Temperature Heat and Expansion Specific Heat Capacity Specific





- Slides: 10

Temperature, Heat, and Expansion

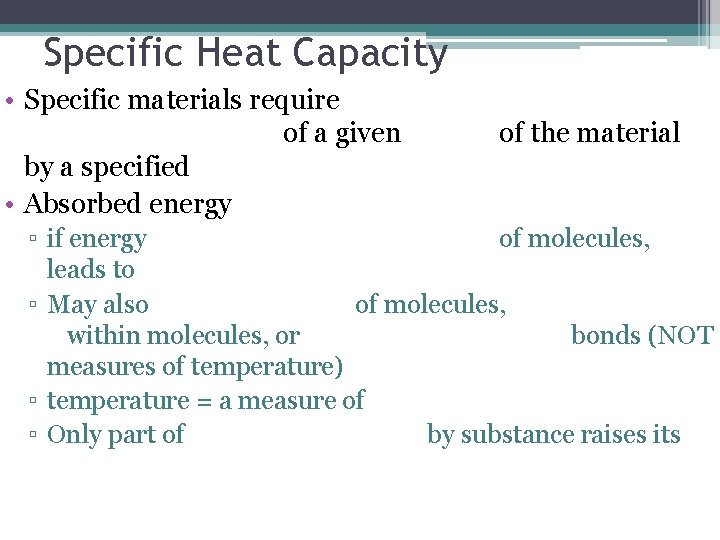
Specific Heat Capacity • Specific materials require of a given by a specified • Absorbed energy of the material ▫ if energy of molecules, leads to ▫ May also of molecules, within molecules, or bonds (NOT measures of temperature) ▫ temperature = a measure of ▫ Only part of by substance raises its

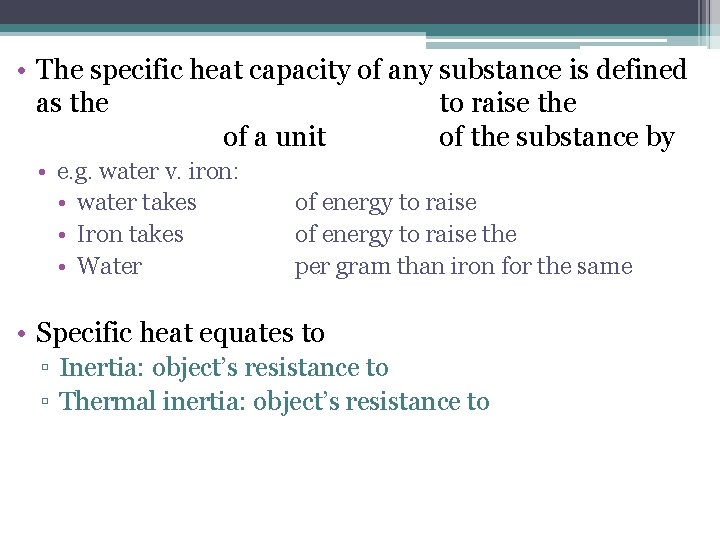
• The specific heat capacity of any substance is defined as the to raise the of a unit of the substance by • e. g. water v. iron: • water takes • Iron takes • Water of energy to raise the per gram than iron for the same • Specific heat equates to ▫ Inertia: object’s resistance to ▫ Thermal inertia: object’s resistance to

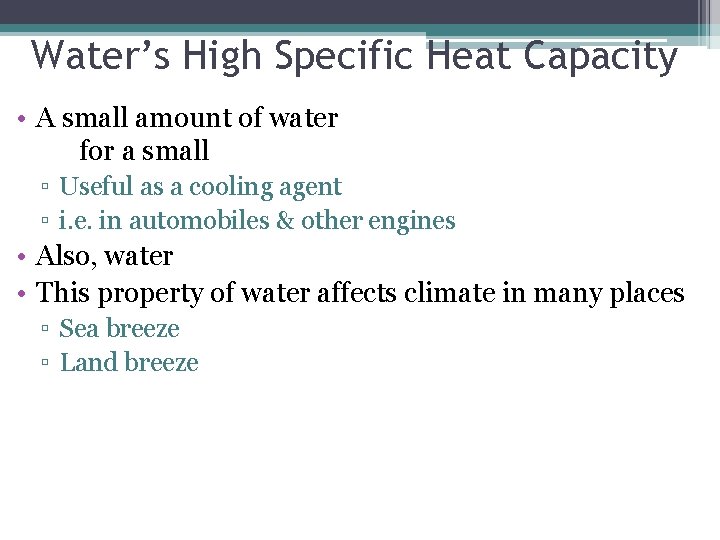
Water’s High Specific Heat Capacity • A small amount of water for a small ▫ Useful as a cooling agent ▫ i. e. in automobiles & other engines • Also, water • This property of water affects climate in many places ▫ Sea breeze ▫ Land breeze

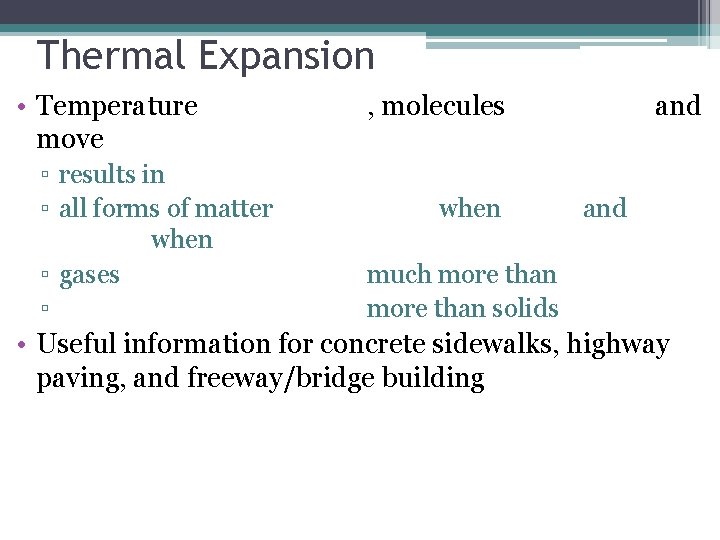
Thermal Expansion • Temperature move ▫ results in ▫ all forms of matter when ▫ gases ▫ , molecules when and much more than solids • Useful information for concrete sidewalks, highway paving, and freeway/bridge building

• Different materials ▫ Bimetallic strip: or riveted together ▫ Strip is , ▫ Useful in are welded is seen �Bimetallic coil �Room cold – coil bends electric switch and �Room hot – coil bends electric switch and • Amount of and closes and opens depends on

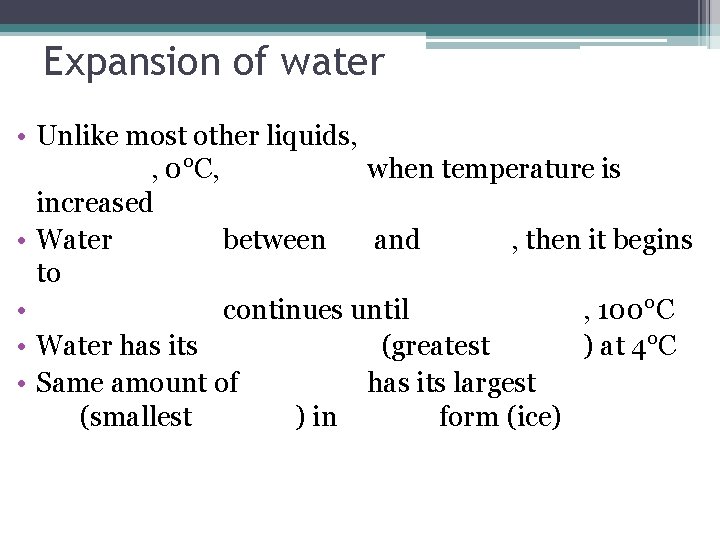
Expansion of water • Unlike most other liquids, , 0°C, when temperature is increased • Water between and , then it begins to • continues until , 100°C • Water has its (greatest ) at 4°C • Same amount of has its largest (smallest ) in form (ice)

WHY? !? • Unlike other solids, ice has of of water molecules ▫ molecules than in the liquid state ▫ ice is b/c of space water • This explains the contraction of water between 0°C and 4°C ▫ ▫ Then,

NATURE • Densest water settles at ▫ 4°C above freezing temperature • Water at freezing point • As water is cooled at surface, Only then can surface water cool to • Because of water’s and , the bottom of deep bodies of water is a

• Read p 318 • Do T/E p 323 #1, 2, 7, 15 ▫ Make sure you not only answer each question…but also EXPLAIN your answer