Thermochemistry Specific Heat Specific Heat n Amount of


- Slides: 8

Thermochemistry Specific Heat

Specific Heat n Amount of heat it takes to raise 1 g of How easy or hard an object a substance by 1°C is to heat up – how fast does it absorb heat energy q q Metals have a low specific heat Water has a high specific heat

Learning Check 1. On a sunny day, the concrete deck around an outdoor swimming pool becomes hot, while the water stays cool. This is because… A. The deck has a higher specific heat than the water B. The deck has a lower specific heat then the water C. Both objects have the same specific heat

Learning Check 2. Two objects are sitting next to each other in the sunlight. Object A gets hotter than object B. A. Object A has a lower specific heat than object B B. Object A has a higher specific heat than object B C. Both objects have the same specific heat

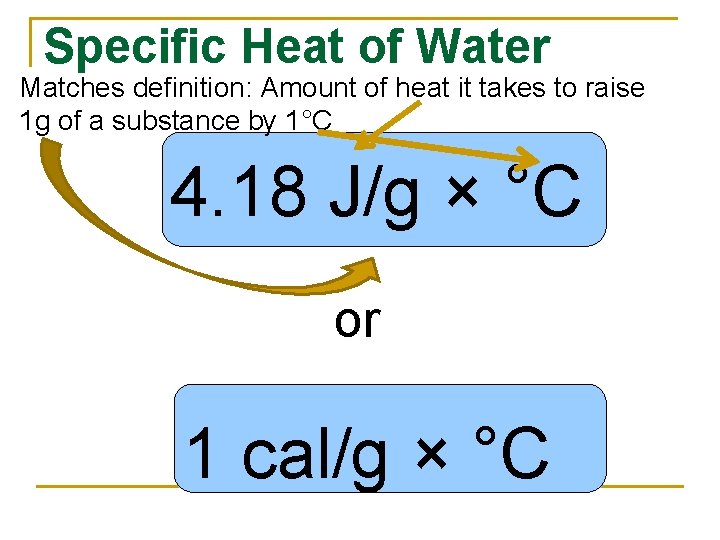
Specific Heat of Water Matches definition: Amount of heat it takes to raise 1 g of a substance by 1°C 4. 18 J/g × °C or 1 cal/g × °C

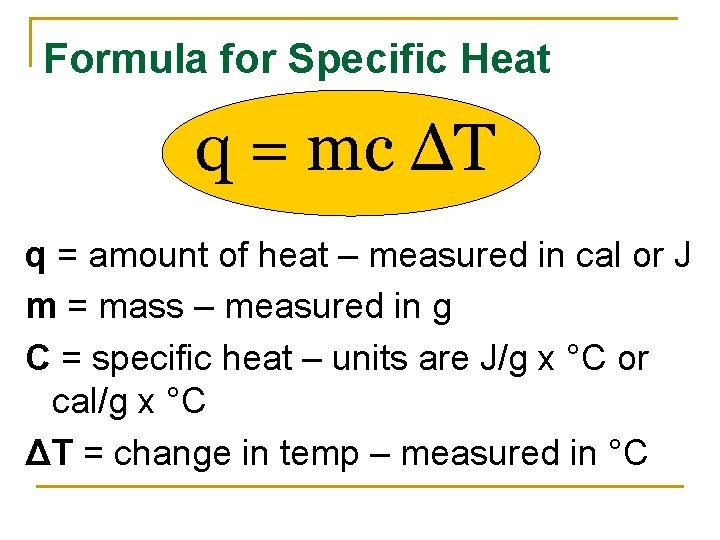
Formula for Specific Heat q = mc ΔT q = amount of heat – measured in cal or J m = mass – measured in g C = specific heat – units are J/g x °C or cal/g x °C ΔT = change in temp – measured in °C

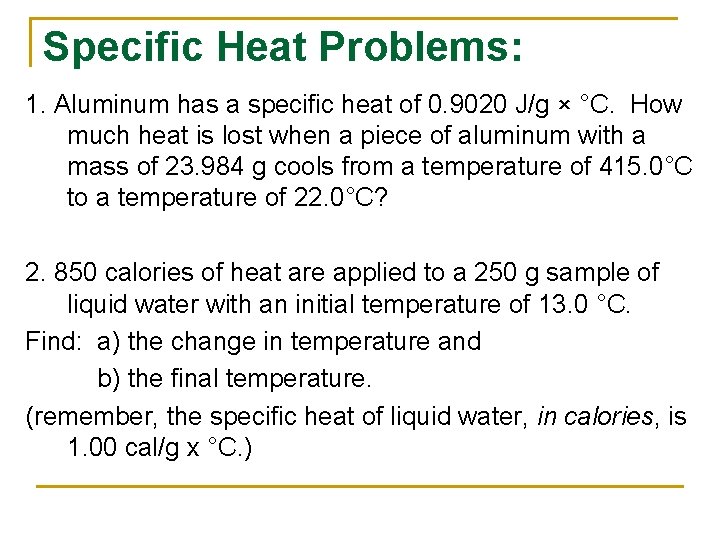
Specific Heat Problems: 1. Aluminum has a specific heat of 0. 9020 J/g × °C. How much heat is lost when a piece of aluminum with a mass of 23. 984 g cools from a temperature of 415. 0°C to a temperature of 22. 0°C? 2. 850 calories of heat are applied to a 250 g sample of liquid water with an initial temperature of 13. 0 °C. Find: a) the change in temperature and b) the final temperature. (remember, the specific heat of liquid water, in calories, is 1. 00 cal/g x °C. )

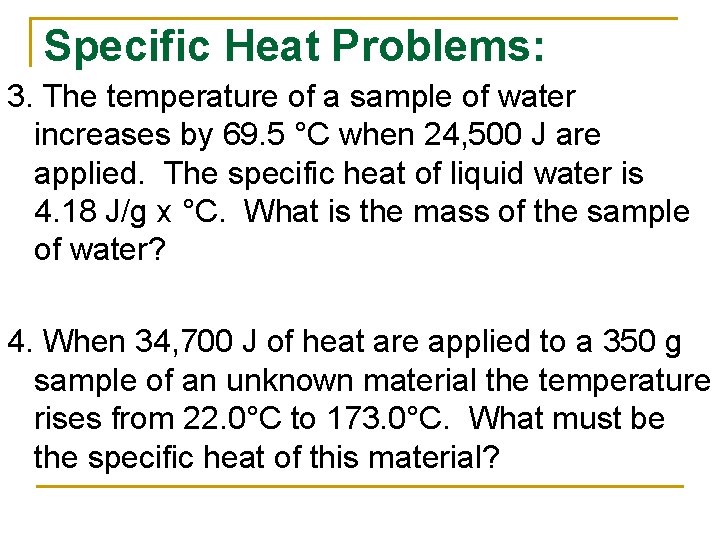
Specific Heat Problems: 3. The temperature of a sample of water increases by 69. 5 °C when 24, 500 J are applied. The specific heat of liquid water is 4. 18 J/g x °C. What is the mass of the sample of water? 4. When 34, 700 J of heat are applied to a 350 g sample of an unknown material the temperature rises from 22. 0°C to 173. 0°C. What must be the specific heat of this material?