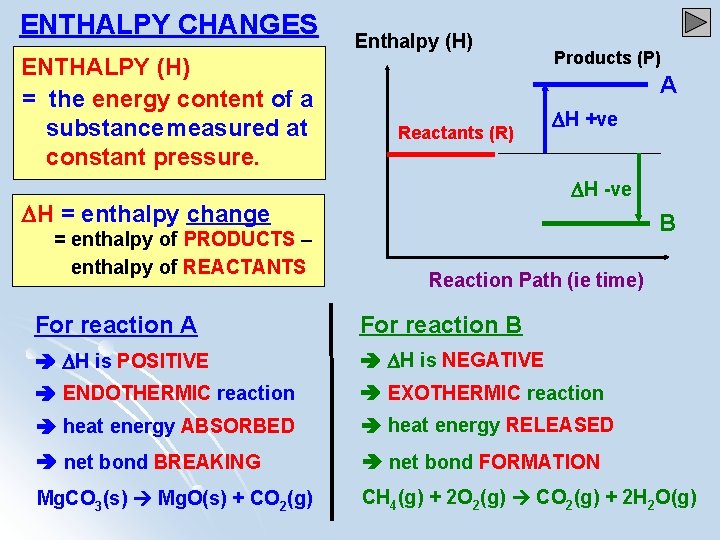
Enthalpy ENTHALPY CHANGES ENTHALPY H the energy content


![BOND ENTHALPY or BOND ENERGY E[X-Y] Q The average energy needed for ONE MOLE BOND ENTHALPY or BOND ENERGY E[X-Y] Q The average energy needed for ONE MOLE](https://slidetodoc.com/presentation_image_h2/5bda57d75e284e93cbc47c41c015e422/image-16.jpg)

![4 Calculate H f [CH 4(g)], given H f [CO 2(g)] = -393. 5 4 Calculate H f [CH 4(g)], given H f [CO 2(g)] = -393. 5](https://slidetodoc.com/presentation_image_h2/5bda57d75e284e93cbc47c41c015e422/image-23.jpg)
- Slides: 26

Enthalpy

ENTHALPY CHANGES ENTHALPY (H) = the energy content of a substancemeasured at constant pressure. Enthalpy (H) A Reactants (R) H +ve H -ve H = enthalpy change = enthalpy of PRODUCTS – enthalpy of REACTANTS Products (P) B Reaction Path (ie time) For reaction A For reaction B H is POSITIVE H is NEGATIVE ENDOTHERMIC reaction EXOTHERMIC reaction heat energy ABSORBED heat energy RELEASED net bond BREAKING net bond FORMATION Mg. CO 3(s) Mg. O(s) + CO 2(g) CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)

BOND ENERGIES • definition? • uses ? • limitations ?

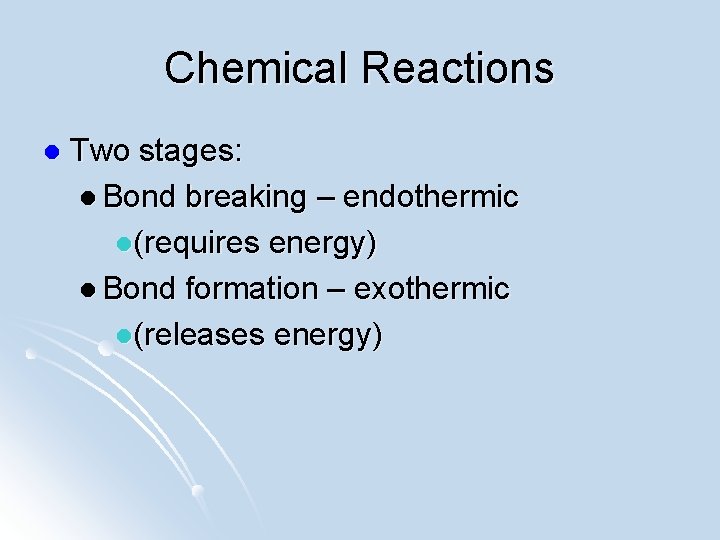
Chemical Reactions l Two stages: l Bond breaking – endothermic l(requires energy) l Bond formation – exothermic l(releases energy)

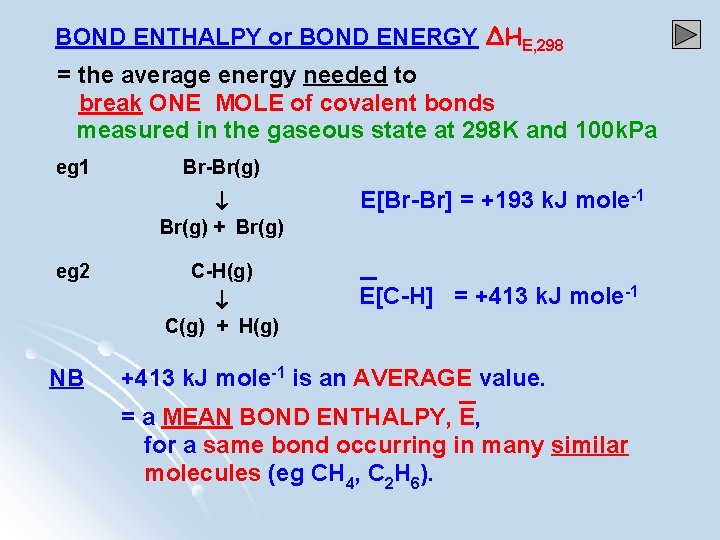
BOND ENTHALPY or BOND ENERGY ΔHE, 298 = the average energy needed to break ONE MOLE of covalent bonds measured in the gaseous state at 298 K and 100 k. Pa eg 1 Br-Br(g) + Br(g) eg 2 C-H(g) C(g) + H(g) NB E[Br-Br] = +193 k. J mole-1 E[C-H] = +413 k. J mole-1 is an AVERAGE value. = a MEAN BOND ENTHALPY, E, for a same bond occurring in many similar molecules (eg CH 4, C 2 H 6).

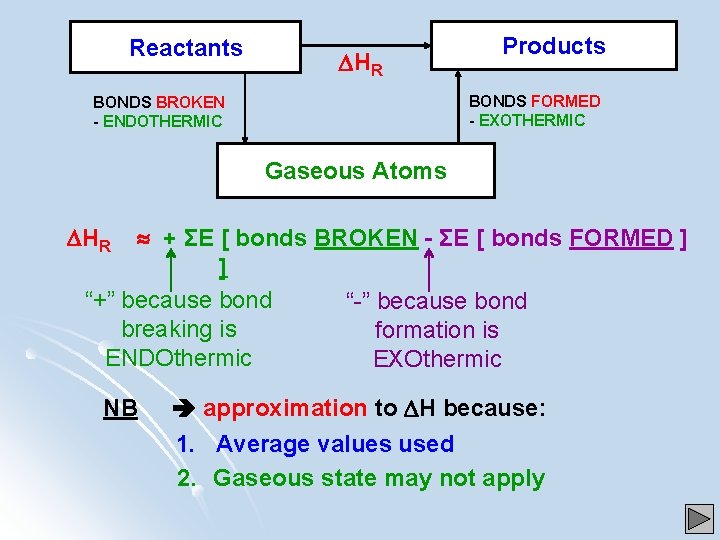
Reactants HR Products BONDS FORMED - EXOTHERMIC BONDS BROKEN - ENDOTHERMIC Gaseous Atoms HR + ΣE [ bonds BROKEN - ΣE [ bonds FORMED ] ] “+” because bond “-” because bond breaking is formation is ENDOthermic EXOthermic NB approximation to H because: 1. Average values used 2. Gaseous state may not apply

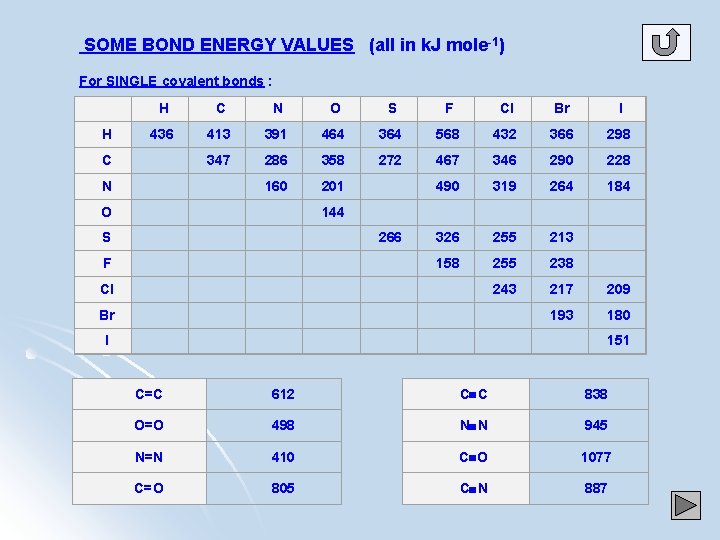
SOME BOND ENERGY VALUES (all in k. J mole-1) For SINGLE covalent bonds : H H C N O S F Cl Br I 436 413 391 464 364 568 432 366 298 347 286 358 272 467 346 290 228 160 201 490 319 264 184 326 255 213 158 255 238 243 217 209 193 180 C N 144 O S 266 F Cl Br I 151 C=C 612 C C 838 O=O 498 N N 945 N=N 410 C O 1077 C=O 805 C N 887

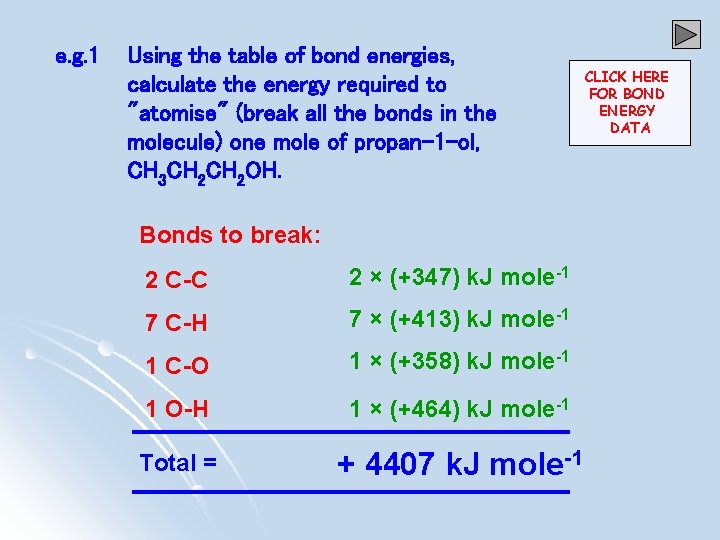
e. g. 1 Using the table of bond energies, calculate the energy required to "atomise" (break all the bonds in the molecule) one mole of propan-1 -ol, CH 3 CH 2 OH. Bonds to break: 2 C-C 2 × (+347) k. J mole-1 7 C-H 7 × (+413) k. J mole-1 1 C-O 1 × (+358) k. J mole-1 1 O-H 1 × (+464) k. J mole-1 Total = + 4407 k. J mole-1 CLICK HERE FOR BOND ENERGY DATA

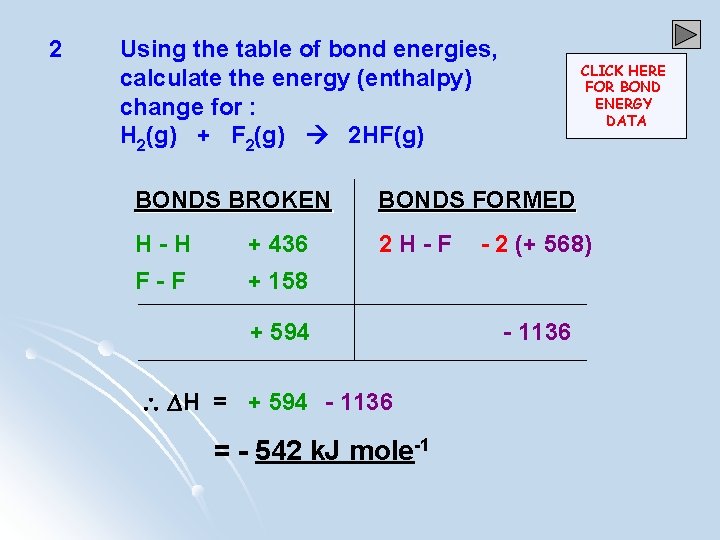
2 Using the table of bond energies, calculate the energy (enthalpy) change for : H 2(g) + F 2(g) 2 HF(g) CLICK HERE FOR BOND ENERGY DATA BONDS BROKEN BONDS FORMED H-H + 436 2 H-F F-F + 158 + 594 H = + 594 - 1136 = - 542 k. J mole-1 - 2 (+ 568) - 1136

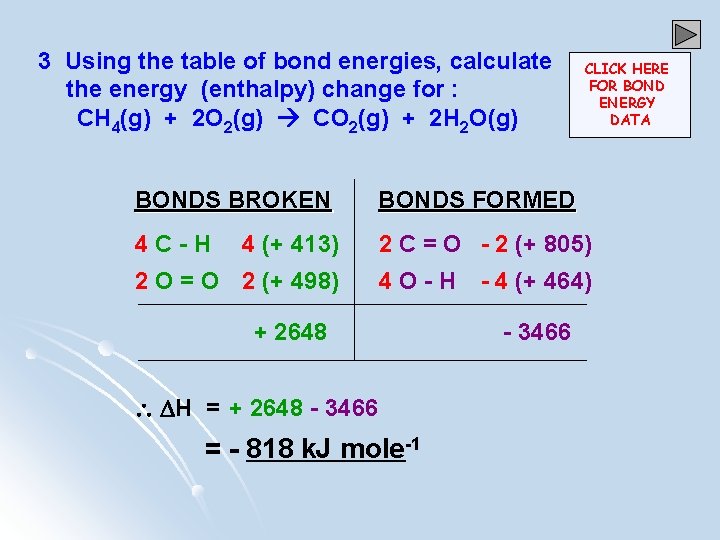
3 Using the table of bond energies, calculate the energy (enthalpy) change for : CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) CLICK HERE FOR BOND ENERGY DATA BONDS BROKEN BONDS FORMED 4 C-H 2 C = O - 2 (+ 805) 4 (+ 413) 2 O = O 2 (+ 498) 4 O-H + 2648 H = + 2648 - 3466 = - 818 k. J mole-1 - 4 (+ 464) - 3466

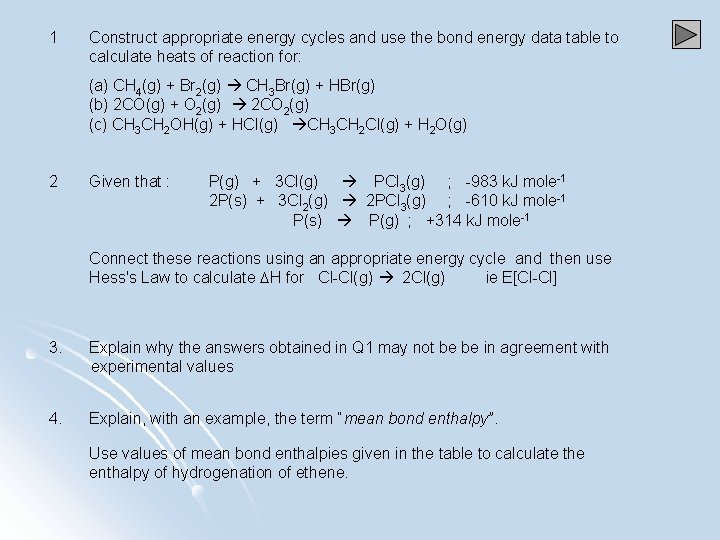
1 Construct appropriate energy cycles and use the bond energy data table to calculate heats of reaction for: (a) CH 4(g) + Br 2(g) CH 3 Br(g) + HBr(g) (b) 2 CO(g) + O 2(g) 2 CO 2(g) (c) CH 3 CH 2 OH(g) + HCl(g) CH 3 CH 2 Cl(g) + H 2 O(g) 2 Given that : P(g) + 3 Cl(g) PCl 3(g) ; -983 k. J mole-1 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ; -610 k. J mole-1 P(s) P(g) ; +314 k. J mole-1 Connect these reactions using an appropriate energy cycle and then use Hess's Law to calculate H for Cl-Cl(g) 2 Cl(g) ie E[Cl-Cl] 3. Explain why the answers obtained in Q 1 may not be be in agreement with experimental values 4. Explain, with an example, the term “mean bond enthalpy”. Use values of mean bond enthalpies given in the table to calculate the enthalpy of hydrogenation of ethene.

STANDARD ENTHALPY CHANGES, ΔH • what are they ? • standardisation? • definitions?

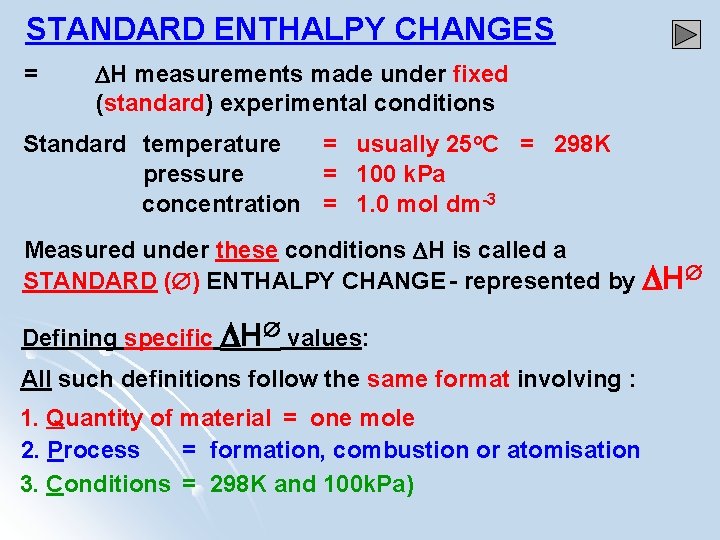
STANDARD ENTHALPY CHANGES = H measurements made under fixed (standard) experimental conditions Standard temperature = usually 25 o. C = 298 K pressure = 100 k. Pa concentration = 1. 0 mol dm-3 Measured under these conditions H is called a STANDARD ( ) ENTHALPY CHANGE - represented by H Defining specific H values: All such definitions follow the same format involving : 1. Quantity of material = one mole 2. Process = formation, combustion or atomisation 3. Conditions = 298 K and 100 k. Pa)

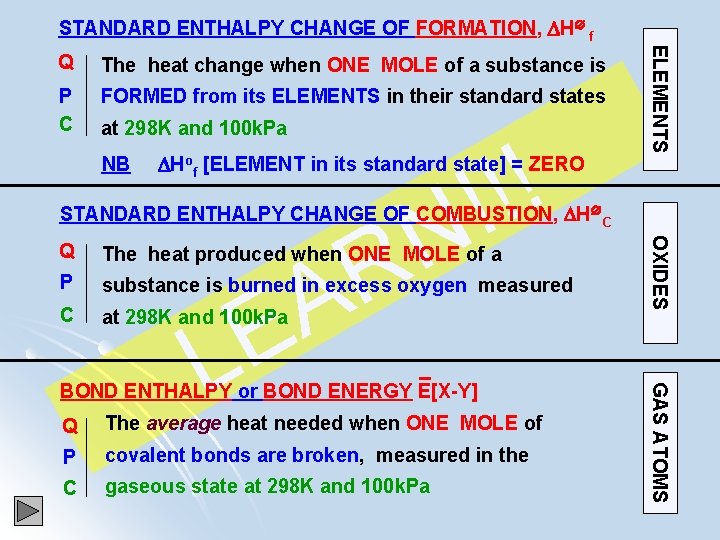
STANDARD ENTHALPY CHANGE OF FORMATION, H f Q The energy change when ONE MOLE of a substance P is FORMED FROM ELEMENTS in their standard states C at fixed temperature (298 K) and 100 k. Pa NB Hof [ELEMENT in its standard state] = ZERO eg Cl 2(g) ; H f[Cl 2(g)] = 0 k. Jmol-1 1. 2 Na(s) + 0. 5 O 2(g) Na 2 O(s) 2. 2 K(s) + Cl 2(g) 2 KCl(s) H f[Na 2 O(s)] 2 H f[KCl(s)] Write the equation corresponding to H f of : a) CH 4(g) : C(s) + 2 H 2(g) CH 4(g) b) NH 4 Cl(s) : ½ N 2(g) + 2 H 2(g) + ½ Cl 2(g) NH 4 Cl(s) c) 2 H 2 O(g) : 2 H 2(g) + O 2(g) 2 H 2 O(g)

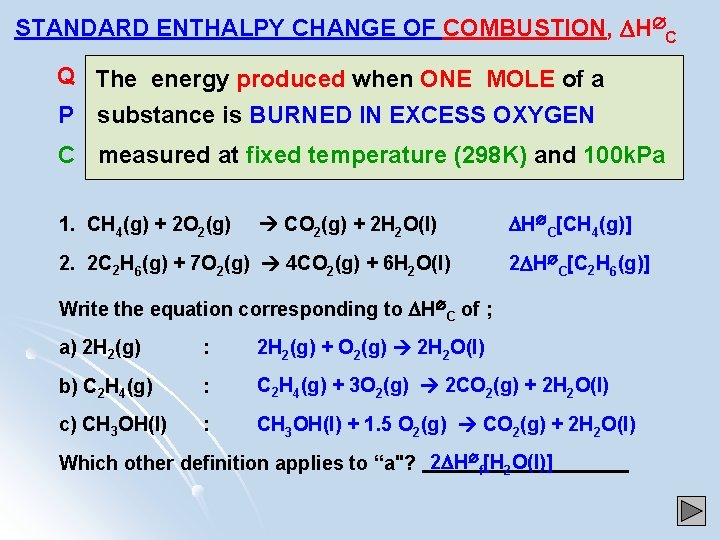
STANDARD ENTHALPY CHANGE OF COMBUSTION, H C Q The energy produced when ONE MOLE of a P substance is BURNED IN EXCESS OXYGEN C measured at fixed temperature (298 K) and 100 k. Pa 1. CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) 2. 2 C 2 H 6(g) + 7 O 2(g) 4 CO 2(g) + 6 H 2 O(l) H C[CH 4(g)] 2 H C[C 2 H 6(g)] Write the equation corresponding to H C of ; a) 2 H 2(g) : 2 H 2(g) + O 2(g) 2 H 2 O(l) b) C 2 H 4(g) : C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) c) CH 3 OH(l) : CH 3 OH(l) + 1. 5 O 2(g) CO 2(g) + 2 H 2 O(l) Which other definition applies to “a"? 2 H f[H 2 O(l)]
![BOND ENTHALPY or BOND ENERGY EXY Q The average energy needed for ONE MOLE BOND ENTHALPY or BOND ENERGY E[X-Y] Q The average energy needed for ONE MOLE](https://slidetodoc.com/presentation_image_h2/5bda57d75e284e93cbc47c41c015e422/image-16.jpg)
BOND ENTHALPY or BOND ENERGY E[X-Y] Q The average energy needed for ONE MOLE P of COVALENT BONDS TO BE BROKEN C in the GASEOUS STATE at fixed T and 100 k. Pa 1. Br-Br(g) + Br(g) 2. C-H(g) C(g) + H(g) NB E[Br-Br] ― E[C-H] MEAN BOND ENTHALPIES, e. g. E [C-H] = average E value for a particular bond occurring in many similar molecules (eg CH 4, C 2 H 6 , C 3 H 8 etc). Write the equation for the splitting of the covalent bond (corresponding to E[X-Y]) in ; g) H-H : H 2(g) 2 H(g) h) 2 O-H in water : H 2 O(g) 2 H(g) + O(g) i) C-C in C 3 H 8 : C 3 H 8 (g) CH 3 (g) + C 2 H 5 (g)

STANDARD ENTHALPY CHANGE OF FORMATION, H f The heat change when ONE MOLE of a substance is P C FORMED from its ELEMENTS in their standard states at 298 K and 100 k. Pa NB ! ! ! N Hof [ELEMENT in its standard state] = ZERO ELEMENTS Q STANDARD ENTHALPY CHANGE OF COMBUSTION, H C The heat produced when ONE MOLE of a P substance is burned in excess oxygen measured C at 298 K and 100 k. Pa BOND ENTHALPY or BOND ENERGY E[X-Y] Q The average heat needed when ONE MOLE of P covalent bonds are broken, measured in the C gaseous state at 298 K and 100 k. Pa GAS ATOMS E L OXIDES R A Q

HESS’S LAW • what is it ? • how is it used ?

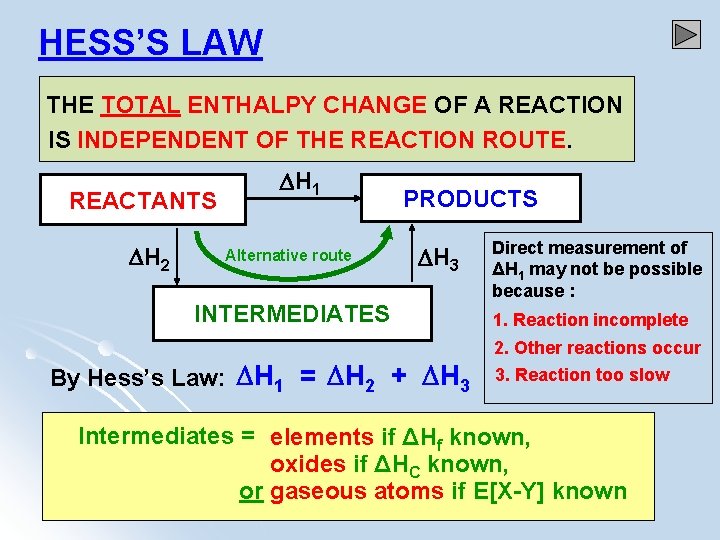
HESS’S LAW THE TOTAL ENTHALPY CHANGE OF A REACTION IS INDEPENDENT OF THE REACTION ROUTE. REACTANTS H 2 H 1 Alternative route PRODUCTS H 3 INTERMEDIATES By Hess’s Law: H 1 = H 2 + H 3 Direct measurement of ΔH 1 may not be possible because : 1. Reaction incomplete 2. Other reactions occur 3. Reaction too slow Intermediates = elements if ΔHf known, oxides if ΔHC known, or gaseous atoms if E[X-Y] known

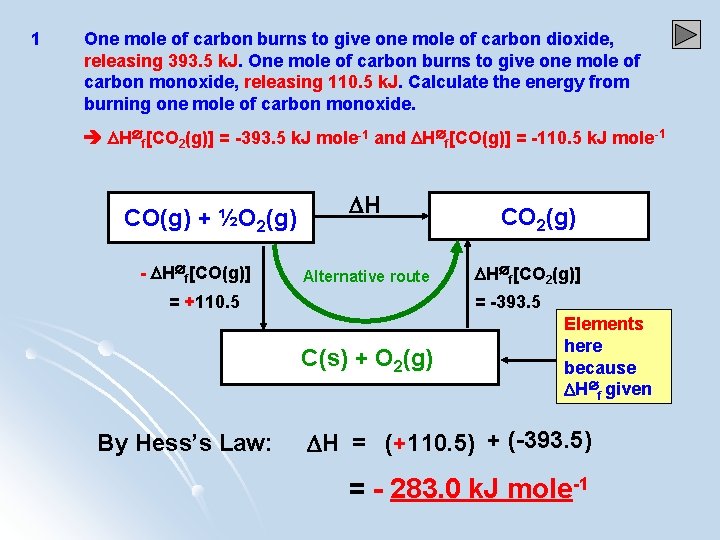
1 One mole of carbon burns to give one mole of carbon dioxide, releasing 393. 5 k. J. One mole of carbon burns to give one mole of carbon monoxide, releasing 110. 5 k. J. Calculate the energy from burning one mole of carbon monoxide. H f[CO 2(g)] = -393. 5 k. J mole-1 and H f[CO(g)] = -110. 5 k. J mole-1 CO(g) + ½O 2(g) - H f[CO(g)] H Alternative route = +110. 5 H f[CO 2(g)] = -393. 5 C(s) + O 2(g) By Hess’s Law: CO 2(g) Elements here because H f given H = (+110. 5) + (-393. 5) = - 283. 0 k. J mole-1

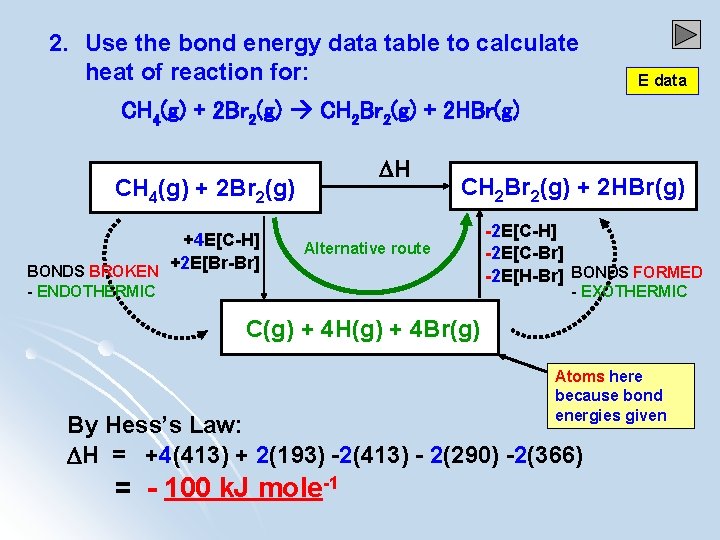
2. Use the bond energy data table to calculate heat of reaction for: E data CH 4(g) + 2 Br 2(g) CH 2 Br 2(g) + 2 HBr(g) H CH 4(g) + 2 Br 2(g) +4 E[C-H] +2 E[Br-Br] BONDS BROKEN CH 2 Br 2(g) + 2 HBr(g) Alternative route - ENDOTHERMIC -2 E[C-H] -2 E[C-Br] -2 E[H-Br] BONDS FORMED - EXOTHERMIC C(g) + 4 H(g) + 4 Br(g) Atoms here because bond energies given By Hess’s Law: H = +4(413) + 2(193) -2(413) - 2(290) -2(366) = - 100 k. J mole-1

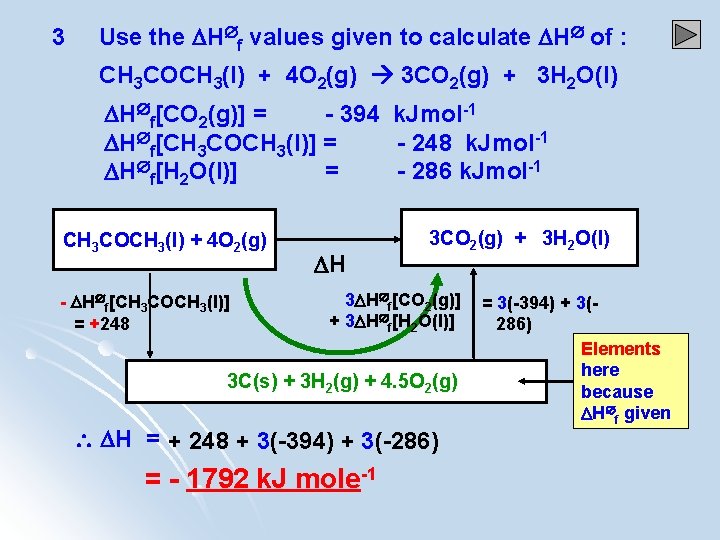
3 Use the H f values given to calculate H of : CH 3 COCH 3(l) + 4 O 2(g) 3 CO 2(g) + 3 H 2 O(l) H f[CO 2(g)] = - 394 k. Jmol-1 H f[CH 3 COCH 3(l)] = - 248 k. Jmol-1 H f[H 2 O(l)] = - 286 k. Jmol-1 CH 3 COCH 3(l) + 4 O 2(g) - H f[CH 3 COCH 3(l)] = +248 H 3 CO 2(g) + 3 H 2 O(l) 3 H f[CO 2(g)] + 3 H f[H 2 O(l)] 3 C(s) + 3 H 2(g) + 4. 5 O 2(g) H = + 248 + 3(-394) + 3(-286) = - 1792 k. J mole-1 = 3(-394) + 3(286) Elements here because H f given
![4 Calculate H f CH 4g given H f CO 2g 393 5 4 Calculate H f [CH 4(g)], given H f [CO 2(g)] = -393. 5](https://slidetodoc.com/presentation_image_h2/5bda57d75e284e93cbc47c41c015e422/image-23.jpg)
4 Calculate H f [CH 4(g)], given H f [CO 2(g)] = -393. 5 k. J mole-1 = H C [C(s)] H f [H 2 O(l)] = -285. 8 k. J mole-1 = H C [H 2(g)] H c[CH 4(g)] = -890. 3 k. J mole-1 C(s) + 2 H 2(g) H H C [C(s)] + 2 H C [H 2(g)] CH 4(g) - H c[CH 4(g)] = + 890. 3 = (-393. 5) + 2(-285. 8) CO 2(g) + 2 H 2 O(l) Oxides here because H C given H = -393. 5 + 2(-285. 8) + 890. 3 = - 74. 8 k. J mole-1

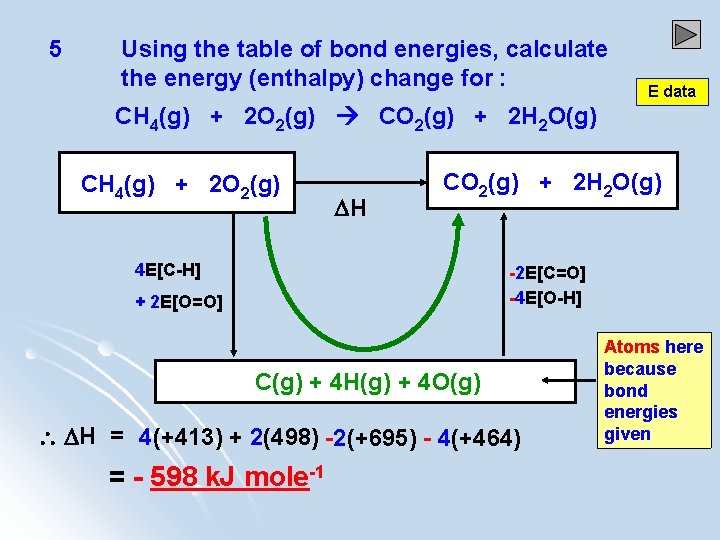
5 Using the table of bond energies, calculate the energy (enthalpy) change for : E data CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) CH 4(g) + 2 O 2(g) H CO 2(g) + 2 H 2 O(g) 4 E[C-H] -2 E[C=O] -4 E[O-H] + 2 E[O=O] C(g) + 4 H(g) + 4 O(g) H = 4(+413) + 2(498) -2(+695) - 4(+464) = - 598 k. J mole-1 Atoms here because bond energies given

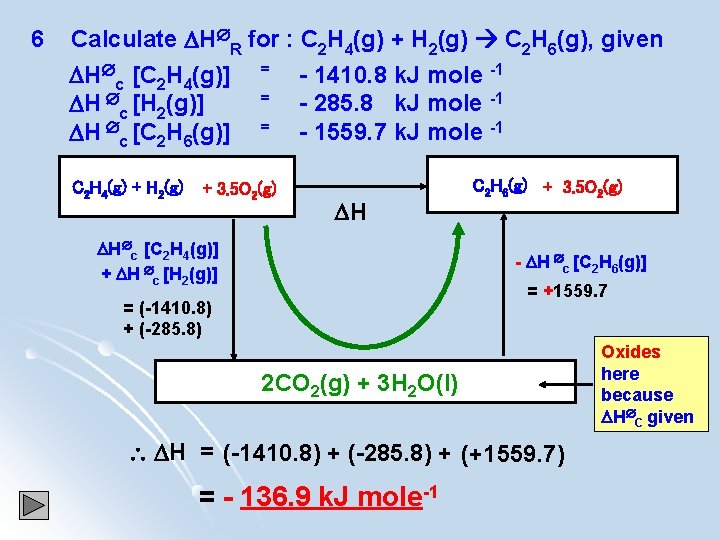
6 Calculate H R for : C 2 H 4(g) + H 2(g) C 2 H 6(g), given H c [C 2 H 4(g)] = - 1410. 8 k. J mole -1 = H c [H 2(g)] - 285. 8 k. J mole -1 H c [C 2 H 6(g)] = - 1559. 7 k. J mole -1 C 2 H 4(g) + H 2(g) + 3. 5 O 2(g) H H c [C 2 H 4(g)] + H c [H 2(g)] C 2 H 6(g) + 3. 5 O 2(g) - H c [C 2 H 6(g)] = +1559. 7 = (-1410. 8) + (-285. 8) 2 CO 2(g) + 3 H 2 O(l) H = (-1410. 8) + (-285. 8) + (+1559. 7) = - 136. 9 k. J mole-1 Oxides here because H C given

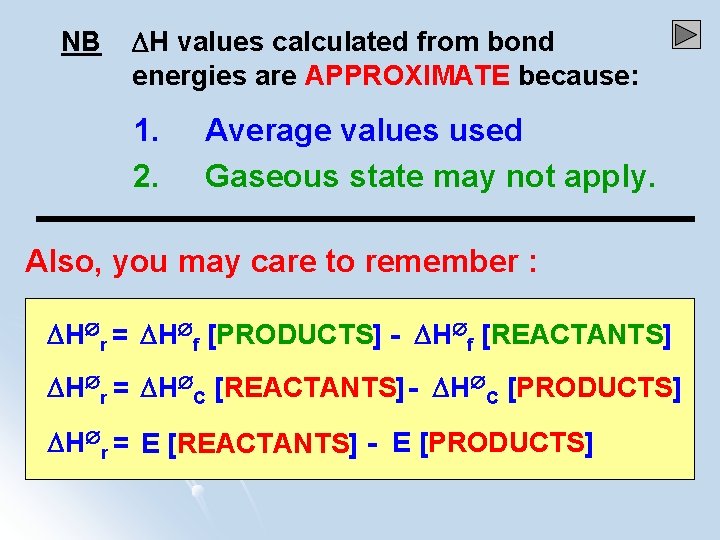
NB H values calculated from bond energies are APPROXIMATE because: 1. 2. Average values used Gaseous state may not apply. Also, you may care to remember : H r = H f [PRODUCTS] - H f [REACTANTS] H r = H C [REACTANTS]- H C [PRODUCTS] H r = E [REACTANTS] - E [PRODUCTS]