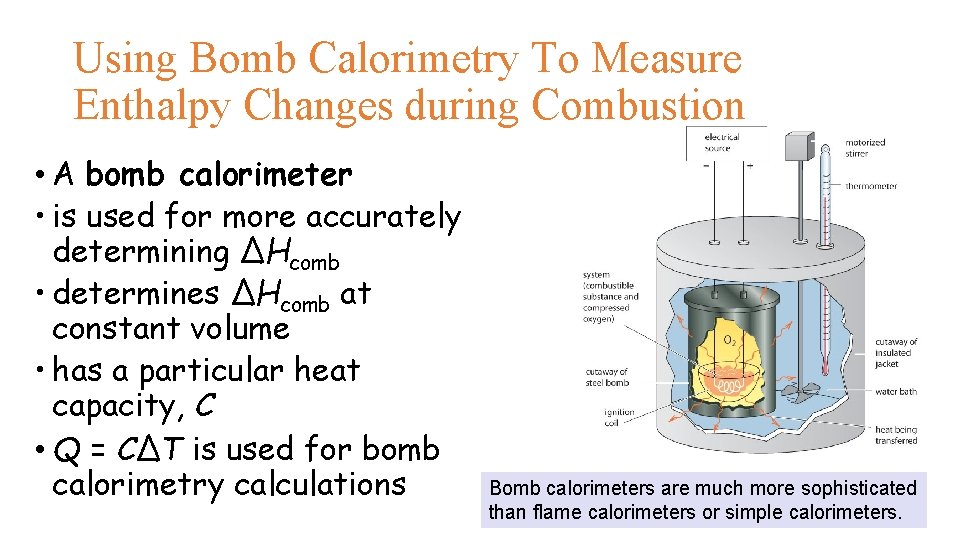
Using Bomb Calorimetry To Measure Enthalpy Changes during Combustion • A bomb calorimeter • is used for more accurately determining ΔHcomb • determines ΔHcomb at constant volume • has a particular heat capacity, C • Q = CΔT is used for bomb calorimetry calculations Bomb calorimeters are much more sophisticated than flame calorimeters or simple calorimeters.

Bomb Calorimeter • Note: Since there is no change • The constant volume calorimeter in volume, then no work is done. measures the system directly. • The specific heat is of the calorimeter itself.

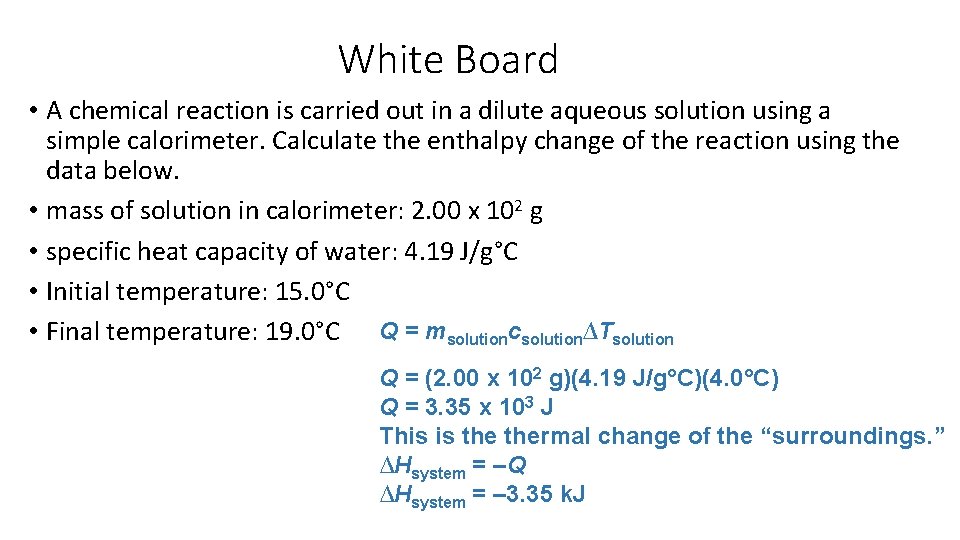
White Board • A chemical reaction is carried out in a dilute aqueous solution using a simple calorimeter. Calculate the enthalpy change of the reaction using the data below. • mass of solution in calorimeter: 2. 00 х 102 g • specific heat capacity of water: 4. 19 J/g°C • Initial temperature: 15. 0°C • Final temperature: 19. 0°C Q = msolutioncsolution∆Tsolution Q = (2. 00 х 102 g)(4. 19 J/g°C)(4. 0°C) Q = 3. 35 х 103 J This is thermal change of the “surroundings. ” ∆Hsystem = –Q ∆Hsystem = – 3. 35 k. J

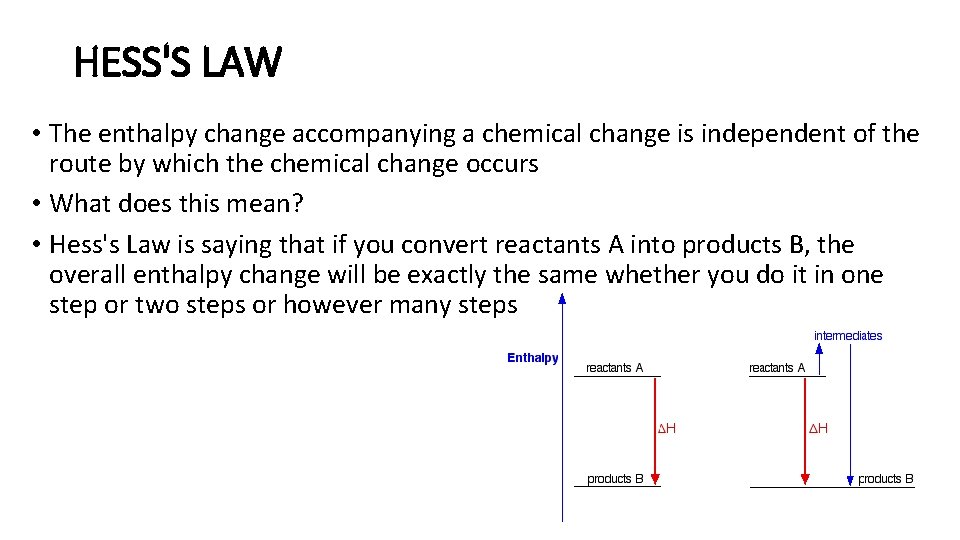
HESS'S LAW • The enthalpy change accompanying a chemical change is independent of the route by which the chemical change occurs • What does this mean? • Hess's Law is saying that if you convert reactants A into products B, the overall enthalpy change will be exactly the same whether you do it in one step or two steps or however many steps

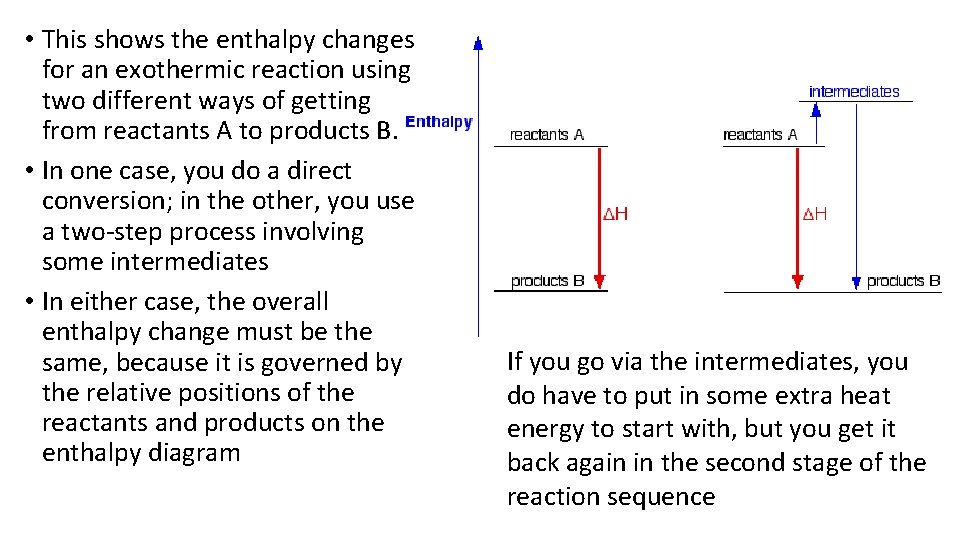
• This shows the enthalpy changes for an exothermic reaction using two different ways of getting from reactants A to products B. • In one case, you do a direct conversion; in the other, you use a two-step process involving some intermediates • In either case, the overall enthalpy change must be the same, because it is governed by the relative positions of the reactants and products on the enthalpy diagram If you go via the intermediates, you do have to put in some extra heat energy to start with, but you get it back again in the second stage of the reaction sequence

Hess’s Law • Since enthalpy is a state function, then • In going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or a series of steps.

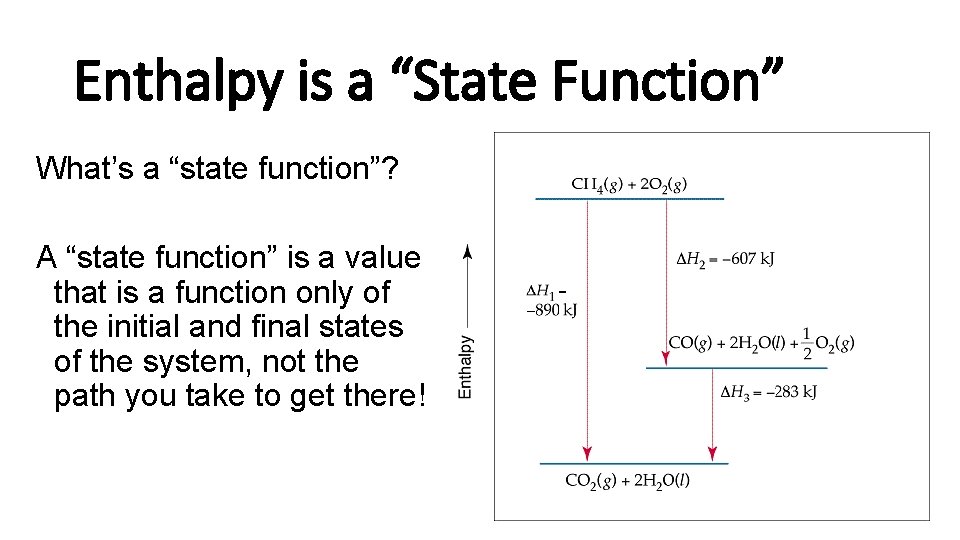
Enthalpy is a “State Function” What’s a “state function”? A “state function” is a value that is a function only of the initial and final states of the system, not the path you take to get there!

Climbing Mt. Everest Suppose you start at Himalayan Base Camp #1, climb to the summit of Everest over the course of 3 weeks, then return to Himalayan Base Camp #1.

Climbing Mt. Everest Back at base camp, I figure out my altitude change. What is it? ZERO – I’m back where I started

Climbing Mt. Everest I did a lot of work along the way, but all that matters is I’m back where I started. The net change in altitude is NADA, ZERO, ZILCH!

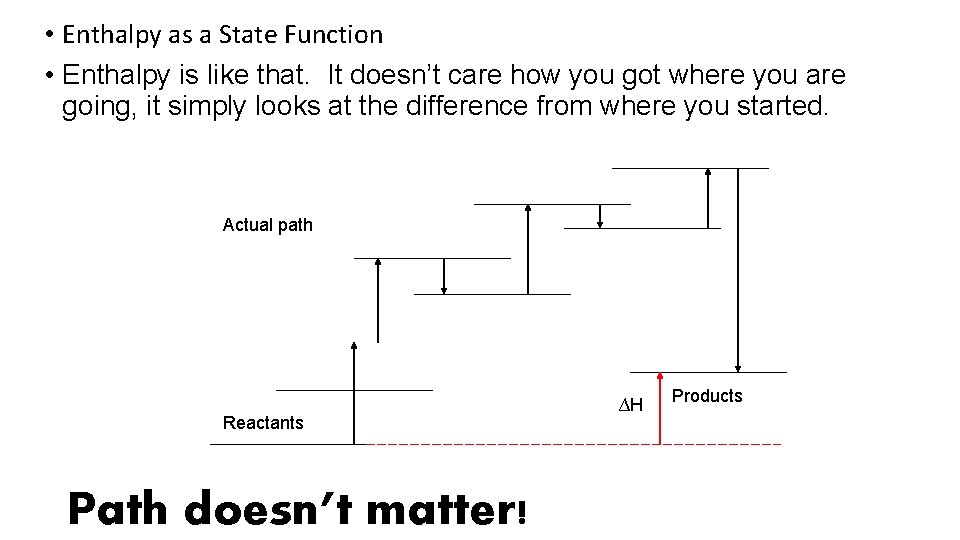
• Enthalpy as a State Function • Enthalpy is like that. It doesn’t care how you got where you are going, it simply looks at the difference from where you started. Actual path Reactants Path doesn’t matter! ∆H Products

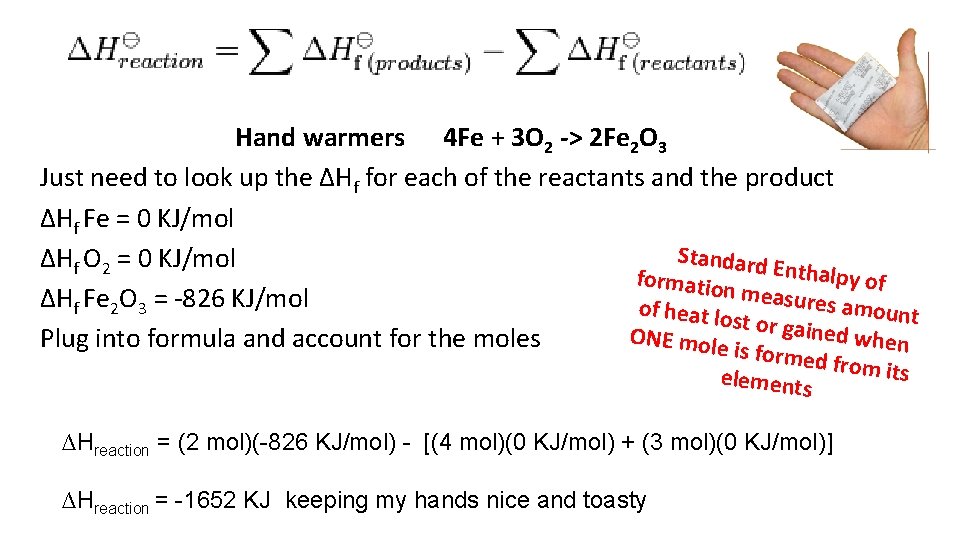
Hand warmers 4 Fe + 3 O 2 -> 2 Fe 2 O 3 Just need to look up the ∆Hf for each of the reactants and the product ∆Hf Fe = 0 KJ/mol Standard ∆Hf O 2 = 0 KJ/mol Enthalpy formatio of n m easures a ∆Hf Fe 2 O 3 = -826 KJ/mol of heat lo mount st or gain ed when ONE mol Plug into formula and account for the moles e is fo rmed fro m its elements ∆Hreaction = (2 mol)(-826 KJ/mol) - [(4 mol)(0 KJ/mol) + (3 mol)(0 KJ/mol)] ∆Hreaction = -1652 KJ keeping my hands nice and toasty

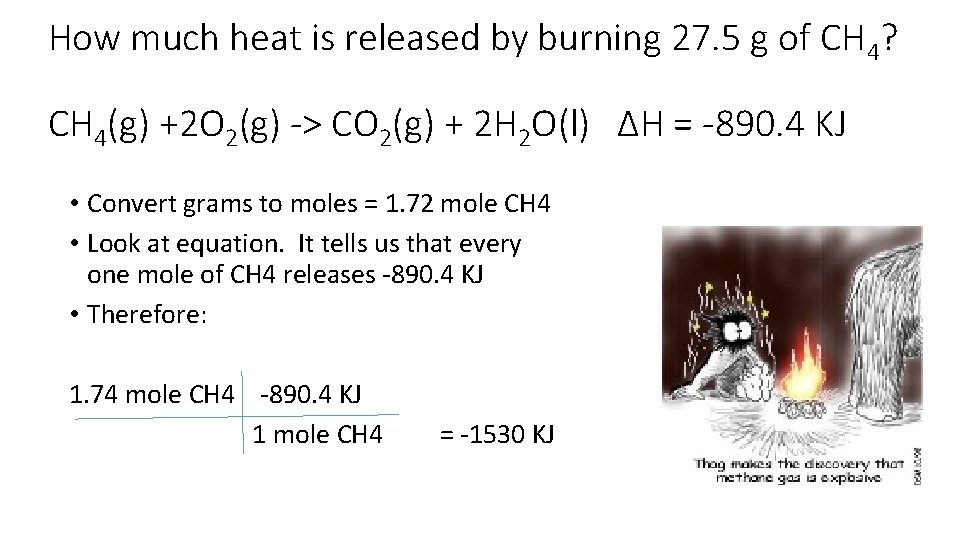
How much heat is released by burning 27. 5 g of CH 4? CH 4(g) +2 O 2(g) -> CO 2(g) + 2 H 2 O(l) ∆H = -890. 4 KJ • Convert grams to moles = 1. 72 mole CH 4 • Look at equation. It tells us that every one mole of CH 4 releases -890. 4 KJ • Therefore: 1. 74 mole CH 4 -890. 4 KJ 1 mole CH 4 = -1530 KJ

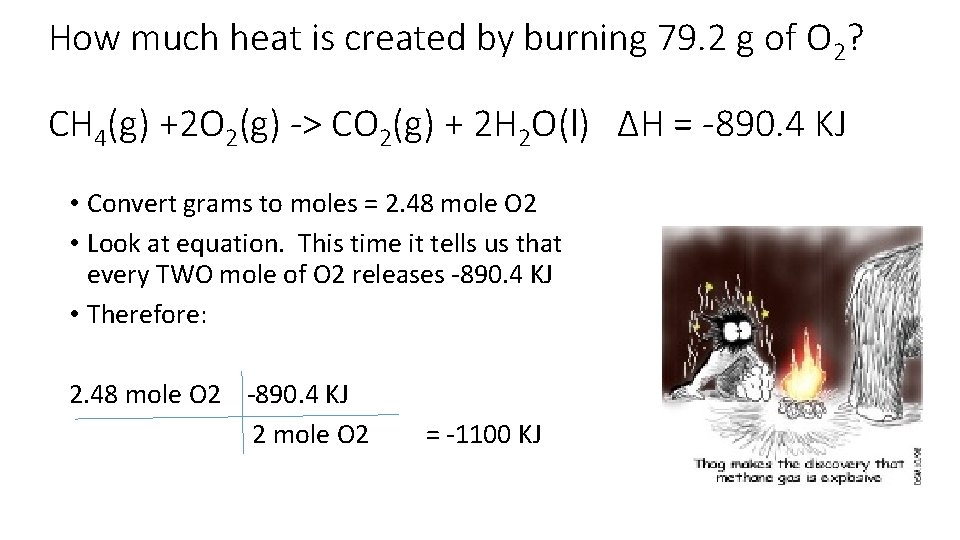
How much heat is created by burning 79. 2 g of O 2? CH 4(g) +2 O 2(g) -> CO 2(g) + 2 H 2 O(l) ∆H = -890. 4 KJ • Convert grams to moles = 2. 48 mole O 2 • Look at equation. This time it tells us that every TWO mole of O 2 releases -890. 4 KJ • Therefore: 2. 48 mole O 2 -890. 4 KJ 2 mole O 2 = -1100 KJ

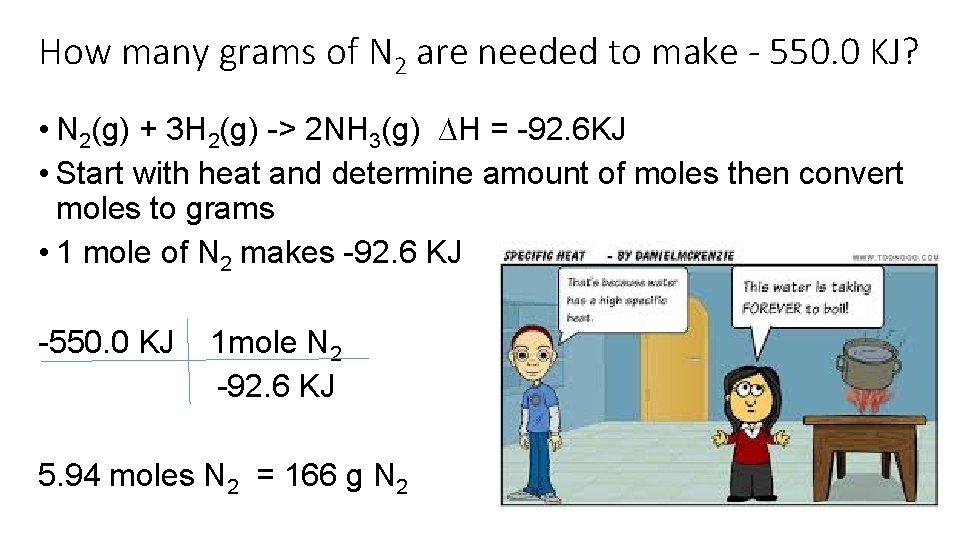
How many grams of N 2 are needed to make - 550. 0 KJ? • N 2(g) + 3 H 2(g) -> 2 NH 3(g) ∆H = -92. 6 KJ • Start with heat and determine amount of moles then convert moles to grams • 1 mole of N 2 makes -92. 6 KJ -550. 0 KJ 1 mole N 2 -92. 6 KJ 5. 94 moles N 2 = 166 g N 2

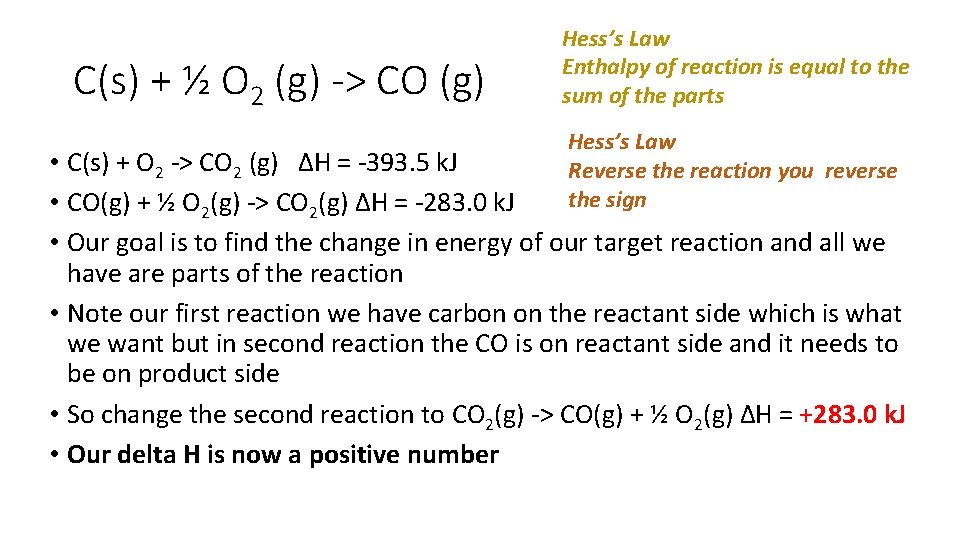
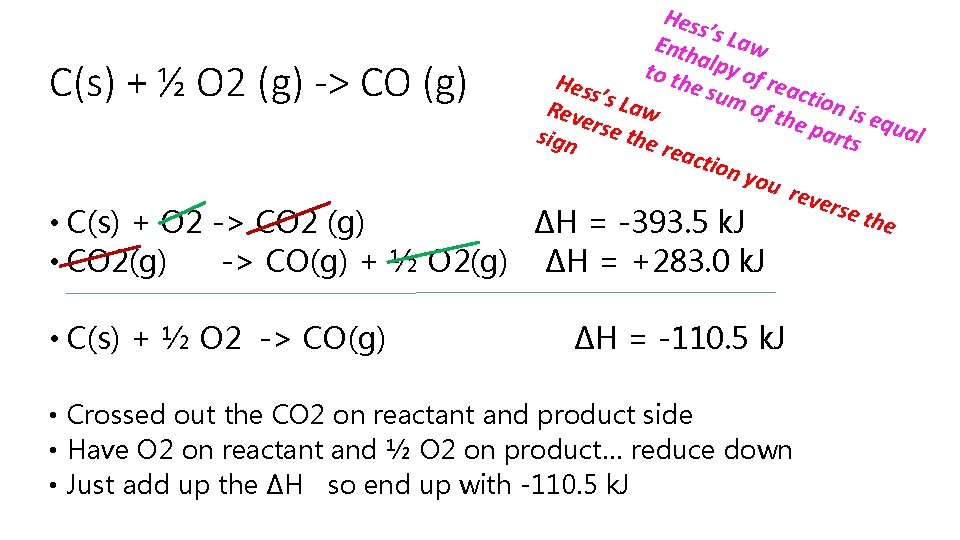
C(s) + ½ O 2 (g) -> CO (g) Hess’s Law Enthalpy of reaction is equal to the sum of the parts Hess’s Law Reverse the reaction you reverse the sign • C(s) + O 2 -> CO 2 (g) ∆H = -393. 5 k. J • CO(g) + ½ O 2(g) -> CO 2(g) ∆H = -283. 0 k. J • Our goal is to find the change in energy of our target reaction and all we have are parts of the reaction • Note our first reaction we have carbon on the reactant side which is what we want but in second reaction the CO is on reactant side and it needs to be on product side • So change the second reaction to CO 2(g) -> CO(g) + ½ O 2(g) ∆H = +283. 0 k. J • Our delta H is now a positive number

C(s) + ½ O 2 (g) -> CO (g) • C(s) + O 2 -> CO 2 (g) • CO 2(g) -> CO(g) + ½ O 2(g) • C(s) + ½ O 2 -> CO(g) Hes s’s L aw Enth alpy t o of re the Hess acti sum ’s La of th on is e Reve w qua e pa l sign rse the r ts reac tion you reve rse t he ∆H = -393. 5 k. J ∆H = +283. 0 k. J ∆H = -110. 5 k. J • Crossed out the CO 2 on reactant and product side • Have O 2 on reactant and ½ O 2 on product… reduce down • Just add up the ∆H so end up with -110. 5 k. J

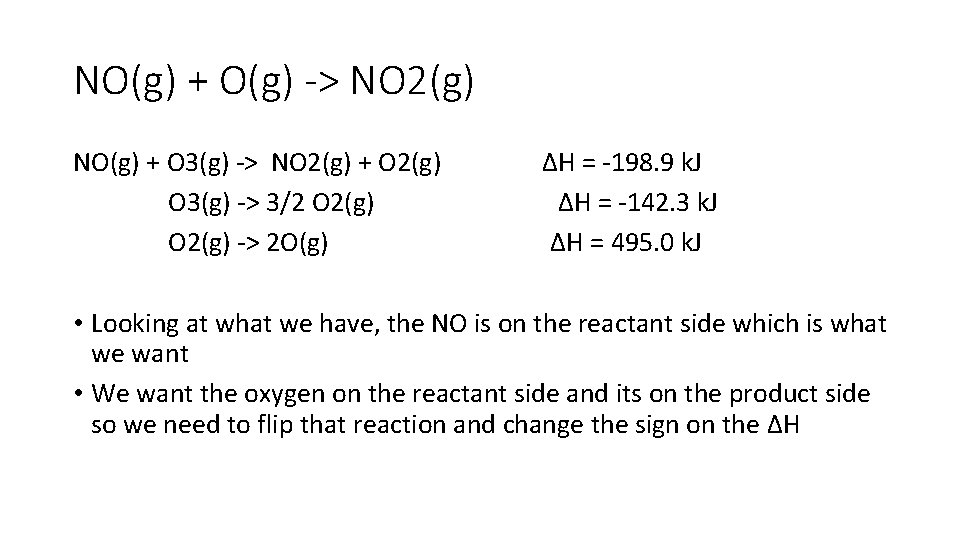
NO(g) + O(g) -> NO 2(g) NO(g) + O 3(g) -> NO 2(g) + O 2(g) ∆H = -198. 9 k. J O 3(g) -> 3/2 O 2(g) ∆H = -142. 3 k. J O 2(g) -> 2 O(g) ∆H = 495. 0 k. J • Looking at what we have, the NO is on the reactant side which is what we want • We want the oxygen on the reactant side and its on the product side so we need to flip that reaction and change the sign on the ∆H

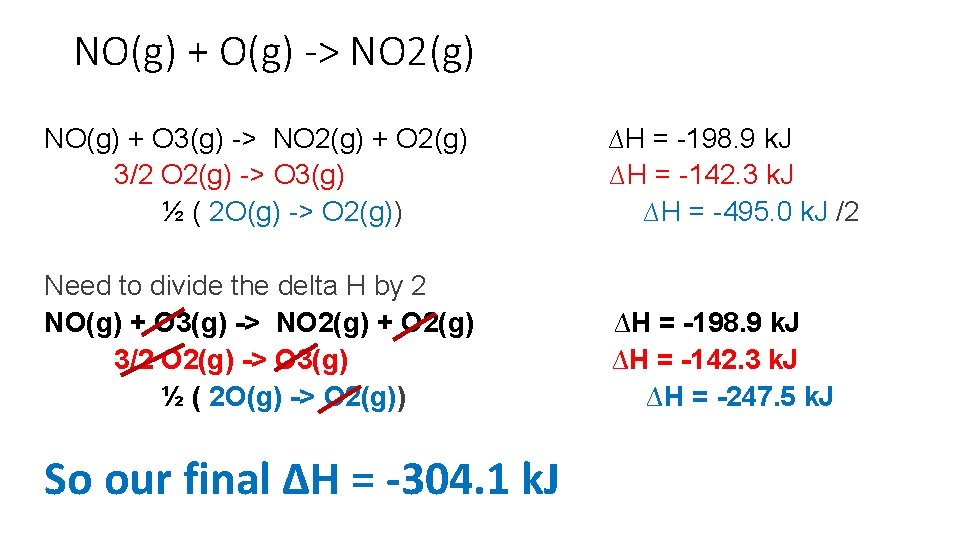
NO(g) + O(g) -> NO 2(g) NO(g) + O 3(g) -> NO 2(g) + O 2(g) ∆H = -198. 9 k. J O 3(g) -> 3/2 O 2(g) ∆H = -142. 3 k. J 2 O(g) -> O 2(g) ∆H = -495. 0 k. J • Notice that we still have two ozones on the reactant side so if we flip the second reaction then we cancel out the ozones

NO(g) + O(g) -> NO 2(g) NO(g) + O 3(g) -> NO 2(g) + O 2(g) ∆H = -198. 9 k. J 3/2 O 2(g) -> O 3(g) ∆H = -142. 3 k. J 2 O(g) -> O 2(g) ∆H = -495. 0 k. J Looking better. NO on reactant side NO 2 on product side But I have 2 oxygens on reactant side and I only want one oxygen so multiple the bottom equation by 1/2

NO(g) + O(g) -> NO 2(g) NO(g) + O 3(g) -> NO 2(g) + O 2(g) 3/2 O 2(g) -> O 3(g) ½ ( 2 O(g) -> O 2(g)) ∆H = -198. 9 k. J ∆H = -142. 3 k. J ∆H = -495. 0 k. J /2 Need to divide the delta H by 2 NO(g) + O 3(g) -> NO 2(g) + O 2(g) 3/2 O 2(g) -> O 3(g) ½ ( 2 O(g) -> O 2(g)) ∆H = -198. 9 k. J ∆H = -142. 3 k. J ∆H = -247. 5 k. J So our final ∆H = -304. 1 k. J

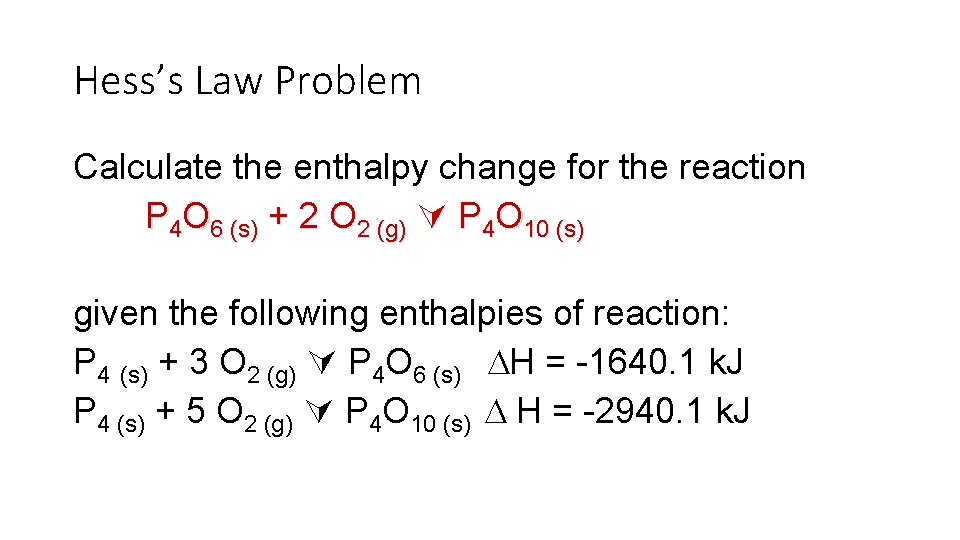
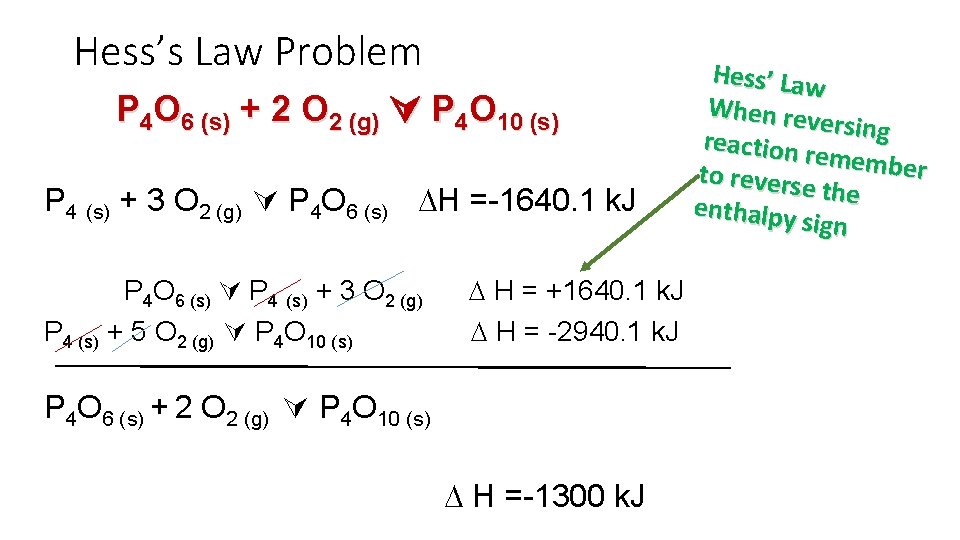
Hess’s Law Problem Calculate the enthalpy change for the reaction P 4 O 6 (s) + 2 O 2 (g) P 4 O 10 (s) given the following enthalpies of reaction: P 4 (s) + 3 O 2 (g) P 4 O 6 (s) H = -1640. 1 k. J P 4 (s) + 5 O 2 (g) P 4 O 10 (s) H = -2940. 1 k. J

Hess’s Law Problem P 4 O 6 (s) + 2 O 2 (g) P 4 O 10 (s) P 4 (s) + 3 O 2 (g) P 4 O 6 (s) H =-1640. 1 k. J P 4 O 6 (s) P 4 (s) + 3 O 2 (g) P 4 (s) + 5 O 2 (g) P 4 O 10 (s) H = +1640. 1 k. J H = -2940. 1 k. J P 4 O 6 (s) + 2 O 2 (g) P 4 O 10 (s) H =-1300 k. J Hess’ Law When rev ersing reaction remembe r to revers e the enthalpy sign

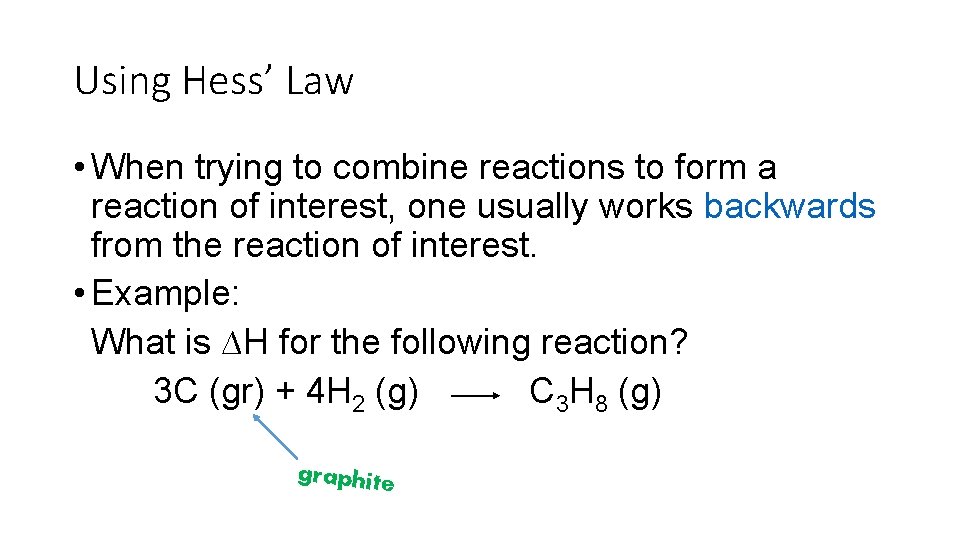
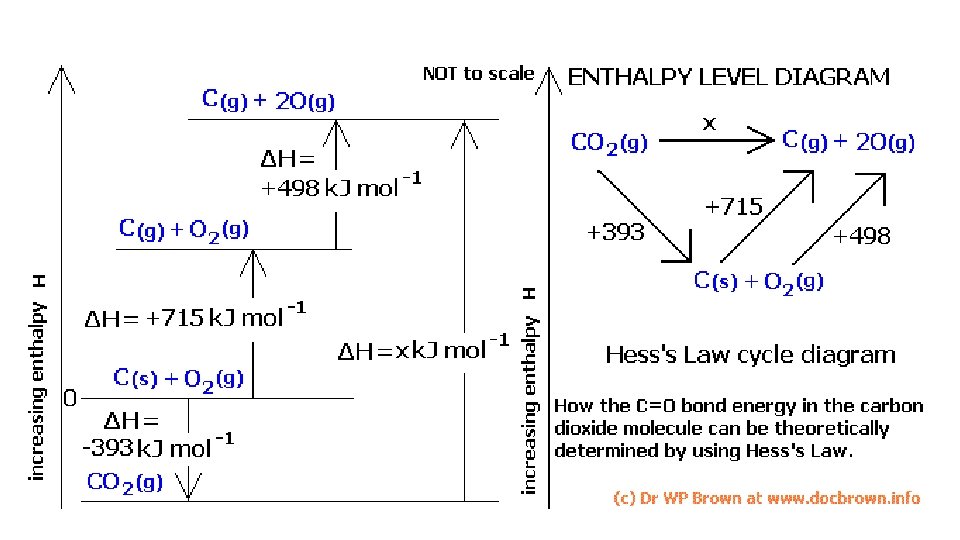
Using Hess’ Law • When trying to combine reactions to form a reaction of interest, one usually works backwards from the reaction of interest. • Example: What is ∆H for the following reaction? 3 C (gr) + 4 H 2 (g) C 3 H 8 (g) graphite
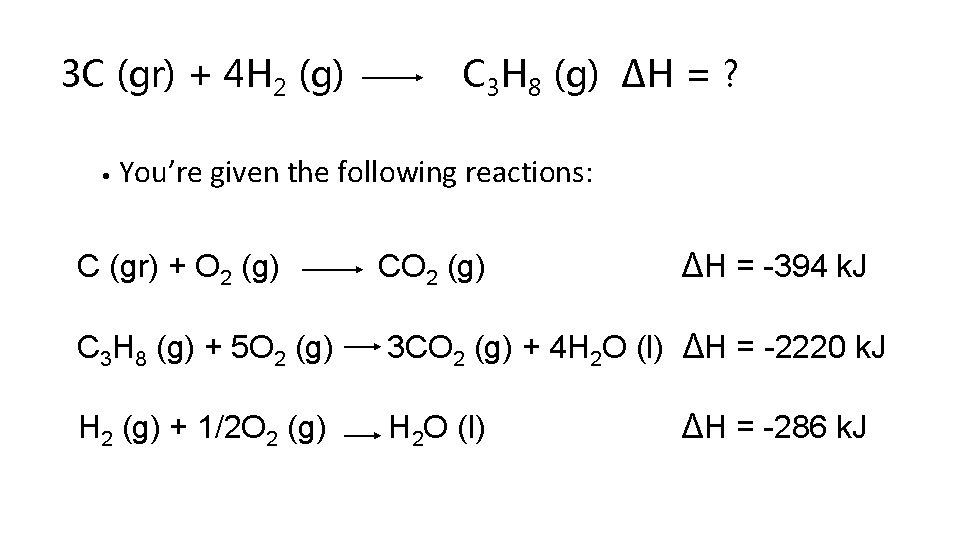
3 C (gr) + 4 H 2 (g) • C 3 H 8 (g) ∆H = ? You’re given the following reactions: ∆H = -394 k. J C (gr) + O 2 (g) C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) ∆H = -2220 k. J H 2 (g) + 1/2 O 2 (g) H 2 O (l) ∆H = -286 k. J
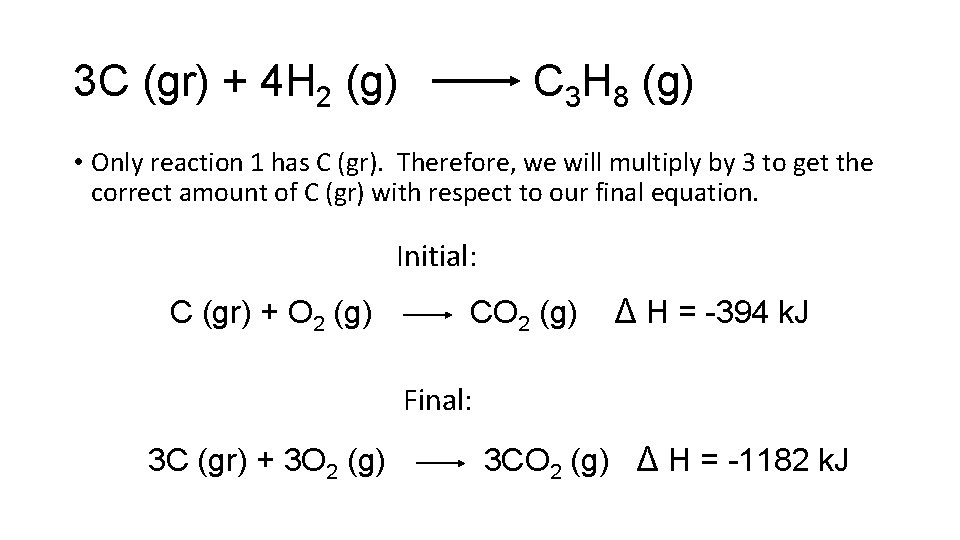
3 C (gr) + 4 H 2 (g) C 3 H 8 (g) • Only reaction 1 has C (gr). Therefore, we will multiply by 3 to get the correct amount of C (gr) with respect to our final equation. Initial: C (gr) + O 2 (g) CO 2 (g) ∆ H = -394 k. J Final: 3 C (gr) + 3 O 2 (g) 3 CO 2 (g) ∆ H = -1182 k. J
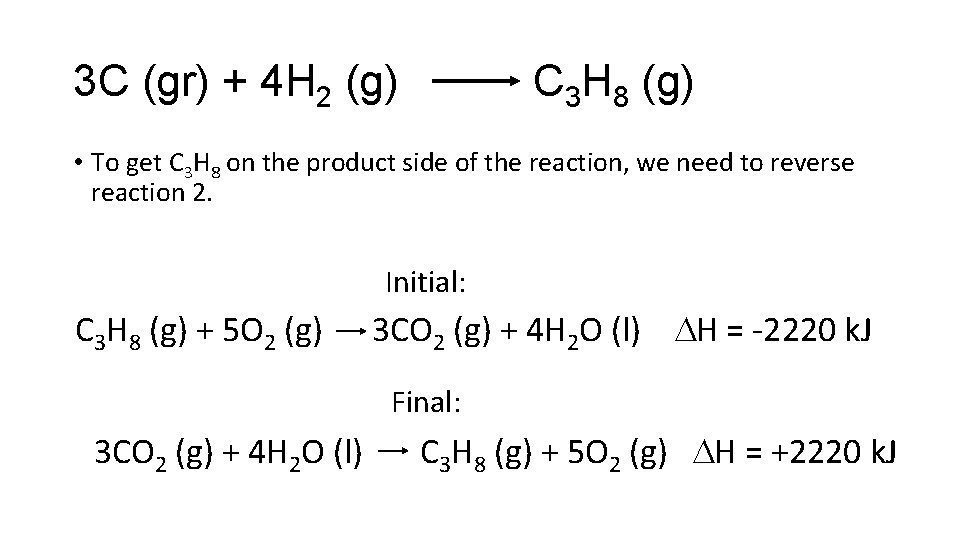
3 C (gr) + 4 H 2 (g) C 3 H 8 (g) • To get C 3 H 8 on the product side of the reaction, we need to reverse reaction 2. Initial: C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) H = -2220 k. J Final: 3 CO 2 (g) + 4 H 2 O (l) C 3 H 8 (g) + 5 O 2 (g) H = +2220 k. J
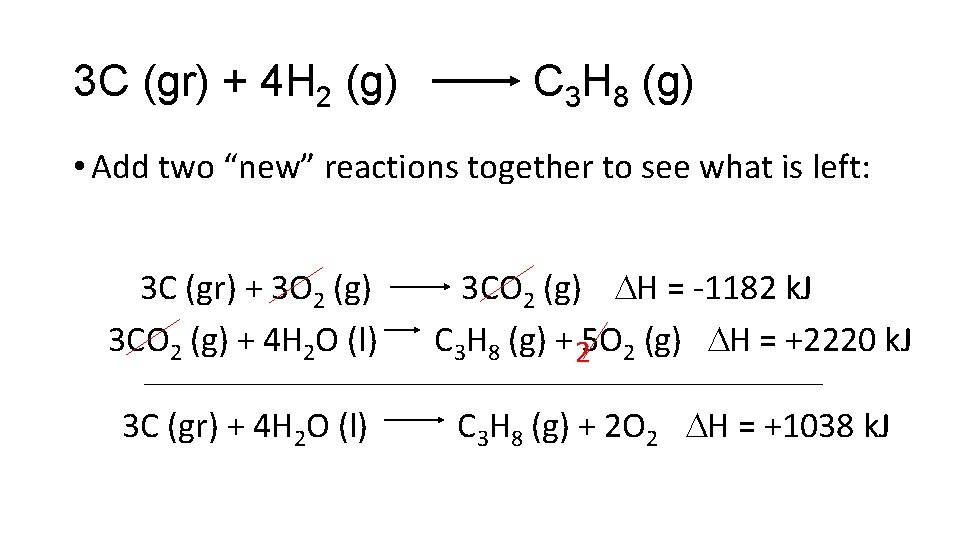
3 C (gr) + 4 H 2 (g) C 3 H 8 (g) • Add two “new” reactions together to see what is left: 3 C (gr) + 3 O 2 (g) 3 CO 2 (g) H = -1182 k. J 3 CO 2 (g) + 4 H 2 O (l) C 3 H 8 (g) + 5 O 2 2 (g) H = +2220 k. J 3 C (gr) + 4 H 2 O (l) C 3 H 8 (g) + 2 O 2 H = +1038 k. J

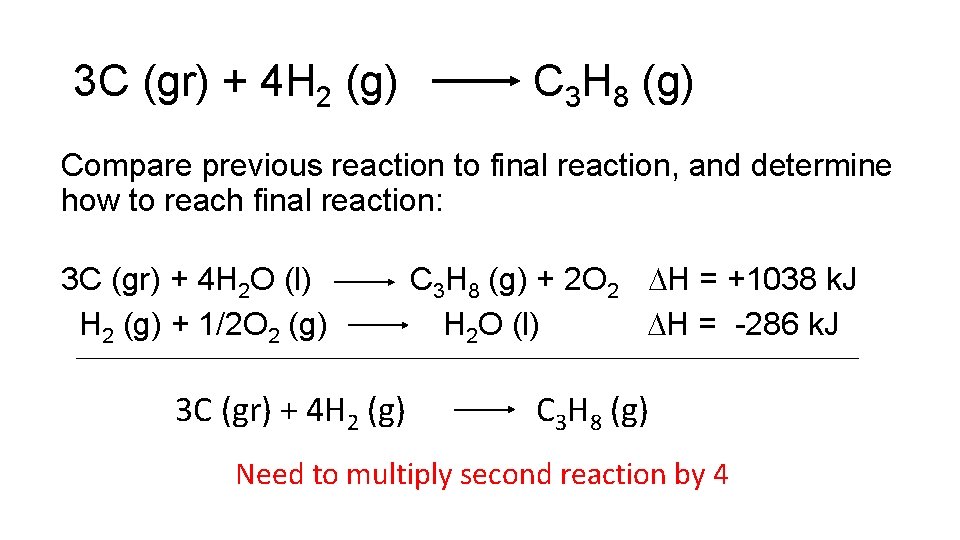
3 C (gr) + 4 H 2 (g) C 3 H 8 (g) Compare previous reaction to final reaction, and determine how to reach final reaction: 3 C (gr) + 4 H 2 O (l) H 2 (g) + 1/2 O 2 (g) C 3 H 8 (g) + 2 O 2 H = +1038 k. J H 2 O (l) H = -286 k. J 3 C (gr) + 4 H 2 (g) C 3 H 8 (g) Need to multiply second reaction by 4
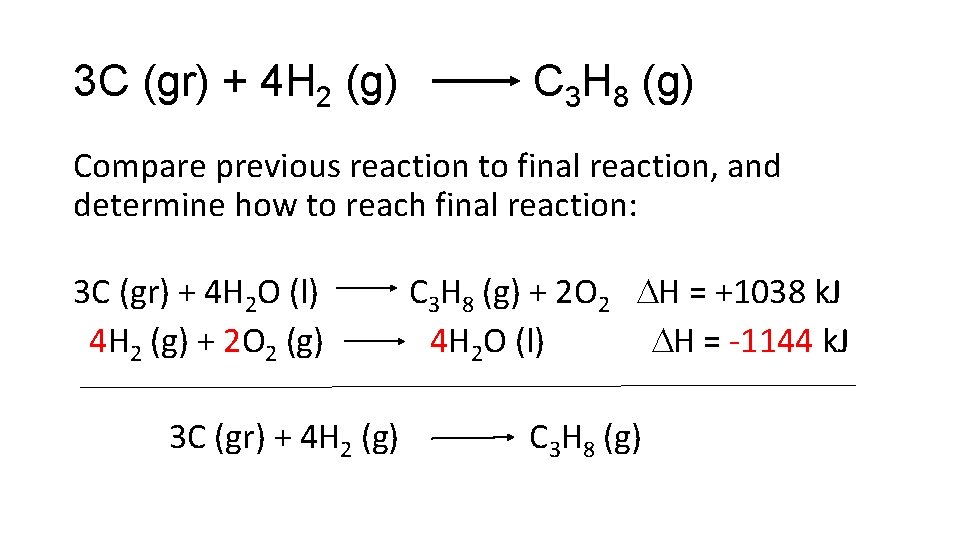
3 C (gr) + 4 H 2 (g) C 3 H 8 (g) Compare previous reaction to final reaction, and determine how to reach final reaction: 3 C (gr) + 4 H 2 O (l) C 3 H 8 (g) + 2 O 2 H = +1038 k. J 4 H 2 (g) + 2 O 2 (g) 4 H 2 O (l) H = -1144 k. J 3 C (gr) + 4 H 2 (g) C 3 H 8 (g)
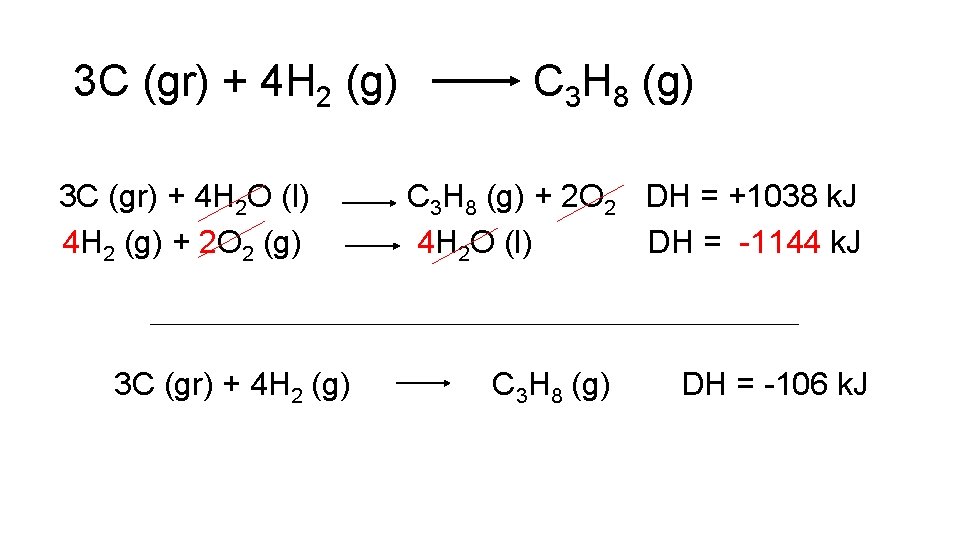
3 C (gr) + 4 H 2 (g) 3 C (gr) + 4 H 2 O (l) 4 H 2 (g) + 2 O 2 (g) 3 C (gr) + 4 H 2 (g) C 3 H 8 (g) + 2 O 2 DH = +1038 k. J 4 H 2 O (l) DH = -1144 k. J C 3 H 8 (g) DH = -106 k. J

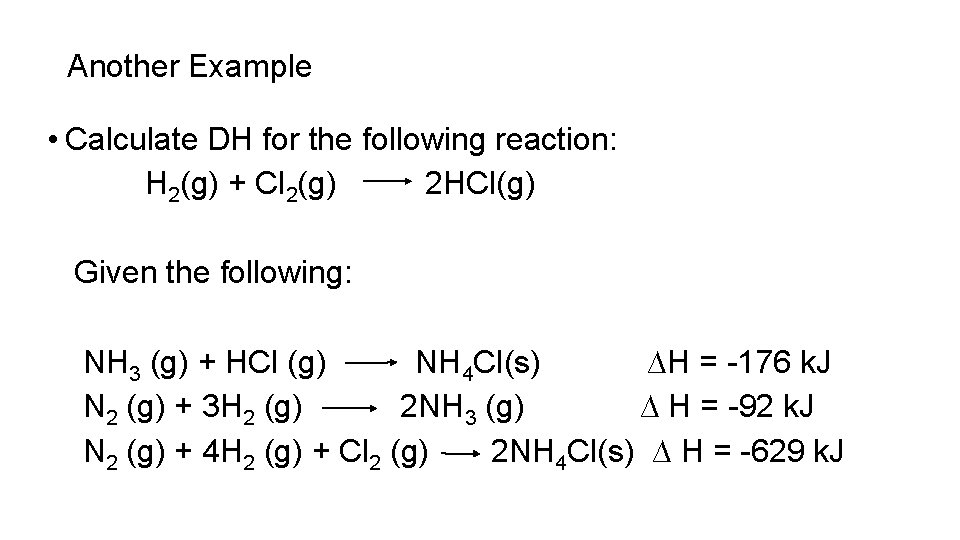
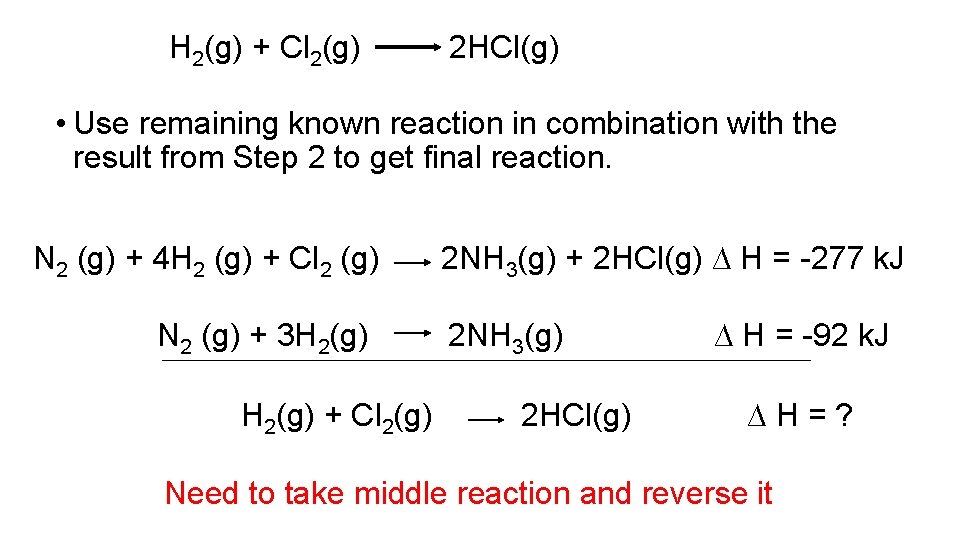
Another Example • Calculate DH for the following reaction: H 2(g) + Cl 2(g) 2 HCl(g) Given the following: NH 3 (g) + HCl (g) NH 4 Cl(s) ∆H = -176 k. J N 2 (g) + 3 H 2 (g) 2 NH 3 (g) ∆ H = -92 k. J N 2 (g) + 4 H 2 (g) + Cl 2 (g) 2 NH 4 Cl(s) ∆ H = -629 k. J
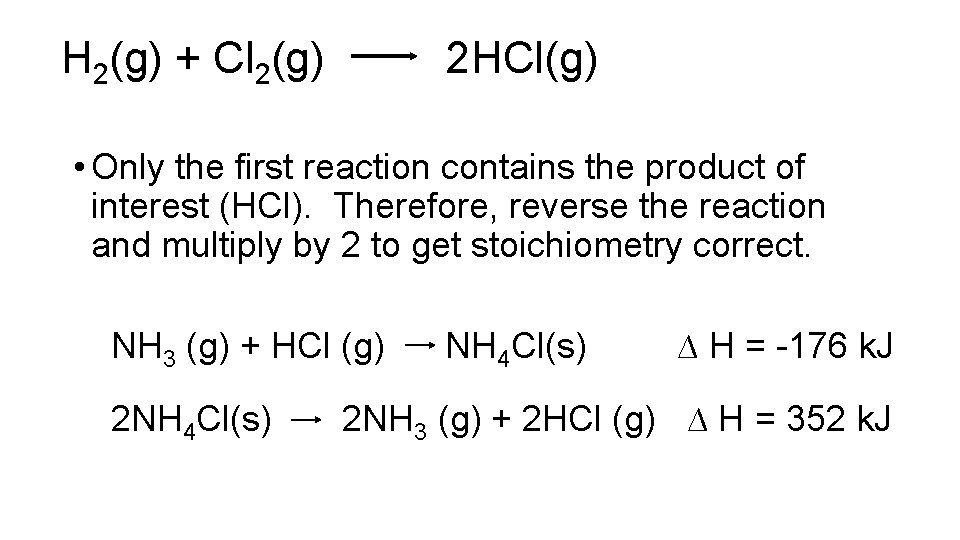
H 2(g) + Cl 2(g) 2 HCl(g) • Only the first reaction contains the product of interest (HCl). Therefore, reverse the reaction and multiply by 2 to get stoichiometry correct. NH 3 (g) + HCl (g) 2 NH 4 Cl(s) ∆ H = -176 k. J 2 NH 3 (g) + 2 HCl (g) ∆ H = 352 k. J
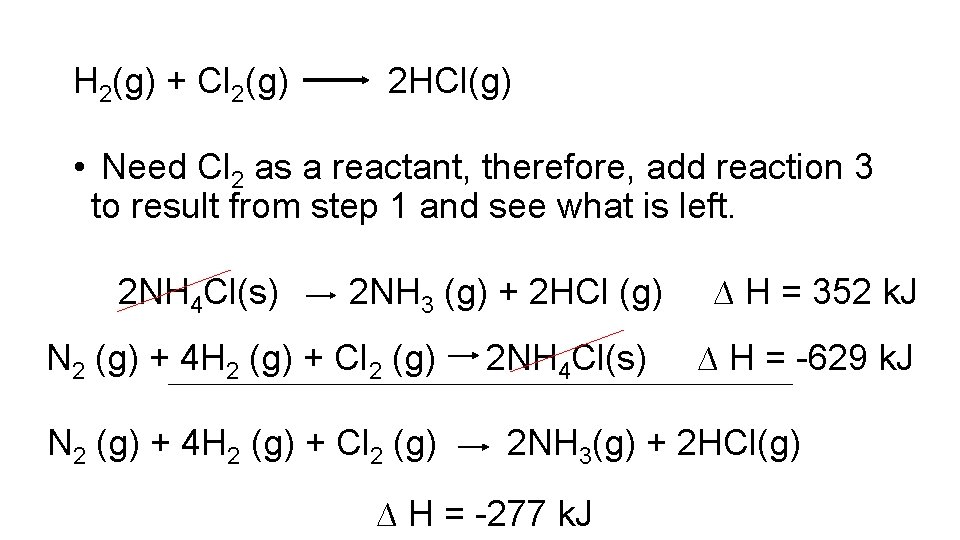
H 2(g) + Cl 2(g) 2 HCl(g) • Need Cl 2 as a reactant, therefore, add reaction 3 to result from step 1 and see what is left. 2 NH 4 Cl(s) 2 NH 3 (g) + 2 HCl (g) N 2 (g) + 4 H 2 (g) + Cl 2 (g) 2 NH 4 Cl(s) ∆ H = 352 k. J ∆ H = -629 k. J 2 NH 3(g) + 2 HCl(g) ∆ H = -277 k. J

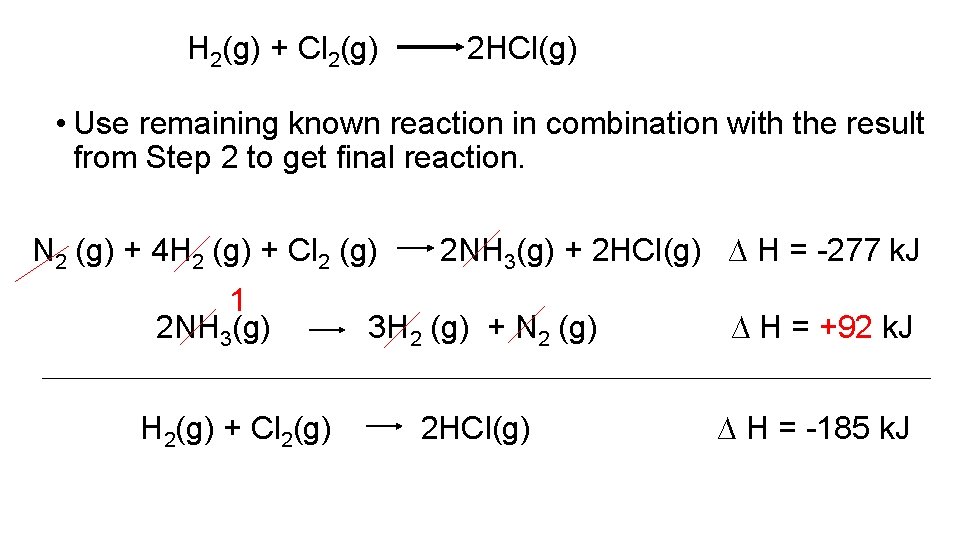
H 2(g) + Cl 2(g) 2 HCl(g) • Use remaining known reaction in combination with the result from Step 2 to get final reaction. N 2 (g) + 4 H 2 (g) + Cl 2 (g) N 2 (g) + 3 H 2(g) + Cl 2(g) 2 NH 3(g) + 2 HCl(g) ∆ H = -277 k. J 2 NH 3(g) 2 HCl(g) ∆ H = -92 k. J ∆H=? Need to take middle reaction and reverse it

H 2(g) + Cl 2(g) 2 HCl(g) • Use remaining known reaction in combination with the result from Step 2 to get final reaction. N 2 (g) + 4 H 2 (g) + Cl 2 (g) 1 2 NH 3(g) H 2(g) + Cl 2(g) 2 NH 3(g) + 2 HCl(g) ∆ H = -277 k. J 3 H 2 (g) + N 2 (g) ∆ H = +92 k. J 2 HCl(g) ∆ H = -185 k. J

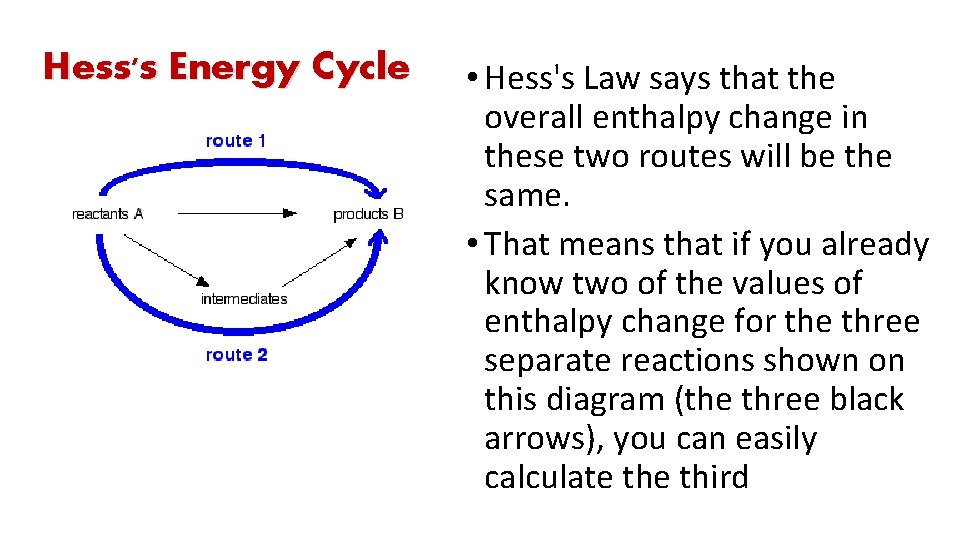
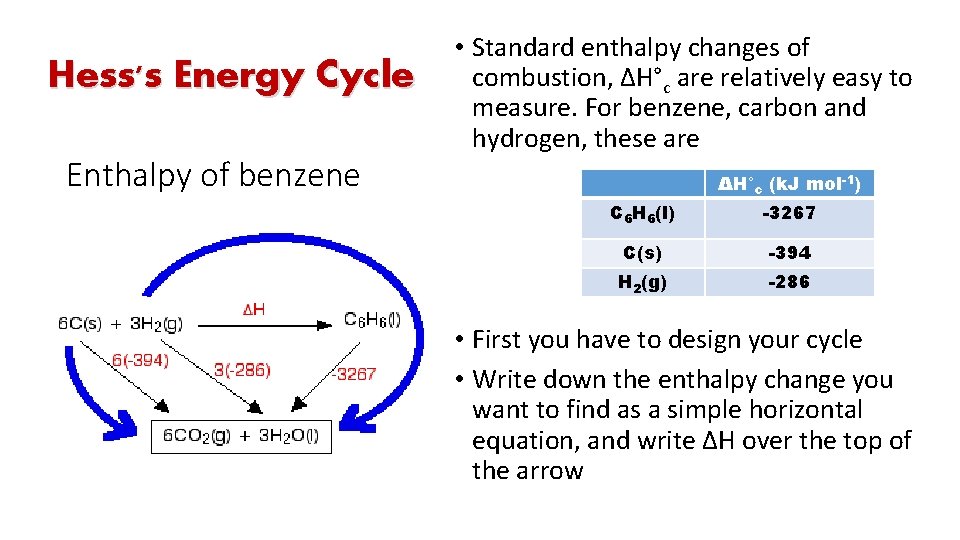
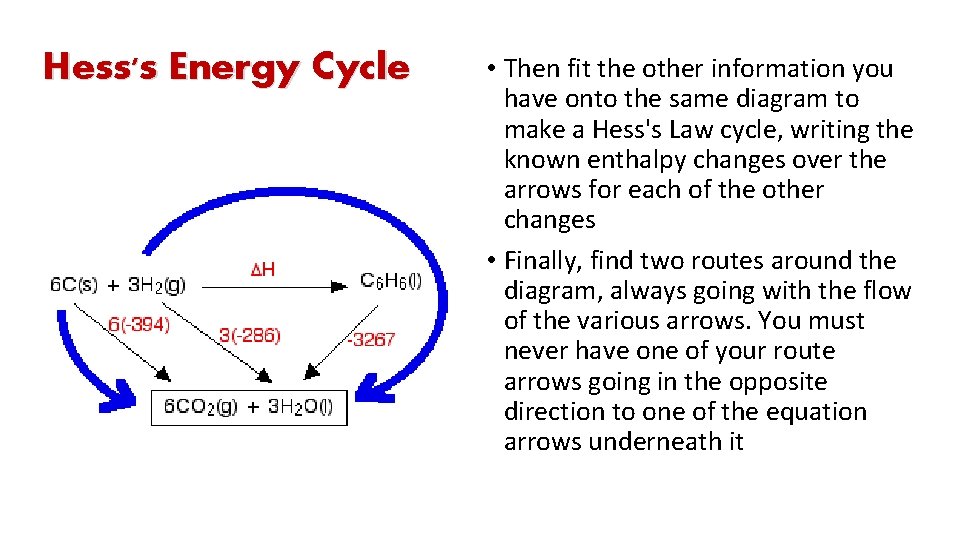
Hess's Energy Cycle • Hess's Law says that the overall enthalpy change in these two routes will be the same. • That means that if you already know two of the values of enthalpy change for the three separate reactions shown on this diagram (the three black arrows), you can easily calculate third

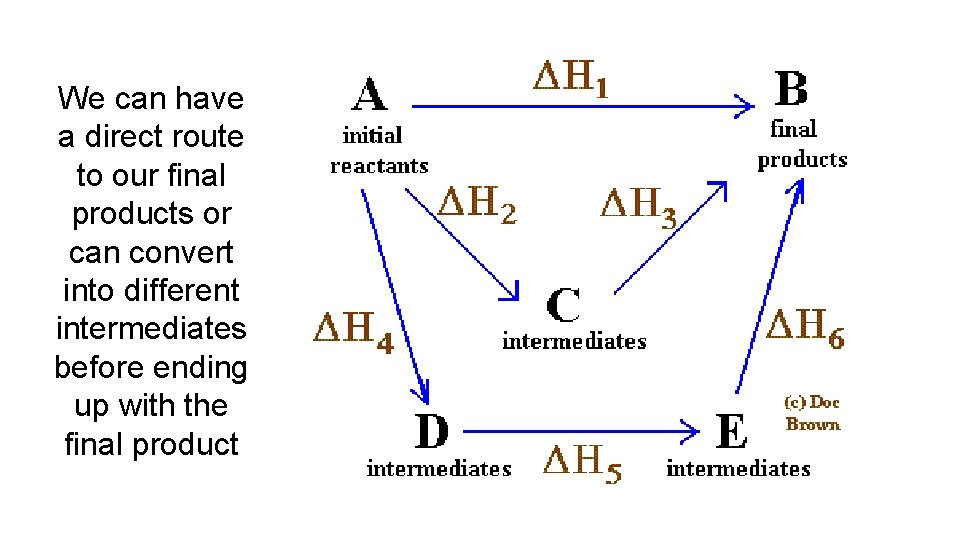
We can have a direct route to our final products or can convert into different intermediates before ending up with the final product

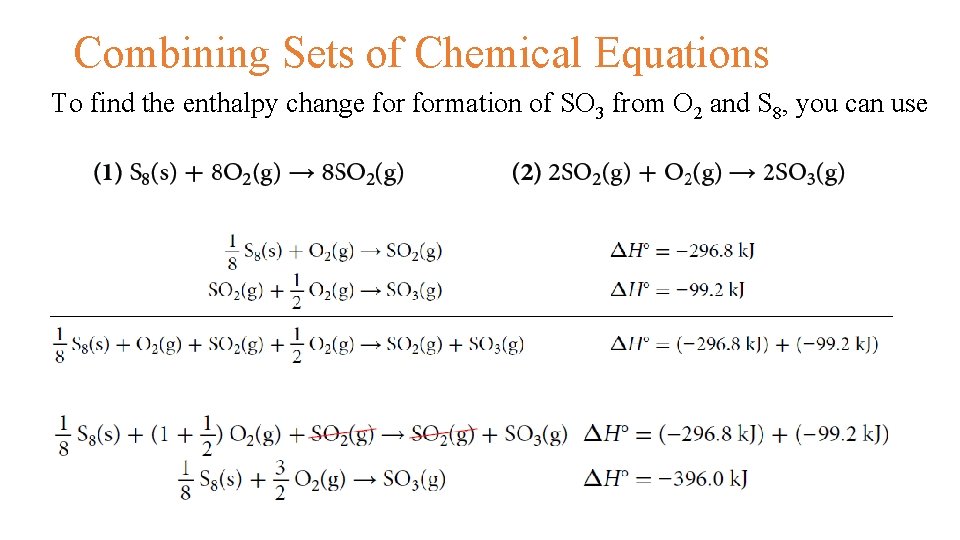
Hess's Energy Cycle Enthalpy of benzene • Standard enthalpy changes of combustion, ΔH°c are relatively easy to measure. For benzene, carbon and hydrogen, these are ΔH°c (k. J mol-1) C 6 H 6(l) -3267 C(s) -394 H 2(g) -286 • First you have to design your cycle • Write down the enthalpy change you want to find as a simple horizontal equation, and write ΔH over the top of the arrow

Hess's Energy Cycle • Then fit the other information you have onto the same diagram to make a Hess's Law cycle, writing the known enthalpy changes over the arrows for each of the other changes • Finally, find two routes around the diagram, always going with the flow of the various arrows. You must never have one of your route arrows going in the opposite direction to one of the equation arrows underneath it

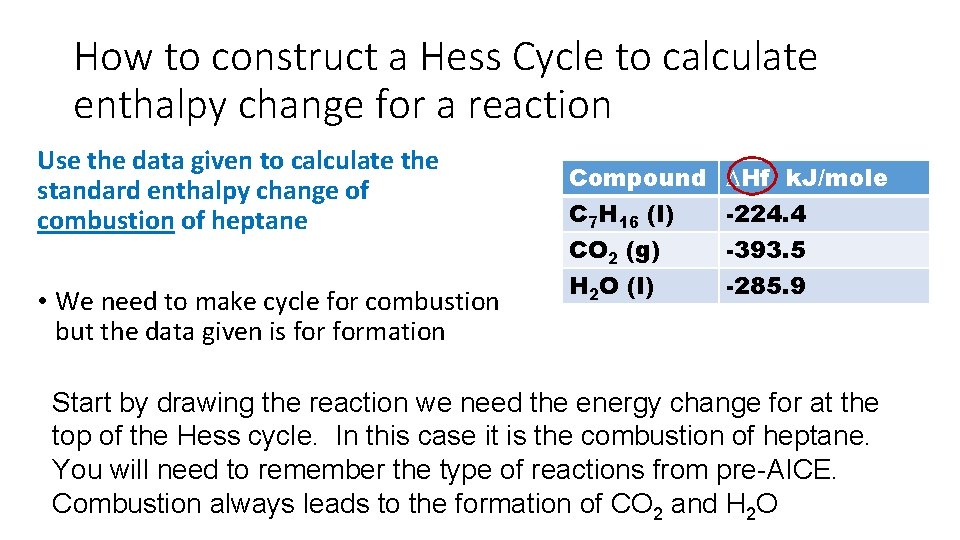
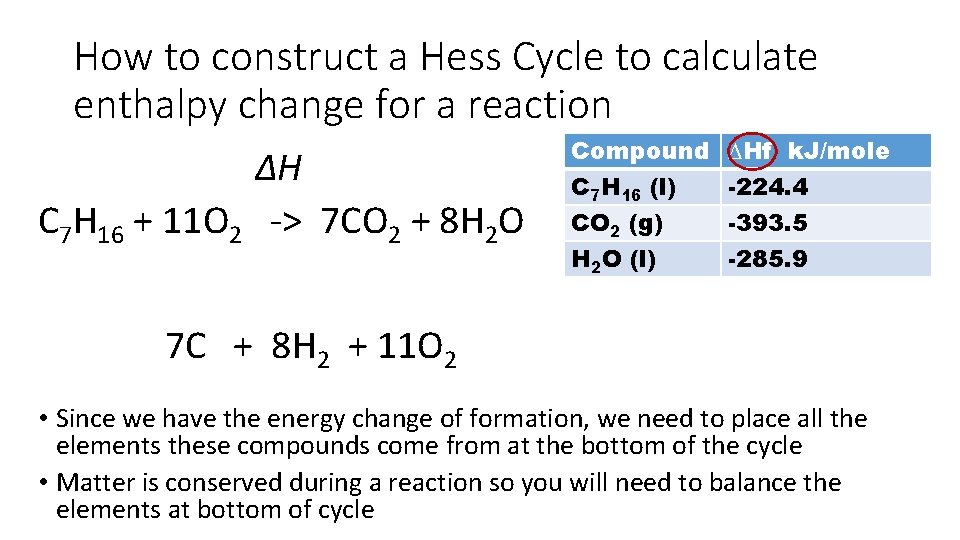
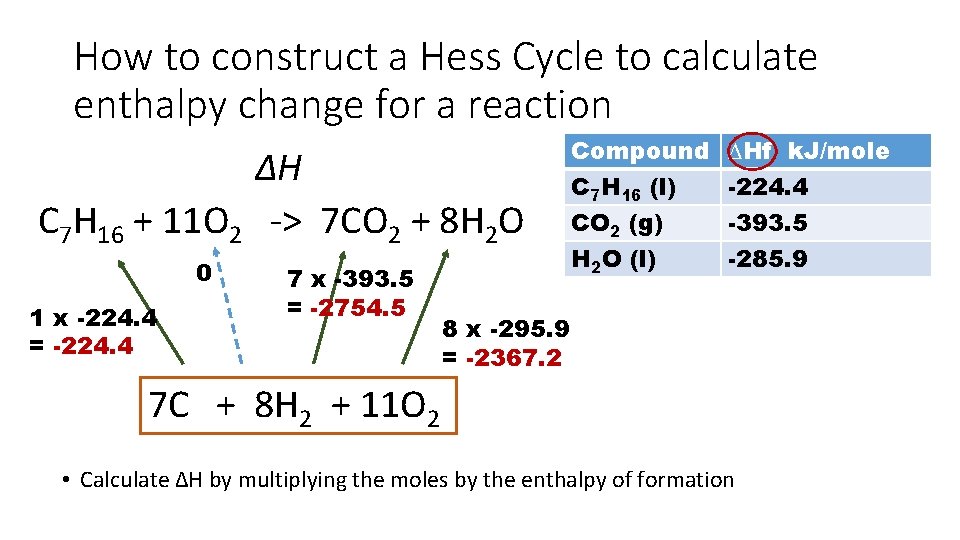
How to construct a Hess Cycle to calculate enthalpy change for a reaction Use the data given to calculate the standard enthalpy change of combustion of heptane • We need to make cycle for combustion but the data given is formation Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 Start by drawing the reaction we need the energy change for at the top of the Hess cycle. In this case it is the combustion of heptane. You will need to remember the type of reactions from pre-AICE. Combustion always leads to the formation of CO 2 and H 2 O

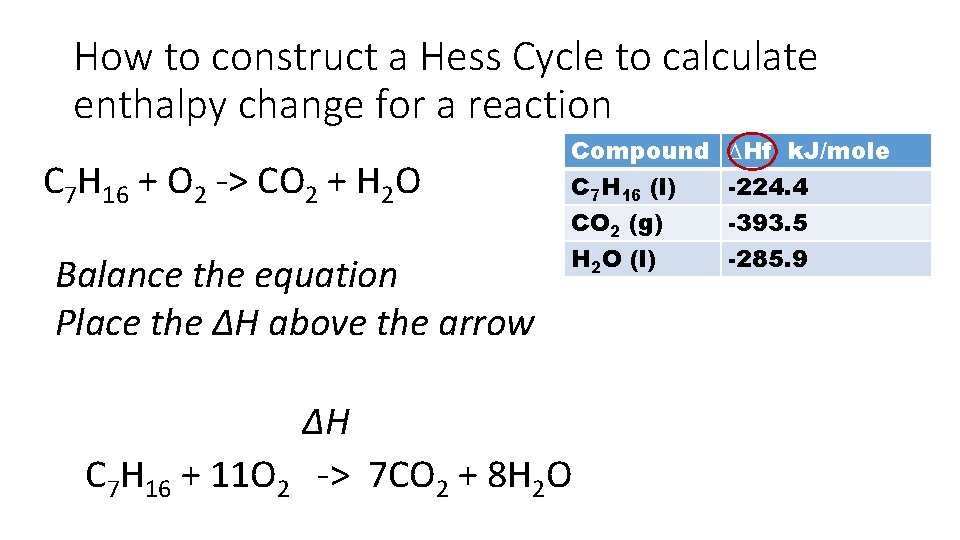
How to construct a Hess Cycle to calculate enthalpy change for a reaction C 7 H 16 + O 2 -> CO 2 + H 2 O Balance the equation Place the ∆H above the arrow Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O ∆Hf k. J/mole -224. 4 -393. 5 -285. 9

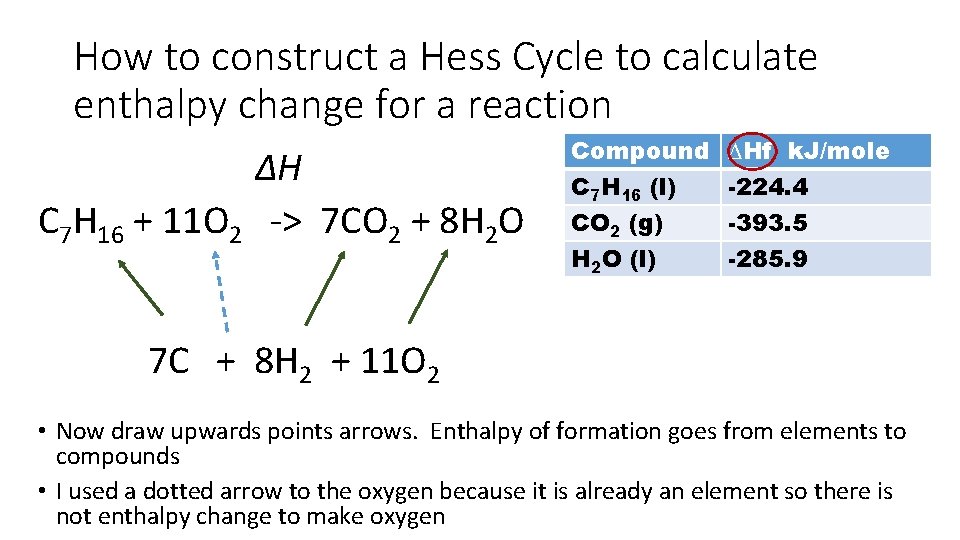
How to construct a Hess Cycle to calculate enthalpy change for a reaction ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 7 C + 8 H 2 + 11 O 2 • Since we have the energy change of formation, we need to place all the elements these compounds come from at the bottom of the cycle • Matter is conserved during a reaction so you will need to balance the elements at bottom of cycle

How to construct a Hess Cycle to calculate enthalpy change for a reaction ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 7 C + 8 H 2 + 11 O 2 • Now draw upwards points arrows. Enthalpy of formation goes from elements to compounds • I used a dotted arrow to the oxygen because it is already an element so there is not enthalpy change to make oxygen

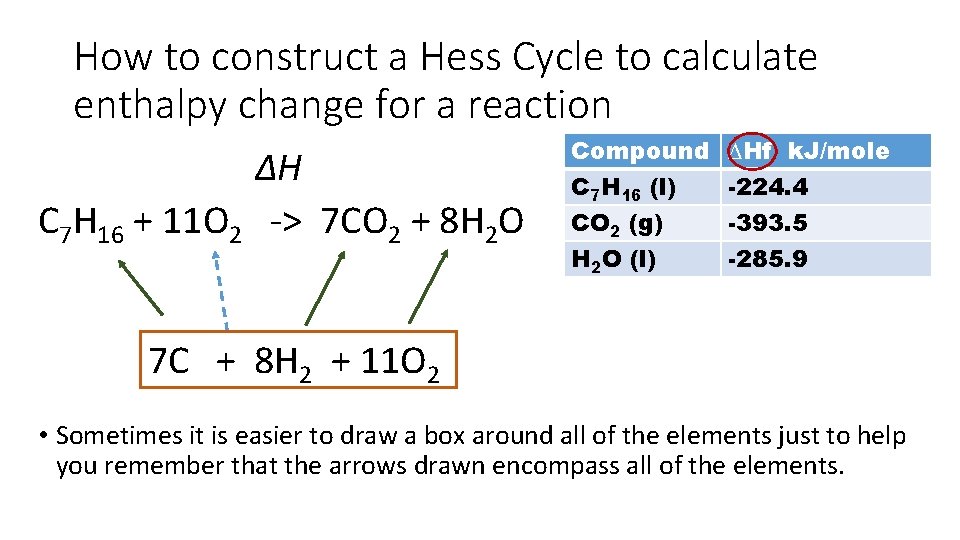
How to construct a Hess Cycle to calculate enthalpy change for a reaction ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 7 C + 8 H 2 + 11 O 2 • Sometimes it is easier to draw a box around all of the elements just to help you remember that the arrows drawn encompass all of the elements.

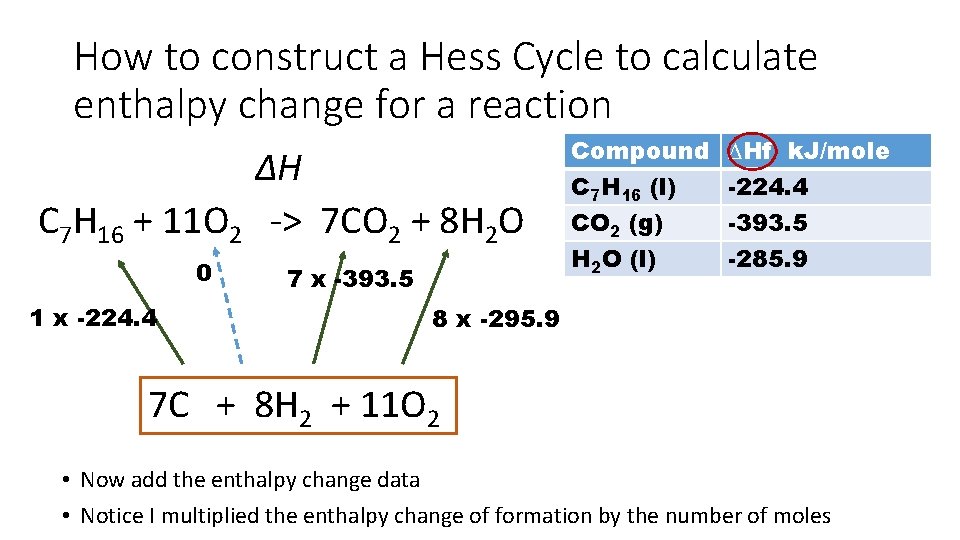
How to construct a Hess Cycle to calculate enthalpy change for a reaction ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O 0 1 x -224. 4 7 x -393. 5 Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 8 x -295. 9 7 C + 8 H 2 + 11 O 2 • Now add the enthalpy change data • Notice I multiplied the enthalpy change of formation by the number of moles

How to construct a Hess Cycle to calculate enthalpy change for a reaction ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O 0 1 x -224. 4 = -224. 4 7 x -393. 5 = -2754. 5 Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 8 x -295. 9 = -2367. 2 7 C + 8 H 2 + 11 O 2 • Calculate ∆H by multiplying the moles by the enthalpy of formation

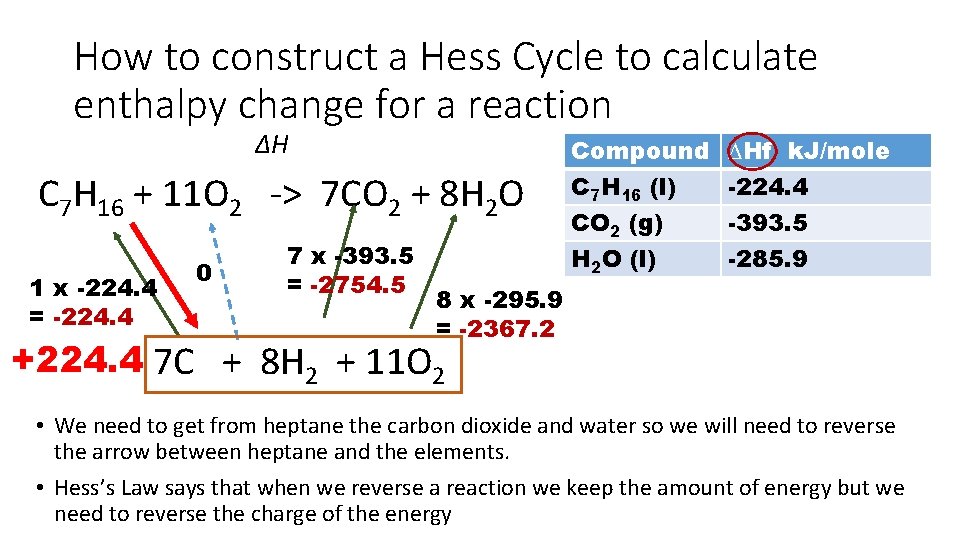
How to construct a Hess Cycle to calculate enthalpy change for a reaction ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O 1 x -224. 4 = -224. 4 0 7 x -393. 5 = -2754. 5 Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 8 x -295. 9 = -2367. 2 +224. 4 7 C + 8 H 2 + 11 O 2 • We need to get from heptane the carbon dioxide and water so we will need to reverse the arrow between heptane and the elements. • Hess’s Law says that when we reverse a reaction we keep the amount of energy but we need to reverse the charge of the energy

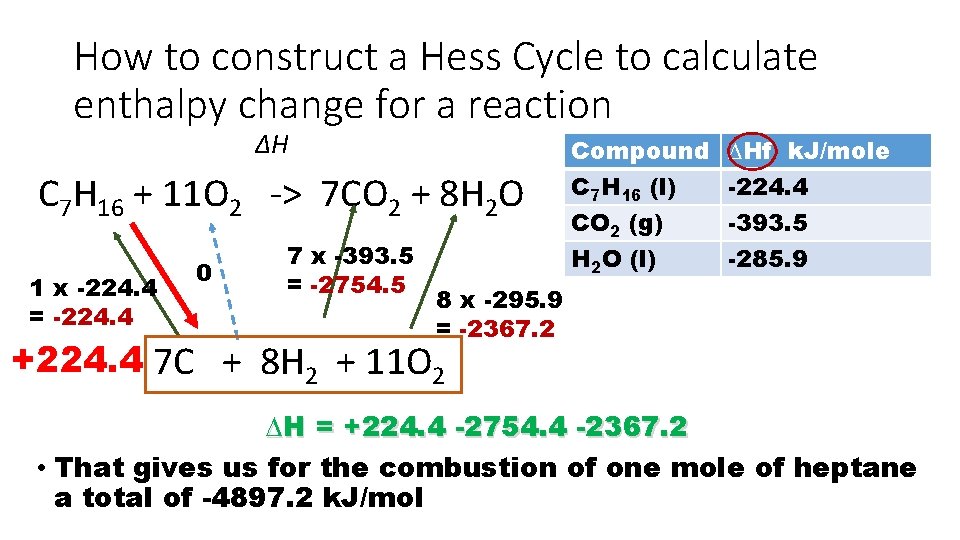
How to construct a Hess Cycle to calculate enthalpy change for a reaction ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O 1 x -224. 4 = -224. 4 0 7 x -393. 5 = -2754. 5 Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 8 x -295. 9 = -2367. 2 +224. 4 7 C + 8 H 2 + 11 O 2 ∆H = +224. 4 -2754. 4 -2367. 2 • That gives us for the combustion of one mole of heptane a total of -4897. 2 k. J/mol

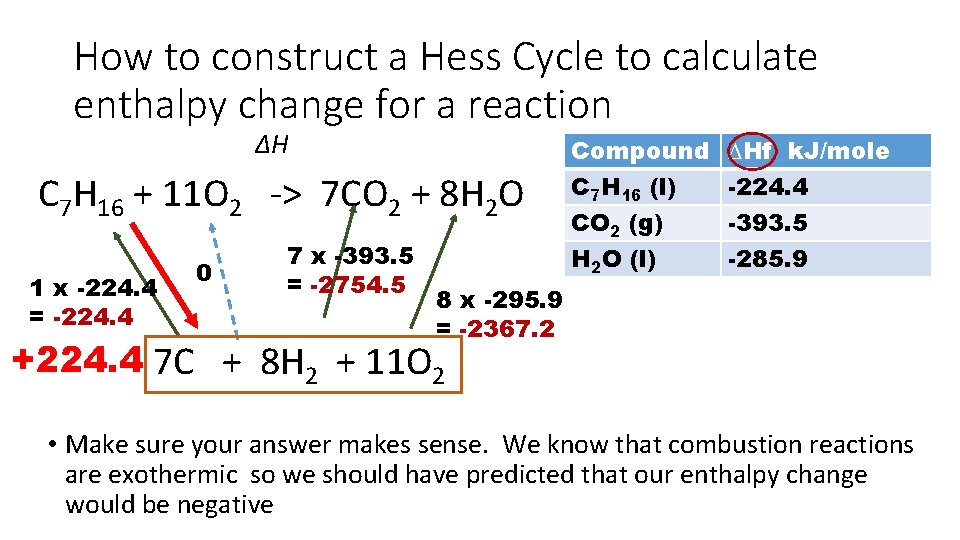
How to construct a Hess Cycle to calculate enthalpy change for a reaction ∆H C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O 1 x -224. 4 = -224. 4 0 7 x -393. 5 = -2754. 5 Compound C 7 H 16 (l) CO 2 (g) H 2 O (l) ∆Hf k. J/mole -224. 4 -393. 5 -285. 9 8 x -295. 9 = -2367. 2 +224. 4 7 C + 8 H 2 + 11 O 2 • Make sure your answer makes sense. We know that combustion reactions are exothermic so we should have predicted that our enthalpy change would be negative

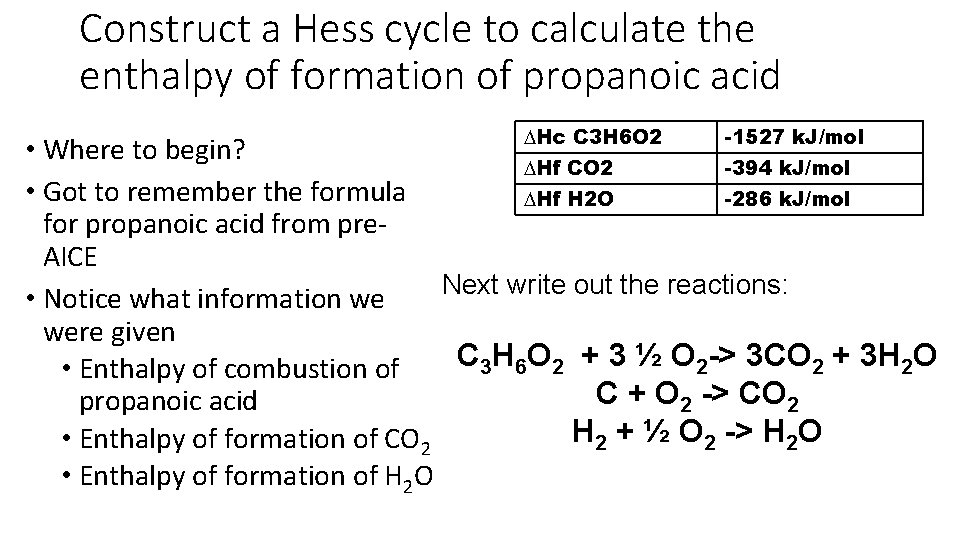
Construct a Hess cycle to calculate the enthalpy of formation of propanoic acid ∆Hc C 3 H 6 O 2 -1527 k. J/mol • Where to begin? ∆Hf CO 2 -394 k. J/mol • Got to remember the formula ∆Hf H 2 O -286 k. J/mol for propanoic acid from pre. AICE Next write out the reactions: • Notice what information we were given C 3 H 6 O 2 + 3 ½ O 2 -> 3 CO 2 + 3 H 2 O • Enthalpy of combustion of C + O 2 -> CO 2 propanoic acid H 2 + ½ O 2 -> H 2 O • Enthalpy of formation of CO 2 • Enthalpy of formation of H 2 O

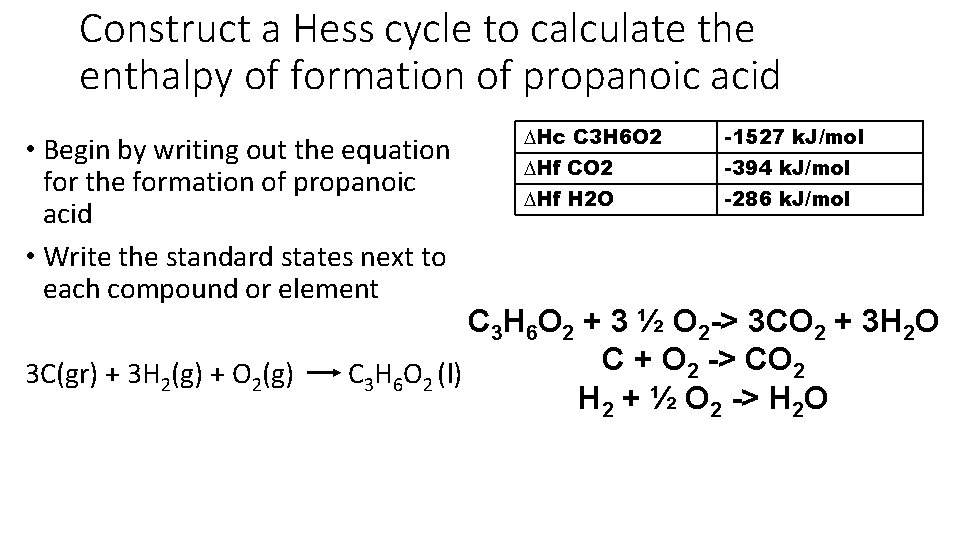
Construct a Hess cycle to calculate the enthalpy of formation of propanoic acid • Begin by writing out the equation for the formation of propanoic acid • Write the standard states next to each compound or element ∆Hc C 3 H 6 O 2 -1527 k. J/mol ∆Hf CO 2 -394 k. J/mol ∆Hf H 2 O -286 k. J/mol C 3 H 6 O 2 + 3 ½ O 2 -> 3 CO 2 + 3 H 2 O C + O 2 -> CO 2 3 C(gr) + 3 H 2(g) + O 2(g) C 3 H 6 O 2 (l) H 2 + ½ O 2 -> H 2 O

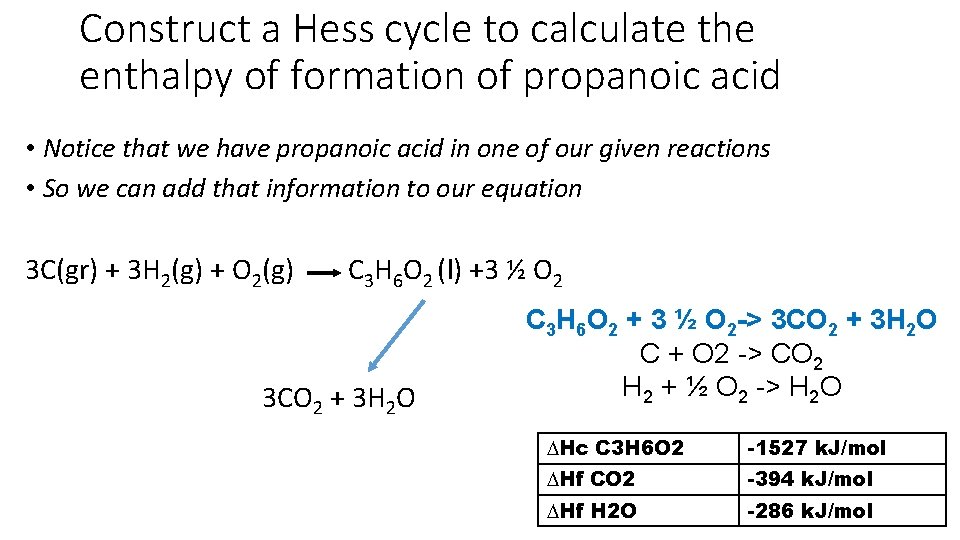
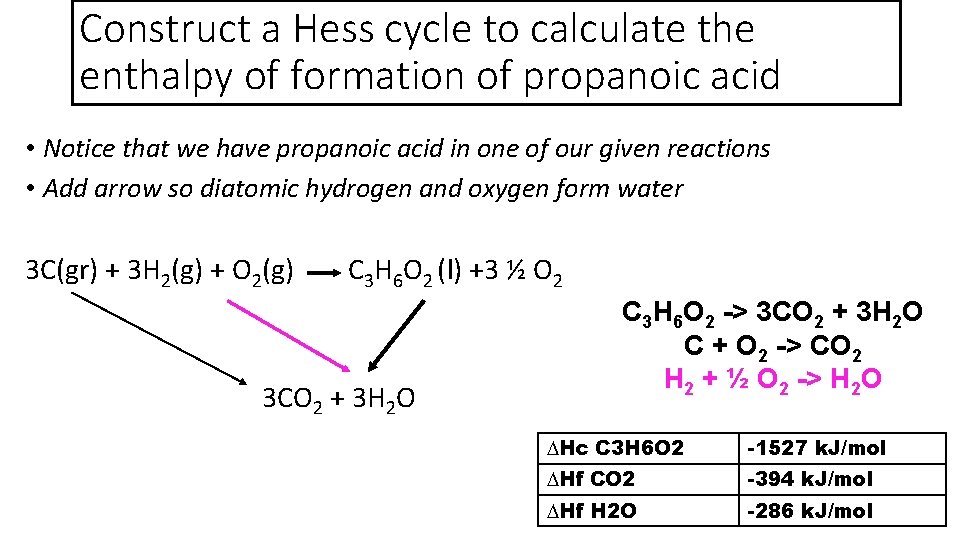
Construct a Hess cycle to calculate the enthalpy of formation of propanoic acid • Notice that we have propanoic acid in one of our given reactions • So we can add that information to our equation 3 C(gr) + 3 H 2(g) + O 2(g) C 3 H 6 O 2 (l) +3 ½ O 2 3 CO 2 + 3 H 2 O C 3 H 6 O 2 + 3 ½ O 2 -> 3 CO 2 + 3 H 2 O C + O 2 -> CO 2 H 2 + ½ O 2 -> H 2 O ∆Hc C 3 H 6 O 2 -1527 k. J/mol ∆Hf CO 2 -394 k. J/mol ∆Hf H 2 O -286 k. J/mol

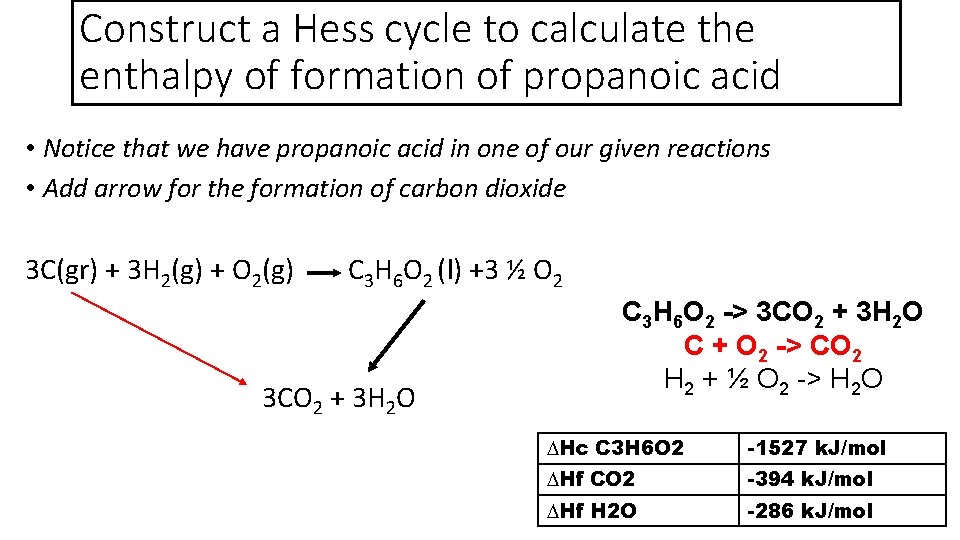
Construct a Hess cycle to calculate the enthalpy of formation of propanoic acid • Notice that we have propanoic acid in one of our given reactions • Add arrow for the formation of carbon dioxide 3 C(gr) + 3 H 2(g) + O 2(g) C 3 H 6 O 2 (l) +3 ½ O 2 3 CO 2 + 3 H 2 O C 3 H 6 O 2 -> 3 CO 2 + 3 H 2 O C + O 2 -> CO 2 H 2 + ½ O 2 -> H 2 O ∆Hc C 3 H 6 O 2 -1527 k. J/mol ∆Hf CO 2 -394 k. J/mol ∆Hf H 2 O -286 k. J/mol

Construct a Hess cycle to calculate the enthalpy of formation of propanoic acid • Notice that we have propanoic acid in one of our given reactions • Add arrow so diatomic hydrogen and oxygen form water 3 C(gr) + 3 H 2(g) + O 2(g) C 3 H 6 O 2 (l) +3 ½ O 2 3 CO 2 + 3 H 2 O C 3 H 6 O 2 -> 3 CO 2 + 3 H 2 O C + O 2 -> CO 2 H 2 + ½ O 2 -> H 2 O ∆Hc C 3 H 6 O 2 -1527 k. J/mol ∆Hf CO 2 -394 k. J/mol ∆Hf H 2 O -286 k. J/mol

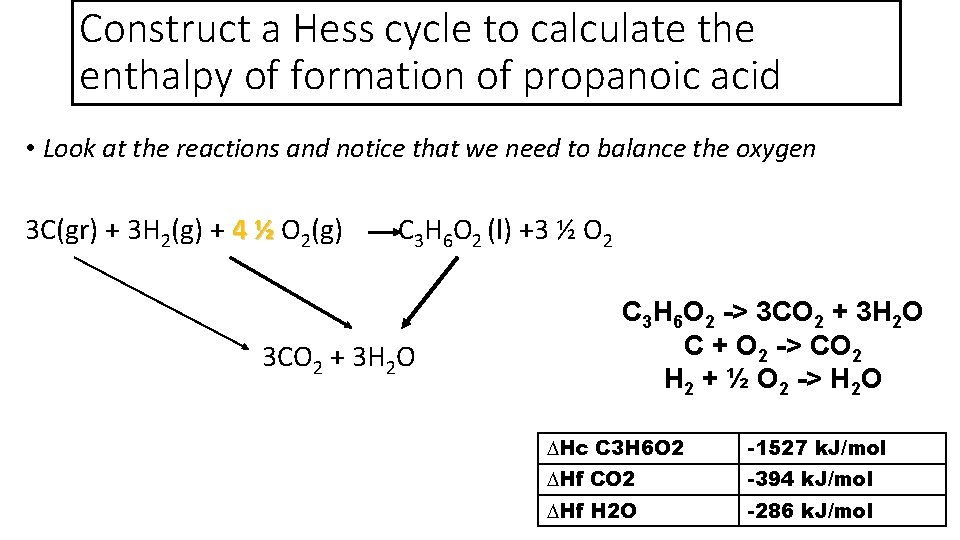
Construct a Hess cycle to calculate the enthalpy of formation of propanoic acid • Look at the reactions and notice that we need to balance the oxygen 3 C(gr) + 3 H 2(g) + 4 ½ O 4 ½ 2(g) C 3 H 6 O 2 (l) +3 ½ O 2 3 CO 2 + 3 H 2 O C 3 H 6 O 2 -> 3 CO 2 + 3 H 2 O C + O 2 -> CO 2 H 2 + ½ O 2 -> H 2 O ∆Hc C 3 H 6 O 2 -1527 k. J/mol ∆Hf CO 2 -394 k. J/mol ∆Hf H 2 O -286 k. J/mol

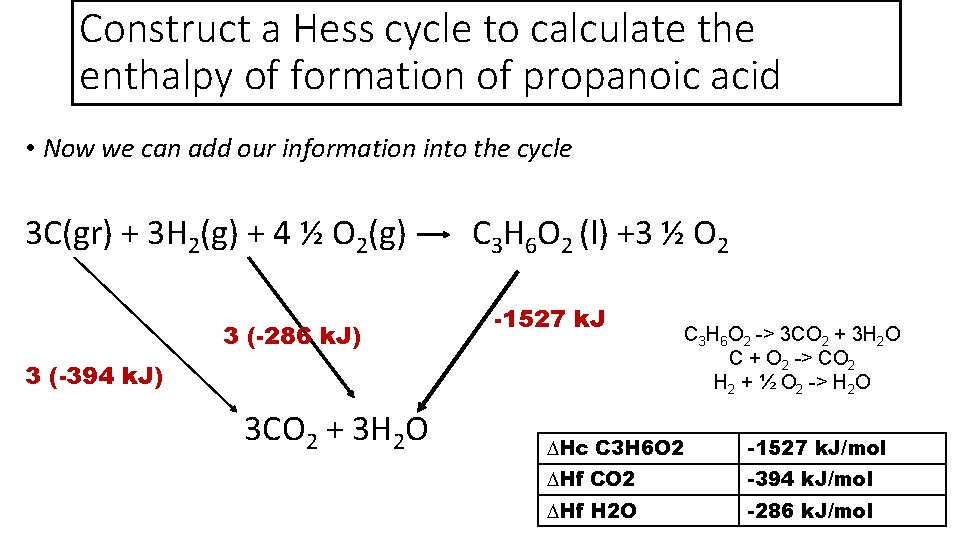
Construct a Hess cycle to calculate the enthalpy of formation of propanoic acid • Now we can add our information into the cycle 3 C(gr) + 3 H 2(g) + 4 ½ O 2(g) C 3 H 6 O 2 (l) +3 ½ O 2 3 (-286 k. J) 3 (-394 k. J) 3 CO 2 + 3 H 2 O -1527 k. J C 3 H 6 O 2 -> 3 CO 2 + 3 H 2 O C + O 2 -> CO 2 H 2 + ½ O 2 -> H 2 O ∆Hc C 3 H 6 O 2 -1527 k. J/mol ∆Hf CO 2 -394 k. J/mol ∆Hf H 2 O -286 k. J/mol

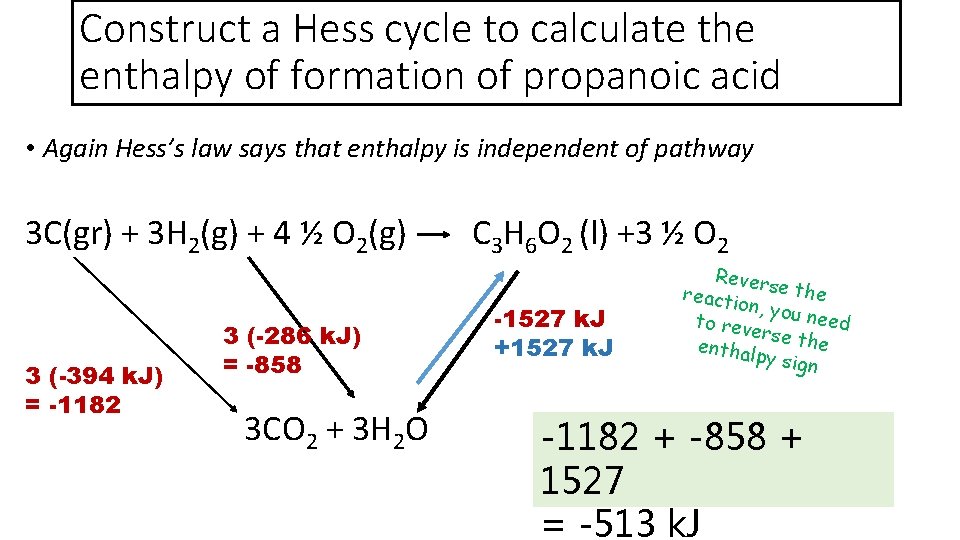
Construct a Hess cycle to calculate the enthalpy of formation of propanoic acid • Again Hess’s law says that enthalpy is independent of pathway 3 C(gr) + 3 H 2(g) + 4 ½ O 2(g) C 3 H 6 O 2 (l) +3 ½ O 2 3 (-286 k. J) = -858 3 (-394 k. J) = -1182 3 CO 2 + 3 H 2 O -1527 k. J +1527 k. J Revers e the reacti on, you need to rev ers enthal e the py sign -1182 + -858 + 1527 = -513 k. J

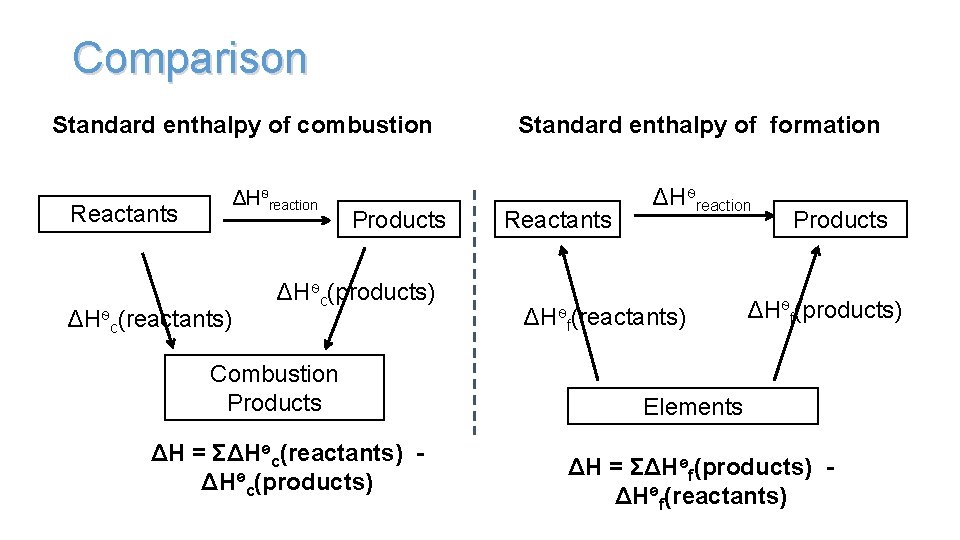
Comparison Standard enthalpy of combustion Reactants ΔHѳreaction c(reactants) Products ΔHѳc(products) Combustion Products ΔH = ΣΔHѳc(reactants) ΔHѳc(products) Standard enthalpy of formation Reactants ΔHѳreaction ΔHѳf(reactants) Products ΔHѳf(products) Elements ΔH = ΣΔHѳf(products) ΔHѳf(reactants)

Calculating the enthalpy change of hydration of an anhydrous salt • Hydrated salt contains water molecules bonded to the compound (not the same as being dissolved in an aqueous solution) • Very difficult to measure the enthalpy change of an anhydrous salt when it becomes hydrated • Again we need to use the energy cycle to help calculate the enthalpy • Enthalpy of hydration is the energy change for converting 1 mole of an anhydrous substance to 1 mole of the hydrated substance • First we need to calculate the enthalpy of dissolution for each substance separately then find the difference between the two

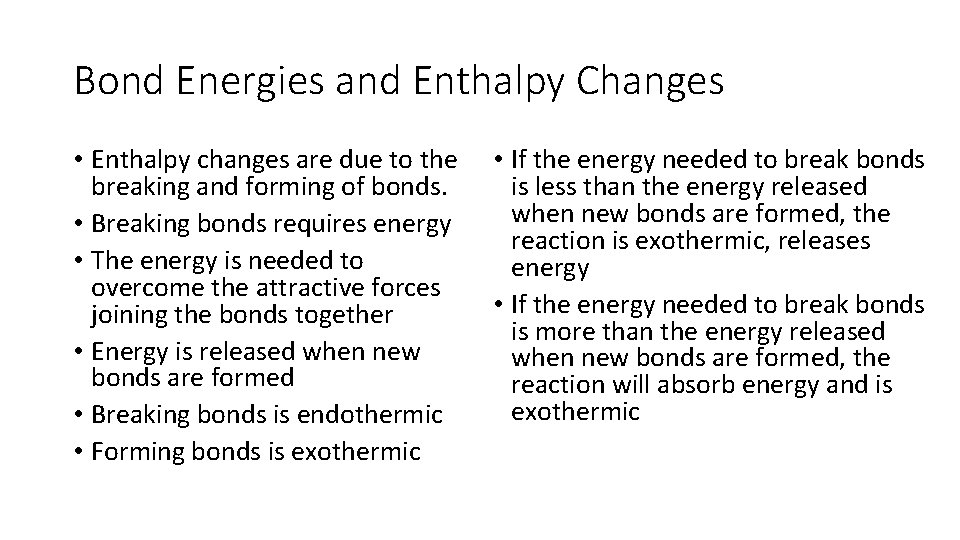
Bond Energies and Enthalpy Changes • Enthalpy changes are due to the breaking and forming of bonds. • Breaking bonds requires energy • The energy is needed to overcome the attractive forces joining the bonds together • Energy is released when new bonds are formed • Breaking bonds is endothermic • Forming bonds is exothermic • If the energy needed to break bonds is less than the energy released when new bonds are formed, the reaction is exothermic, releases energy • If the energy needed to break bonds is more than the energy released when new bonds are formed, the reaction will absorb energy and is exothermic

Bond Energy • The amount of energy needed to break a specific covalent bond is called bond dissociation energy also called bond energy or bond enthalpy. • Symbol for bond energy is E

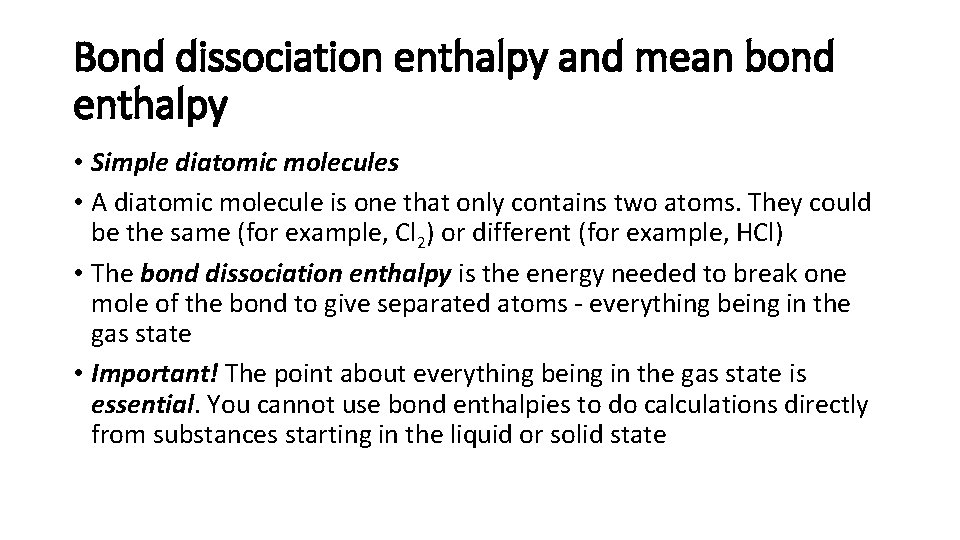
Bond dissociation enthalpy and mean bond enthalpy • Simple diatomic molecules • A diatomic molecule is one that only contains two atoms. They could be the same (for example, Cl 2) or different (for example, HCl) • The bond dissociation enthalpy is the energy needed to break one mole of the bond to give separated atoms - everything being in the gas state • Important! The point about everything being in the gas state is essential. You cannot use bond enthalpies to do calculations directly from substances starting in the liquid or solid state

bond enthalpy quoted is an average value • Methane, CH 4 contains four identical C-H bonds, and it seems reasonable that they should all have the same bond enthalpy • If you took methane to pieces one hydrogen at a time, it needs a different amount of energy to break each of the four C-H bonds • Every time you break a hydrogen off the carbon, the environment of those left behind changes. And the strength of a bond is affected by what else is around it • Tables of bond enthalpies give average values particularly in organic chemistry. The bond enthalpy of, say, the C-H bond varies depending on what is around it in the molecule

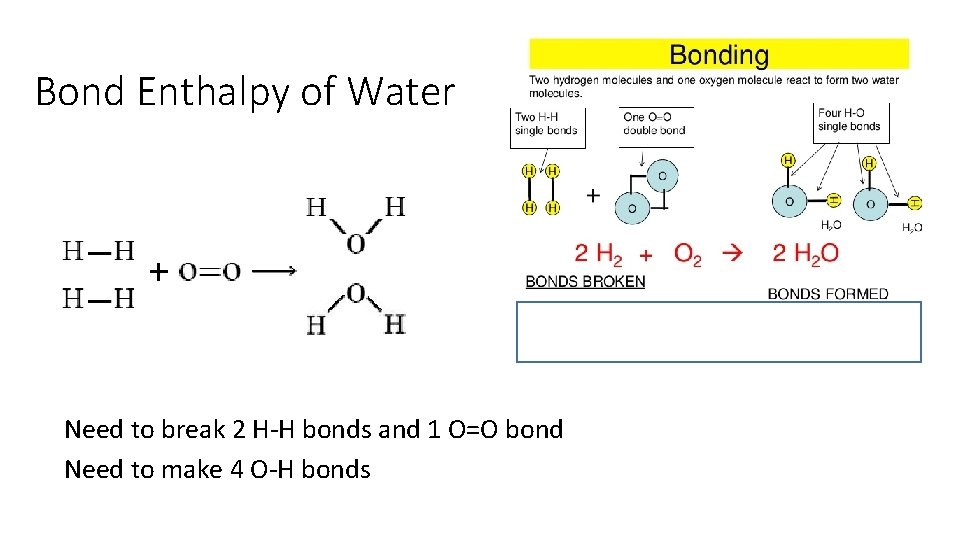
Bond Enthalpy of Water Need to break 2 H-H bonds and 1 O=O bond Need to make 4 O-H bonds

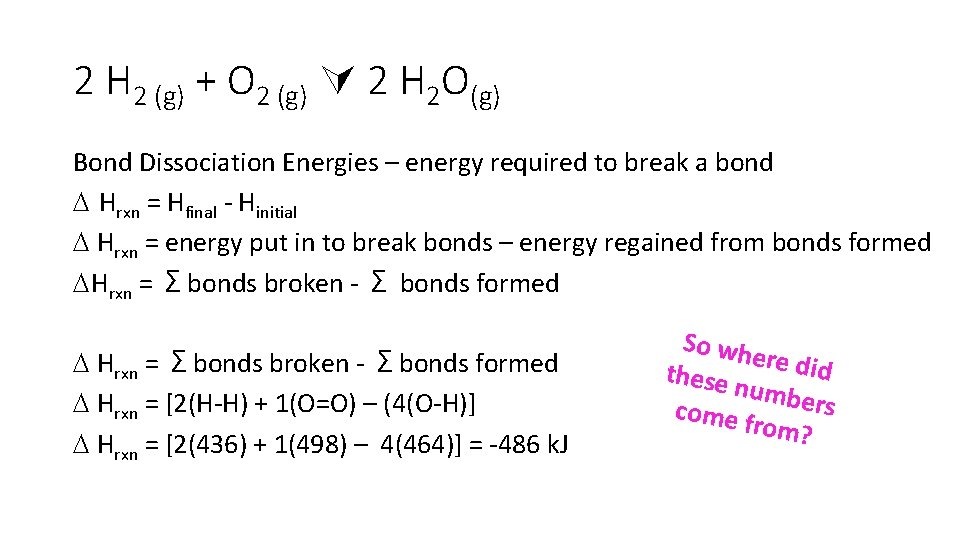
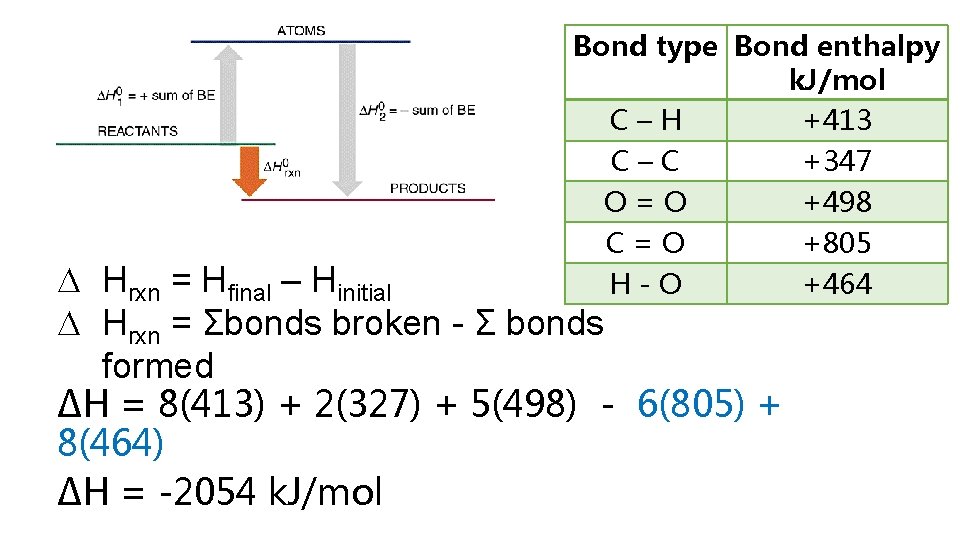
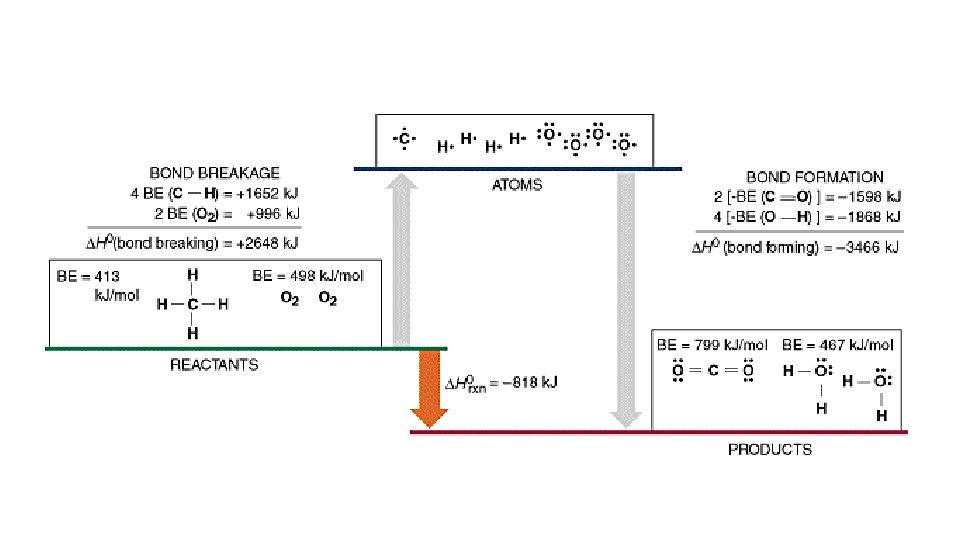
2 H 2 (g) + O 2 (g) 2 H 2 O(g) Bond Dissociation Energies – energy required to break a bond Hrxn = Hfinal - Hinitial Hrxn = energy put in to break bonds – energy regained from bonds formed Hrxn = Σbonds broken - Σ bonds formed Hrxn = Σbonds broken - Σbonds formed Hrxn = [2(H-H) + 1(O=O) – (4(O-H)] Hrxn = [2(436) + 1(498) – 4(464)] = -486 k. J So wh ere did these numbe rs come f rom?

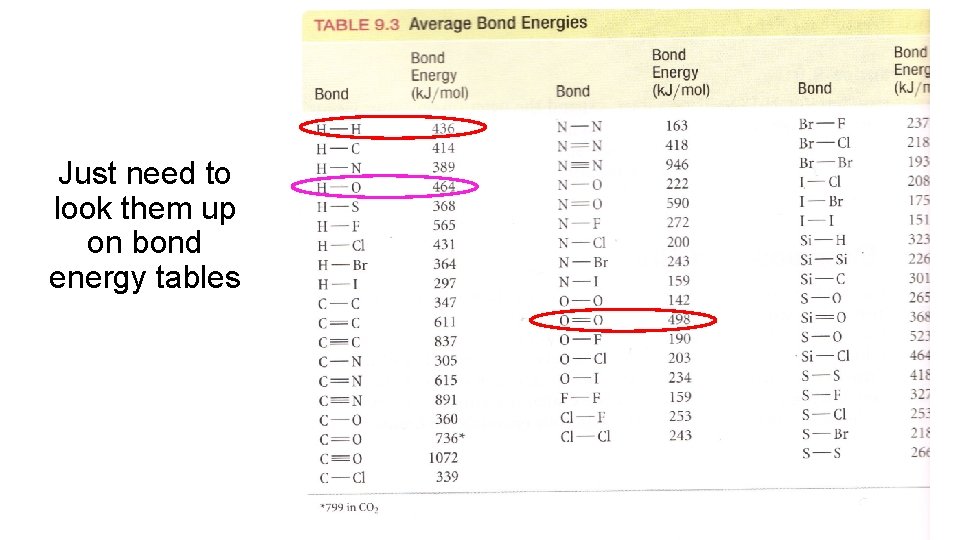
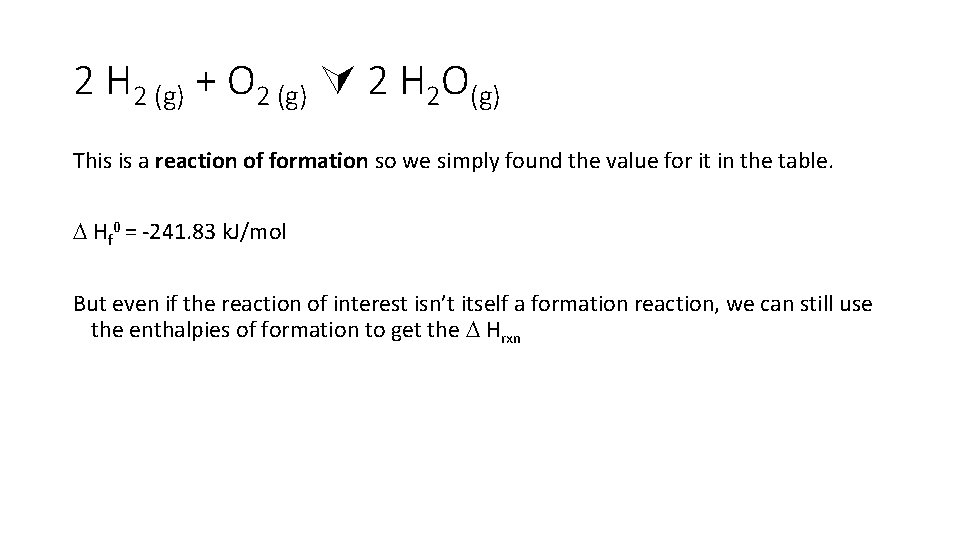
Just need to look them up on bond energy tables

2 H 2 (g) + O 2 (g) 2 H 2 O(g) This is a reaction of formation so we simply found the value for it in the table. Hf 0 = -241. 83 k. J/mol But even if the reaction of interest isn’t itself a formation reaction, we can still use the enthalpies of formation to get the Hrxn

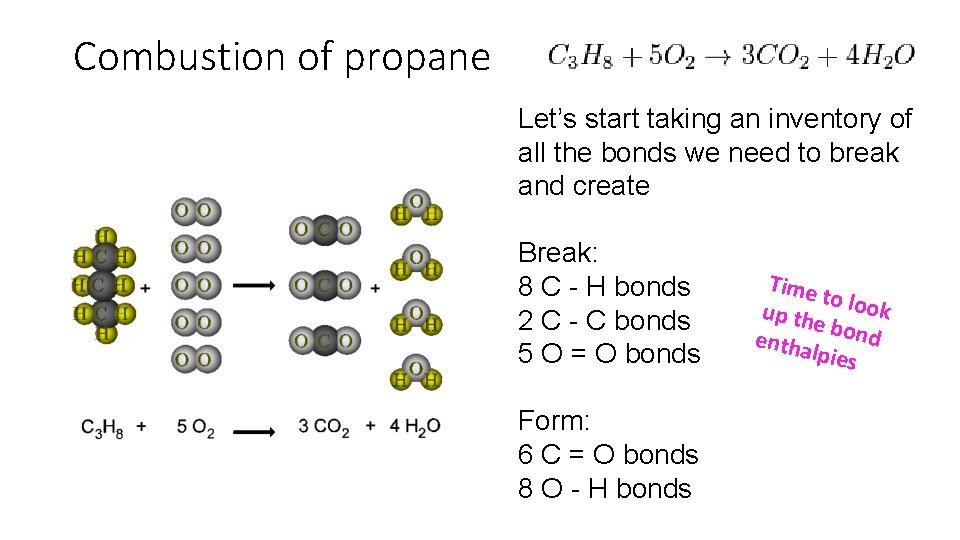
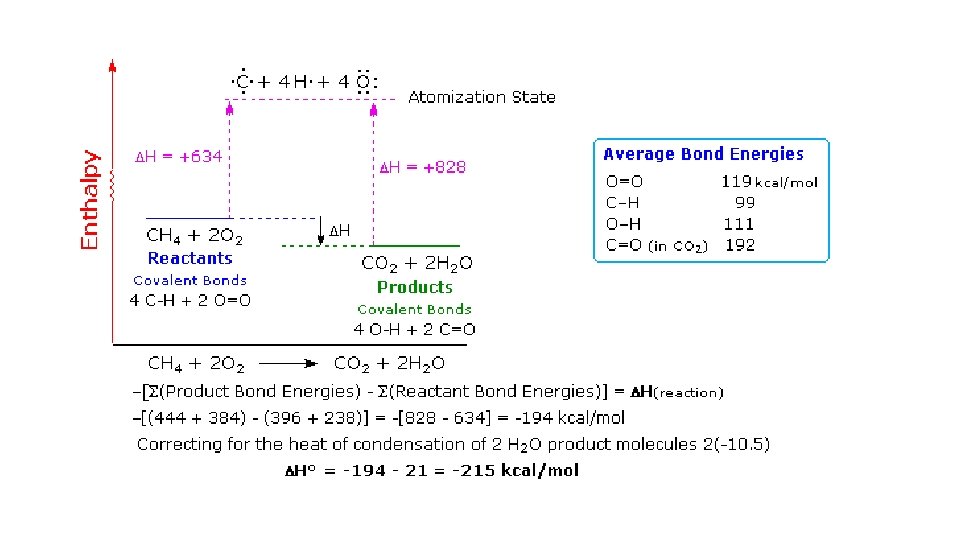
Combustion of propane Let’s start taking an inventory of all the bonds we need to break and create Break: 8 C - H bonds 2 C - C bonds 5 O = O bonds Form: 6 C = O bonds 8 O - H bonds Time to loo k up th e bon d entha lpies

Bond type Bond enthalpy k. J/mol C–H +413 C–C +347 O=O +498 C=O +805 H-O +464 Hrxn = Hfinal – Hinitial Hrxn = Σbonds broken - Σ bonds formed ∆H = 8(413) + 2(327) + 5(498) - 6(805) + 8(464) ∆H = -2054 k. J/mol

Combining Sets of Chemical Equations To find the enthalpy change formation of SO 3 from O 2 and S 8, you can use


White Board • A 1. 0 g sample of copper is heated from 25. 0°C to 31. 0°C. How much heat did the sample absorb? • Q = mc∆T • Q = (1. 0 g)(0. 385 g/J°C)(6. 0°C) • Q = 2. 3 J

White Board Answer: A May 2010 Paper

White Board Answer: D Nov 2007 Paper

White board! Answer: C May 2010 Paper 1

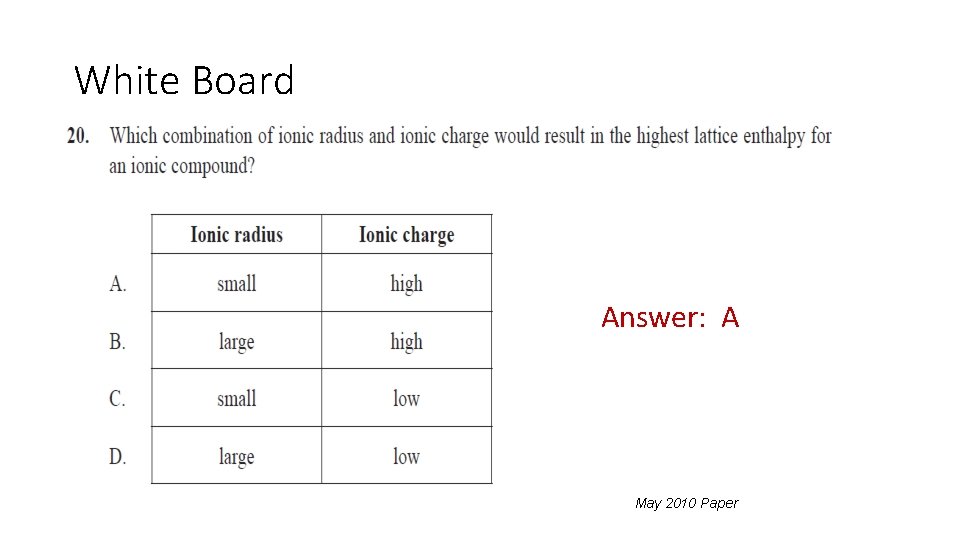
White Board Answer: A May 2010 Paper 1

White Board Answer B May 2010 Paper 1

White Board Answer: A May 2008 Paper 1

White Board Answer: C Nov 2009 Paper 1


Ways to determine H 1. Find H 0 in a table 2. Find Hf 0 in a table 3. Calculate from Bond Energies And… 4. Calculate from Hf 0 5. Calculate from other H that you already know (Hess’s Law)

