Thermal Properties Heat Capacity Specific Heat Thermal Properties
















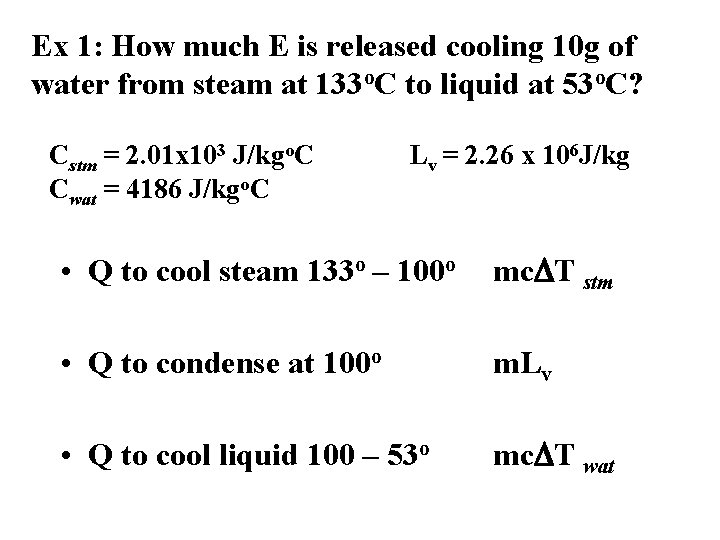
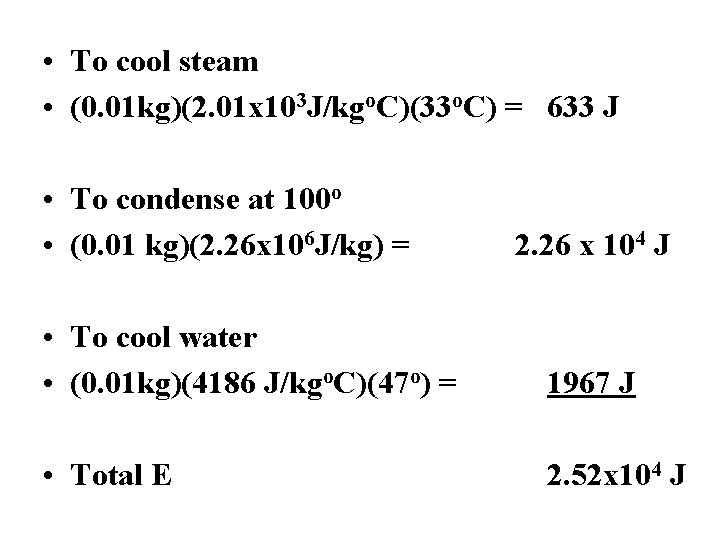















- Slides: 59

Thermal Properties, Heat Capacity, Specific Heat.

Thermal Properties of Matter When objects absorb heat (Q) their T rises. When objects release/lose (Q) heat their T decreases. Different materials undergo different DT for same heat absorbed/released.

Thermal Capacity (C)= amt of heat E (Q) needed to raise a body’s temperature 1 o. C. • Water has high heat C, • takes lots of E to change its T. • Metals have low capacities. A little heat E gives a lot of DT

Land heats and cools (DT) more quickly than water

Why? Temp is measure of average KE of particles. 1 kg of different materials contain different: • # of particles, KE must be spread over varying numbers of particles. • Weights of particles, it takes more E to move heavier particles. • Bond strengths, some materials have particles that are easy to move.

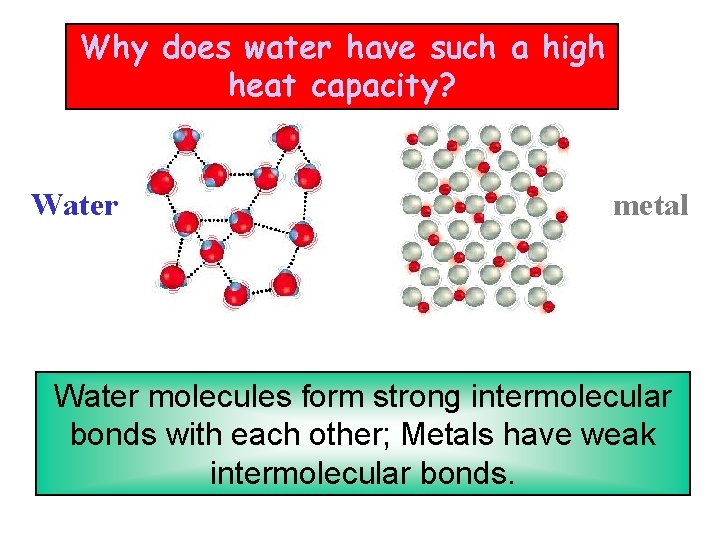
Why does water have such a high heat capacity? Water metal Water molecules form strong intermolecular bonds with each other; Metals have weak intermolecular bonds.

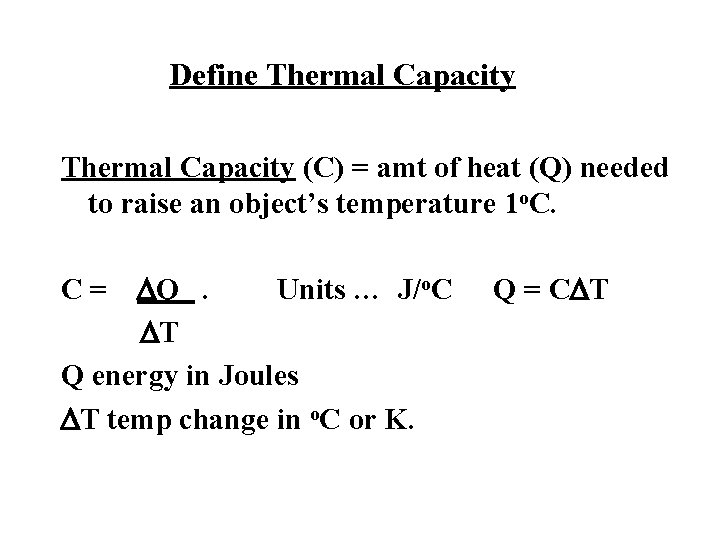
Define Thermal Capacity (C) = amt of heat (Q) needed to raise an object’s temperature 1 o. C. DQ. Units … J/o. C DT Q energy in Joules DT temp change in o. C or K. C= Q = CDT

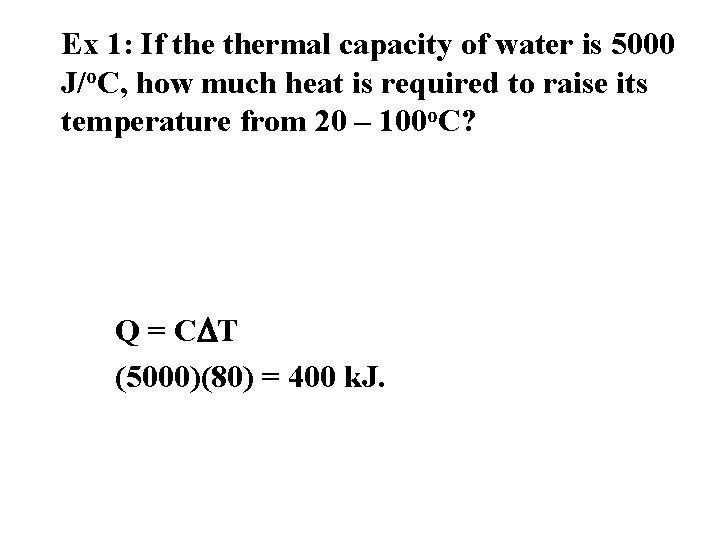
Ex 1: If thermal capacity of water is 5000 J/o. C, how much heat is required to raise its temperature from 20 – 100 o. C? Q = CDT (5000)(80) = 400 k. J.

Ex 2: How much heat is lost from a block of metal of C = 800 J/o. C when it cools from 60 – 20 o. C? 32 k. J

Heat Capacity vs. Specific Heat • Heat Capacity C, relates to objects w/o correcting for mass. Small C Large C

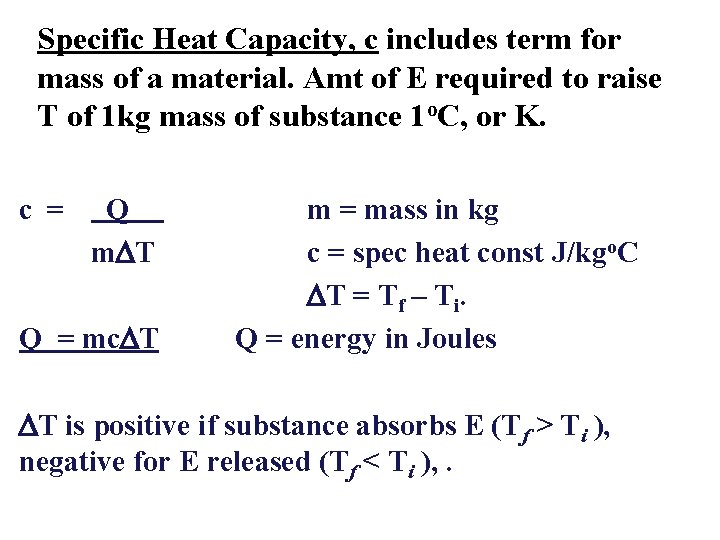
Specific Heat Capacity, c includes term for mass of a material. Amt of E required to raise T of 1 kg mass of substance 1 o. C, or K. c = Q m. DT Q = mc. DT m = mass in kg c = spec heat const J/kgo. C DT = Tf – Ti. Q = energy in Joules DT is positive if substance absorbs E (Tf > Ti ), negative for E released (Tf < Ti ), .

Both Iron same c. Same c

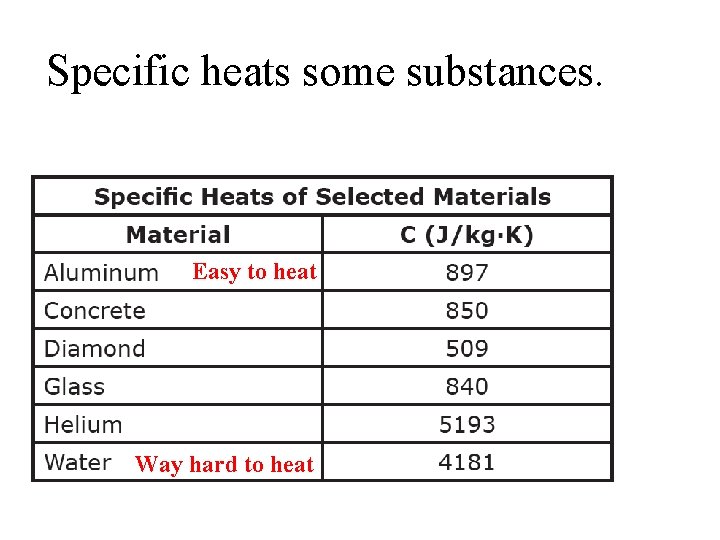
Specific heats some substances. Easy to heat Way hard to heat

Ex 3. A 45 -g piece of aluminum is heated from 25 – 55 o. C. How much E is required? • Q = mc DT • Q = (0. 045 kg)(897 J/kgo. C)(55 -25) o. C = • 1200 J.

4. The SH of water is ~4200 J/kg o. C. How much heat will be required to raise the temperature of 300 g from 20 – 60 o. C? • 50. 4 k. J

5. A metal block has a mass of 1. 5 kg loses 20 k. J of heat. Its temperature drops from 60 – 45 o. What is the specific heat capacity of the metal? • 888. 9 ~ 900 J/kgo. C.

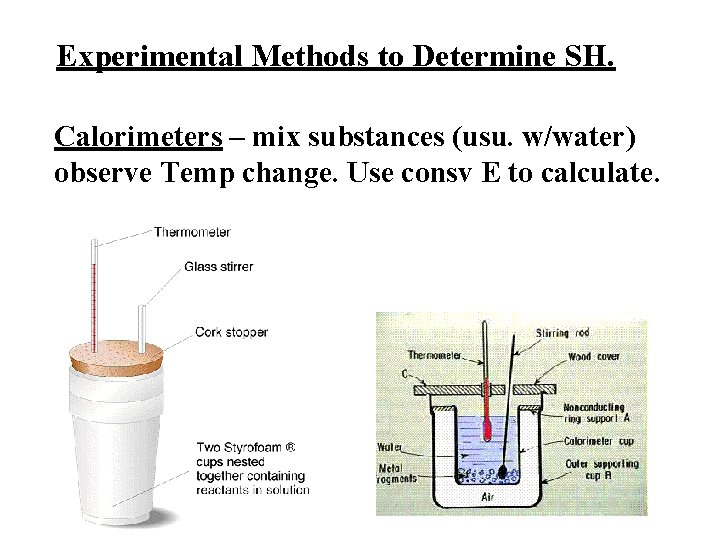
Experimental Methods to Determine SH. Calorimeters – mix substances (usu. w/water) observe Temp change. Use consv E to calculate.

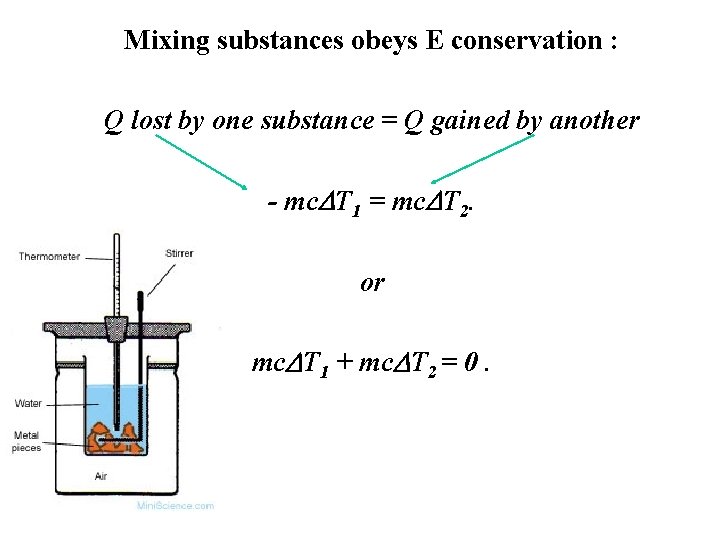
Mixing substances obeys E conservation : Q lost by one substance = Q gained by another - mc. DT 1 = mc. DT 2. or mc. DT 1 + mc. DT 2 = 0.

Throw hot metal into cold water then Q lost by metal = Q gained by water (+calorimeter). For solids with unknown c, liq with known c.

6. A 0. 05 kg metal bolt is heated to an unknown temperature. It is then dropped into a calorimeter containing 0. 15 kg of water of 21. 0 o. C. The bolt and the water reach a final temperature of 25. 0 o. C. If the SH of the metal is 899 J/kgo. C, find the initial temperature of the metal. Ignore the heat lost to the cup. • 81 o. C

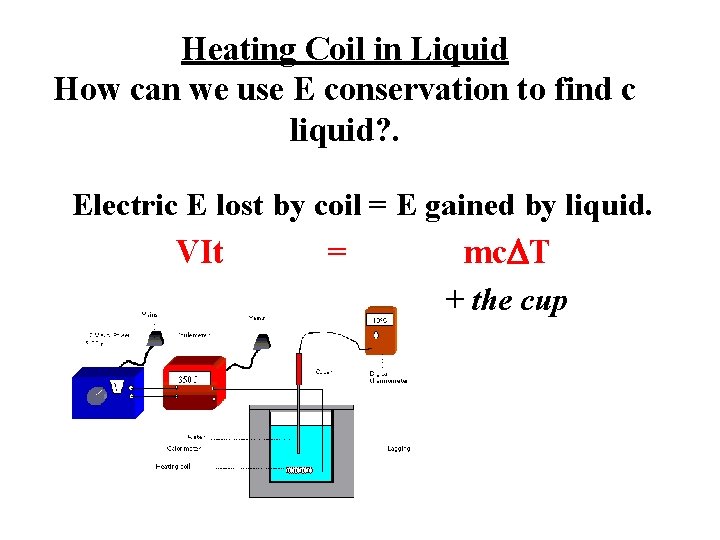
Heating Coil in Liquid How can we use E conservation to find c liquid? . Electric E lost by coil = E gained by liquid. VIt = mc. DT + the cup

7. A heating coil with a voltage potential of 6 V and current of 3 A is immersed in. 7 kg of water. How long will it take to heat the water from 20 o. C to 35 o. C? Assume the cup absorbs no heat. • 2442 sec • 41 min

Read Hamper 50– 57 Do pg 56 - 57 All Write out all work and equations.

Phases/States of Matter

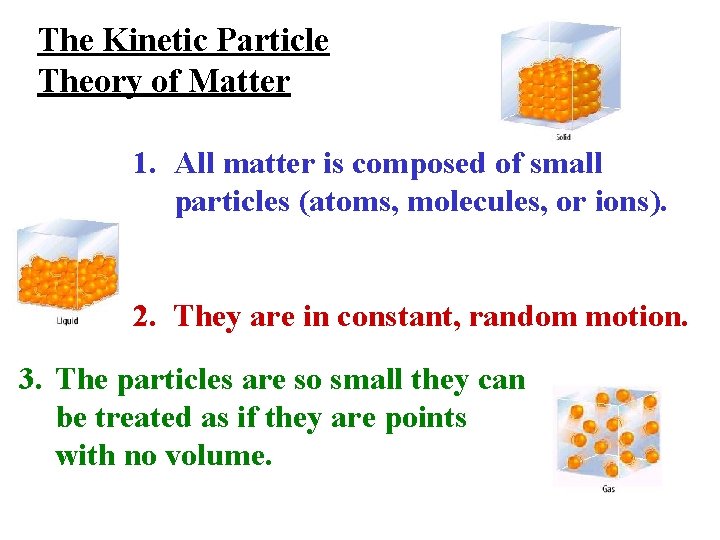
The Kinetic Particle Theory of Matter 1. All matter is composed of small particles (atoms, molecules, or ions). 2. They are in constant, random motion. 3. The particles are so small they can be treated as if they are points with no volume.

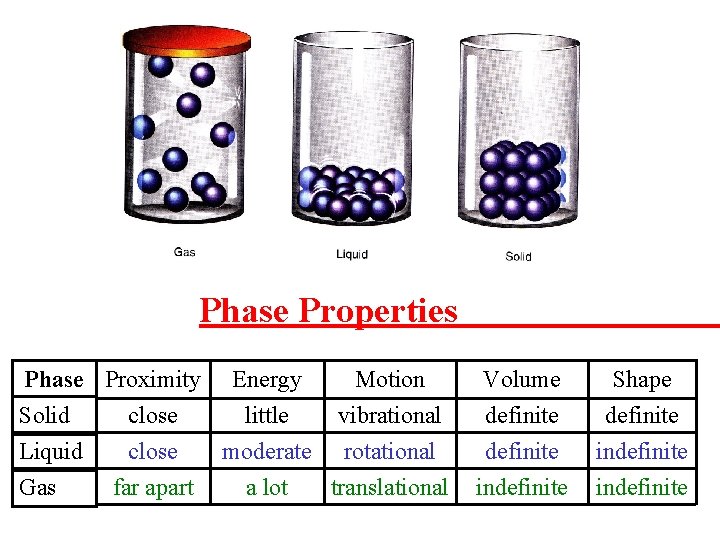
Phase Properties Phase Proximity Energy Motion Solid close little vibrational Liquid close moderate rotational Gas far apart a lot translational Volume definite indefinite Shape definite indefinite

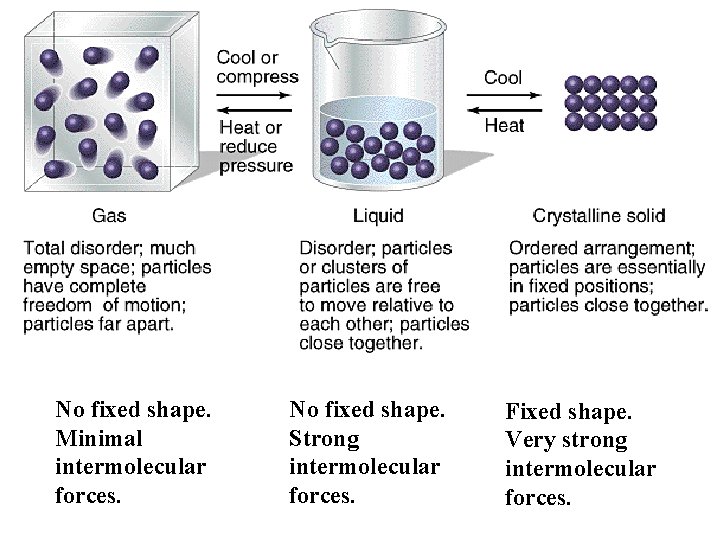
No fixed shape. Minimal intermolecular forces. No fixed shape. Strong intermolecular forces. Fixed shape. Very strong intermolecular forces.

Phases/States of Matter Molecular Distance & Motion

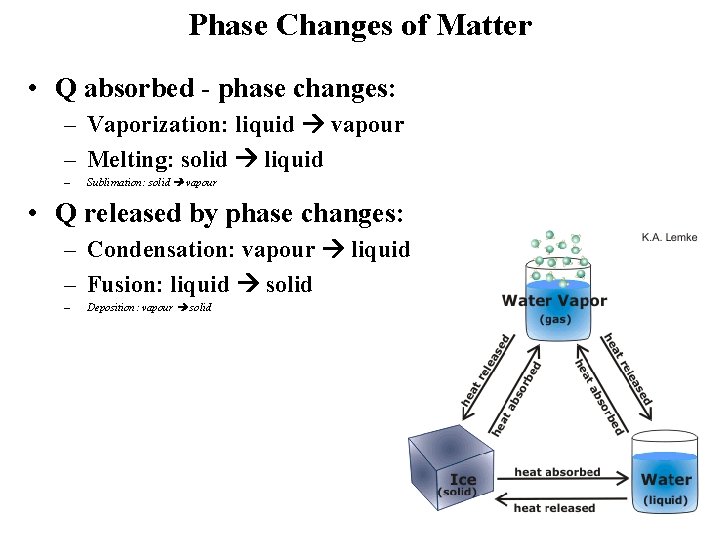
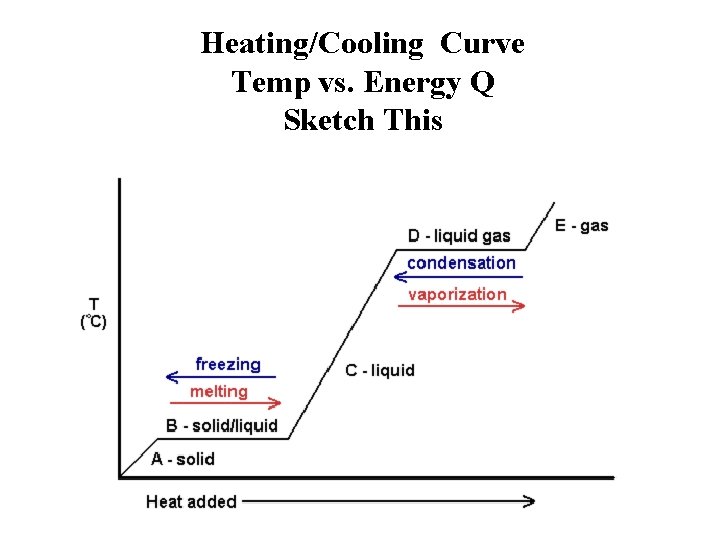
Phase Changes of Matter • Q absorbed - phase changes: – Vaporization: liquid vapour – Melting: solid liquid – Sublimation: solid vapour • Q released by phase changes: – Condensation: vapour liquid – Fusion: liquid solid – Deposition: vapour solid

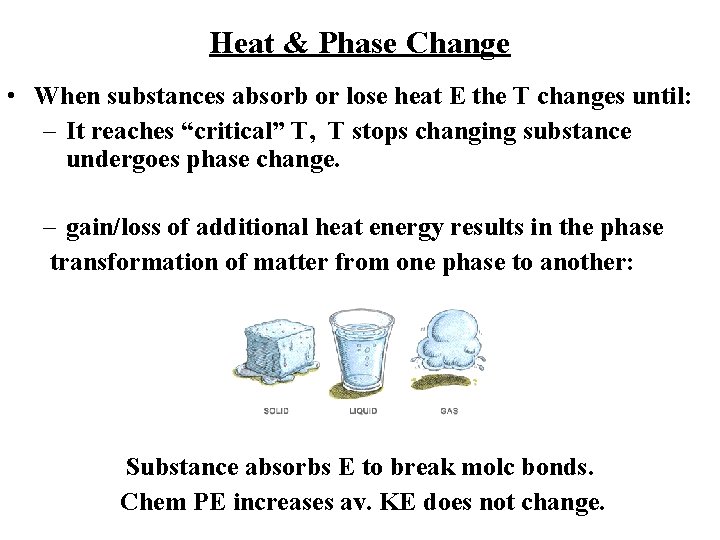
Heat & Phase Change • When substances absorb or lose heat E the T changes until: – It reaches “critical” T, T stops changing substance undergoes phase change. – gain/loss of additional heat energy results in the phase transformation of matter from one phase to another: Solid ® liquid ® gas Substance absorbs E to break molc bonds. Chem PE increases av. KE does not change.

• Consider melting: as heat is absorbed T goes up particles vibrate more. The KE increases. At the melting point, particles vibrate enough to slip from their fixed positions breaking intermolecular bonds. More and more particles break solid bonds until all are slipping past each other in the liquid phase. • The PE of the system increases but the av KE stays constant at the melting (ice) & boiling points (steam).

Phase equilibrium Multiple phases exist at 1 T. Molecules leave and enter phases at equal rates. Ice / liquid water: 0 °C Liquid water / steam: 100 °C Ice water

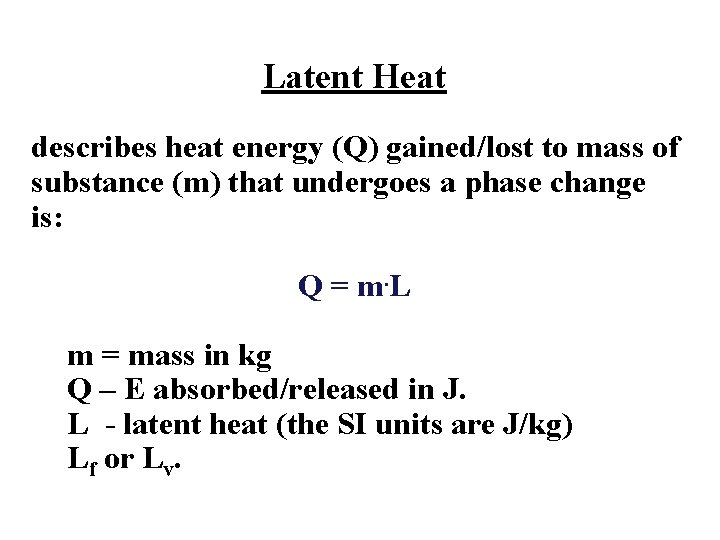
Latent heat is a physical property that describes how much E is required to transform the mass of a substance from one phase to another. Latent heat of fusion Lf – heat absorbed during melting or released during freezing. Latent heat of vaporization Lv – heat absorbed during vaporization, released during condensation.

Latent Heat describes heat energy (Q) gained/lost to mass of substance (m) that undergoes a phase change is: Q = m. L m = mass in kg Q – E absorbed/released in J. L - latent heat (the SI units are J/kg) Lf or Lv.

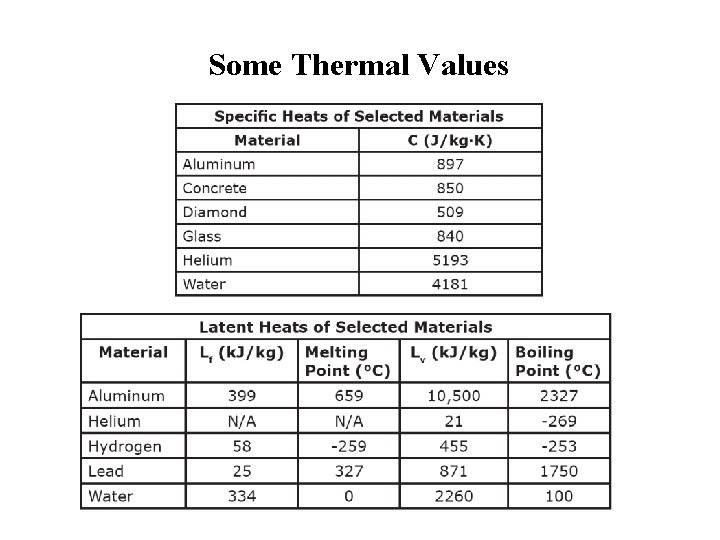
Some Thermal Values
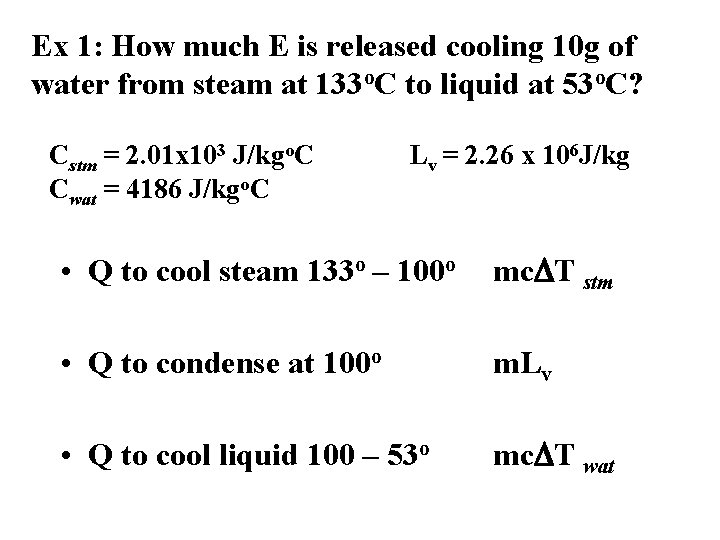
Ex 1: How much E is released cooling 10 g of water from steam at 133 o. C to liquid at 53 o. C? Cstm = 2. 01 x 103 J/kgo. C Cwat = 4186 J/kgo. C Lv = 2. 26 x 106 J/kg • Q to cool steam 133 o – 100 o mc. DT stm • Q to condense at 100 o m. Lv • Q to cool liquid 100 – 53 o mc. DT wat
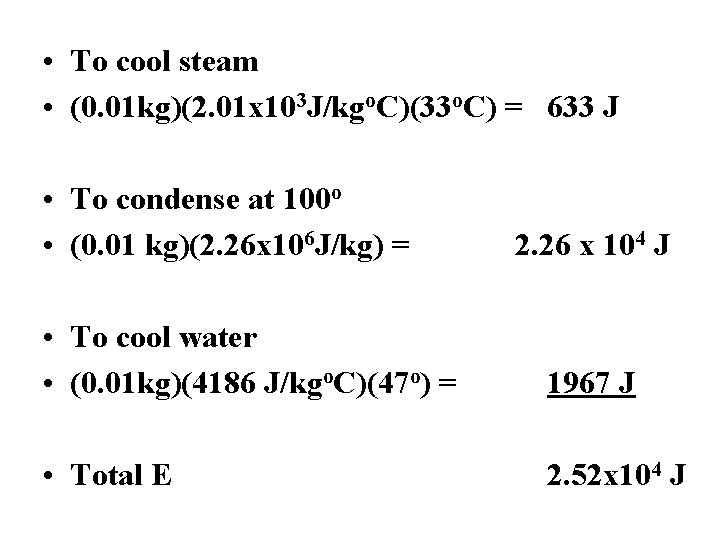
• To cool steam • (0. 01 kg)(2. 01 x 103 J/kgo. C)(33 o. C) = 633 J • To condense at 100 o • (0. 01 kg)(2. 26 x 106 J/kg) = 2. 26 x 104 J • To cool water • (0. 01 kg)(4186 J/kgo. C)(47 o) = 1967 J • Total E 2. 52 x 104 J

Ex 2. How much energy is released when 1. 5 kg of water vapor at 100 o. C is placed in a freezer and converted to ice at -7 o. C. • Specific Heat – c ice = 2. 1 x 103 J/K kg. – c. Wat = 4. 2 x 103 J/K kg. –. • Lf = 3. 34 x 105 J/kg • Lv = 22. 5 x 105 J/kg

4. 5 x 6 10 J

• IB Set Specific, Latent Heat 2014


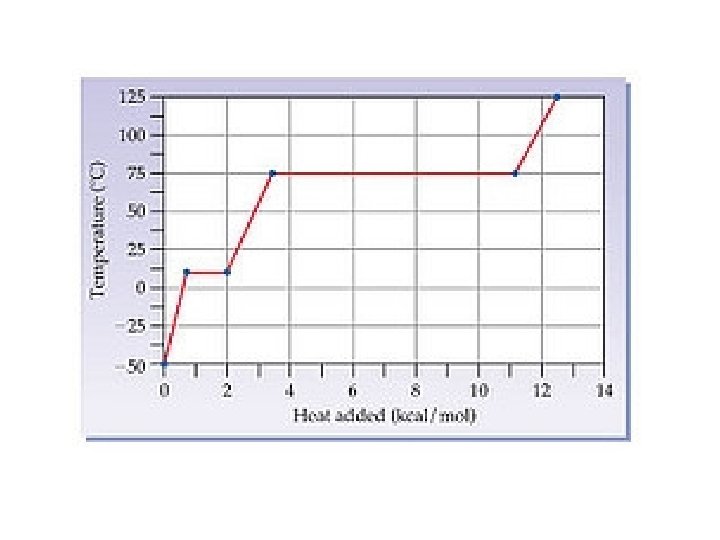
Heating Curves show temperature change with absorbed energy for a substance.

Heating/Cooling Curve Temp vs. Energy Q Sketch This

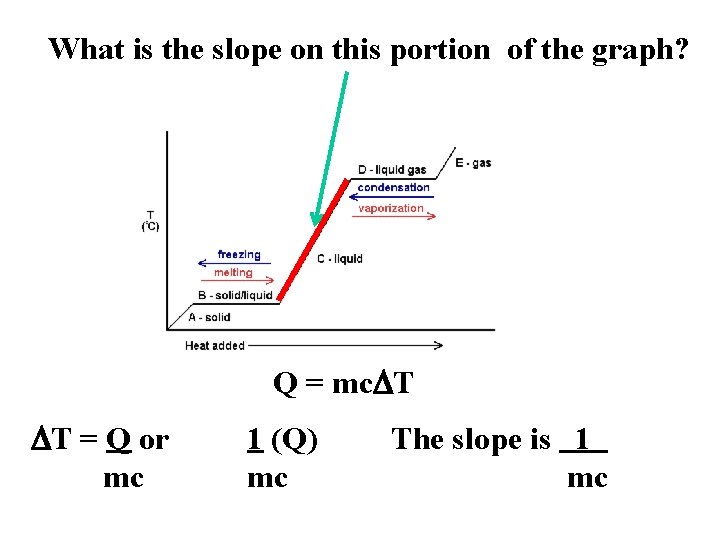
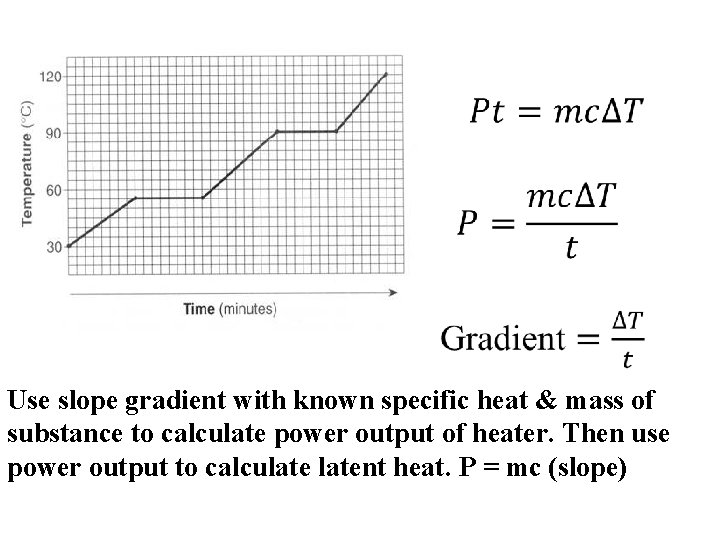
What is the slope on this portion of the graph? Q = mc. DT DT = Q or mc 1 (Q) mc The slope is 1 mc

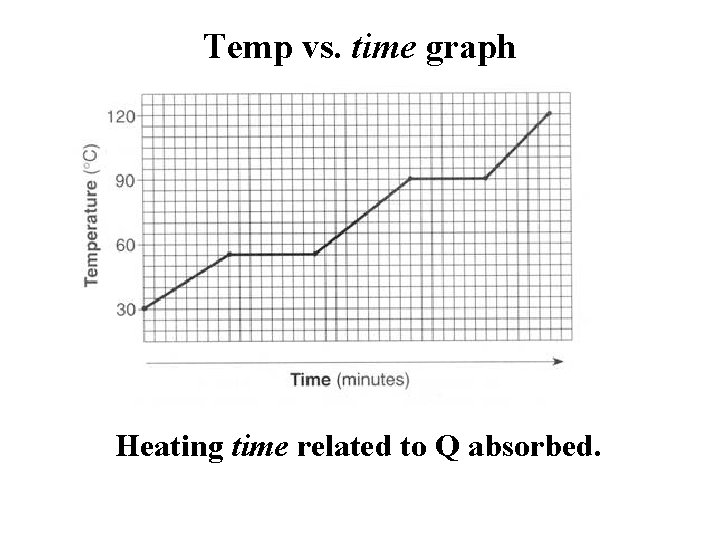
Temp vs. time graph Heating time related to Q absorbed.

Use slope gradient with known specific heat & mass of substance to calculate power output of heater. Then use power output to calculatent heat. P = mc (slope)

Example Cooling Curve • Hypothetical substance. • Phase Changes for ice IB Prob. • Kerr pg 91 #3 - 6, 8, 11.

Determining Lf for Ice • Mixing Method: • Add ice known mass & T water. • Let it melt. Thermal E lost by water = thermal E gained by ice. -mc(DT)w = mc. DTice + m. Lf ice + mc. DT liq ice.



Go to evaporation.

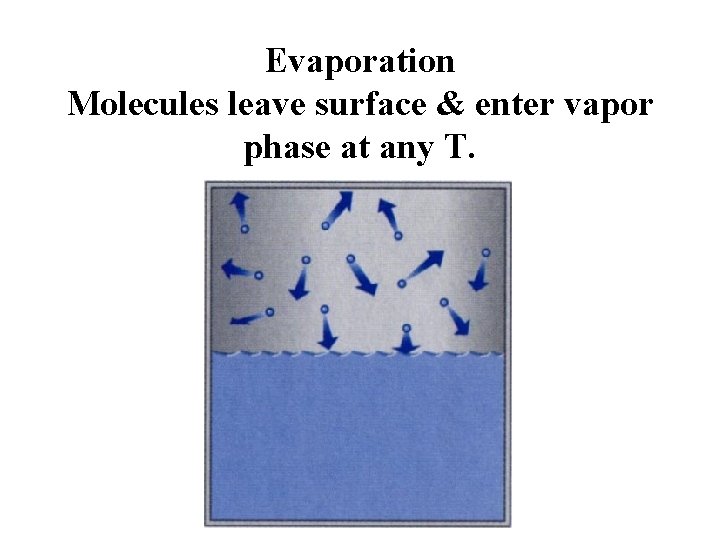
Evaporation Molecules leave surface & enter vapor phase at any T.

Evaporation The molecules in a liquid have a distribution of speeds. The average speed determines the T of the liquid. The fastest molecules have enough energy to overcome the attraction between the molecules in the liquid. They evaporate and become vapor.

Evaporative cooling: as the fastest molecules evaporate, they leave behind the coolest molecules and the average temperature of the liquid decreases. Example: sweat

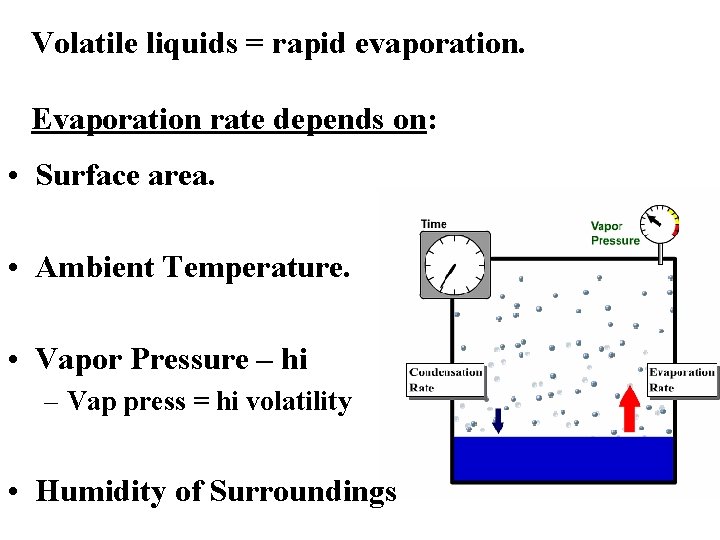
Volatile liquids = rapid evaporation. Evaporation rate depends on: • Surface area. • Ambient Temperature. • Vapor Pressure – hi – Vap press = hi volatility • Humidity of Surroundings

Boiling • Boiling occurs when the average motion of particles is fast enough to overcome the forces holding them close together. • This happens evenly throughout a boiling liquid. You will see bubbles form. • The temperature is uniform throughout.

Boiling vs. Evaporation • In the cases of both boiling and evaporation, intermolecular forces between particles are present. • The greater the space between the particles, the weaker the intermolecular force. • To break the bond between two particles, one particle has to be moving fast enough to overcome the pull of the other, until it gets so far away that pull is diminished.


Film Elem Chem Gas Solid Liq.