CHEM 3310 Thermodynamics Change in Enthalpy H Enthalpy











- Slides: 39

CHEM 3310 Thermodynamics Change in Enthalpy, H

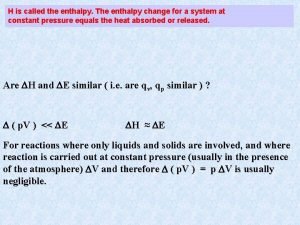
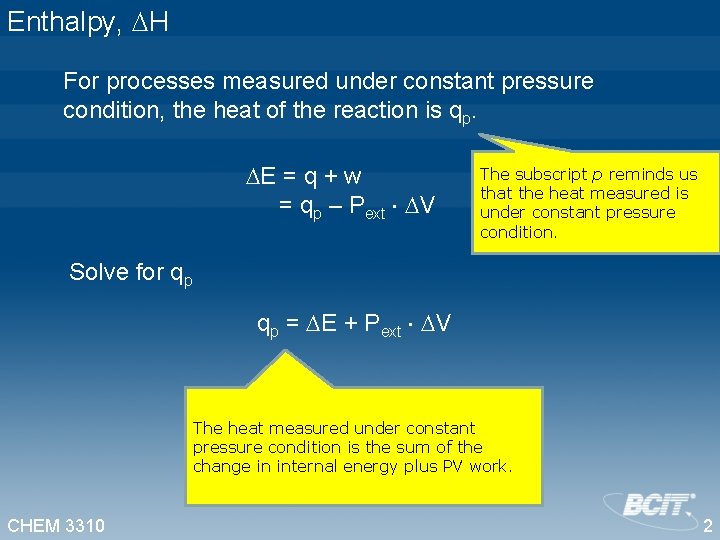
Enthalpy, H For processes measured under constant pressure condition, the heat of the reaction is qp. E = q + w = qp – Pext V The subscript p reminds us that the heat measured is under constant pressure condition. Solve for qp = E + Pext V The heat measured under constant pressure condition is the sum of the change in internal energy plus PV work. CHEM 3310 2

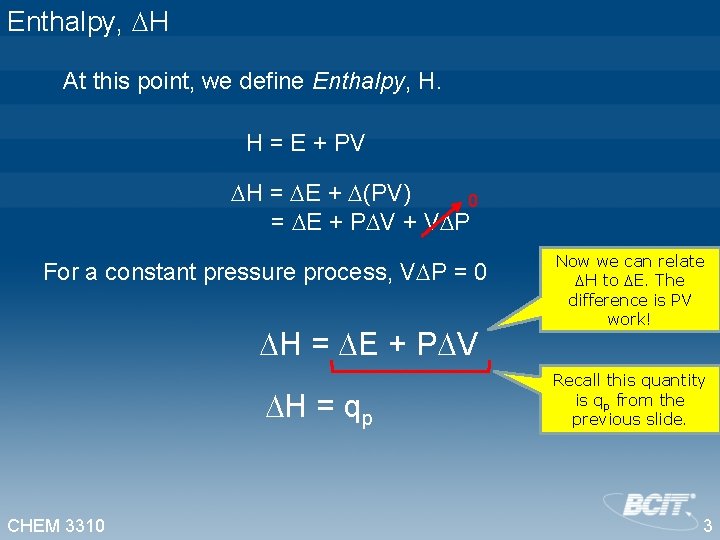
Enthalpy, H At this point, we define Enthalpy, H. H = E + PV H = E + (PV) 0 = E + P V + V P For a constant pressure process, V P = 0 H = E + P V H = qp CHEM 3310 Now we can relate H to E. The difference is PV work! Recall this quantity is qp from the previous slide. 3

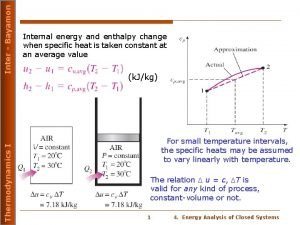
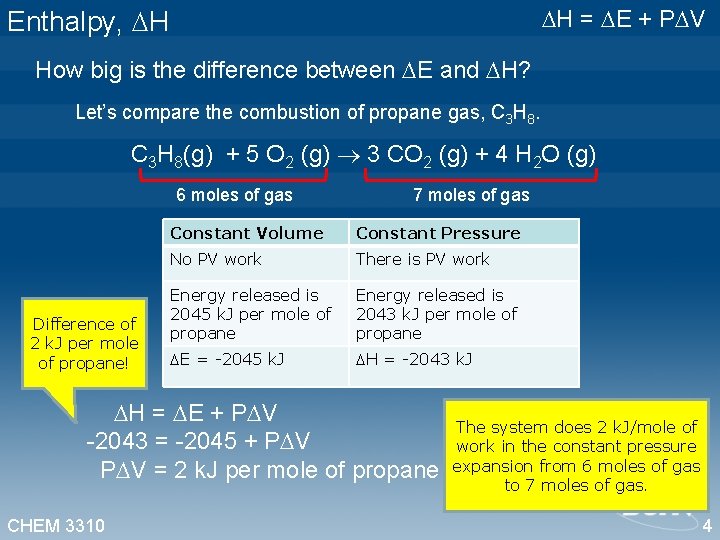
H = E + P V Enthalpy, H How big is the difference between E and H? Let’s compare the combustion of propane gas, C 3 H 8(g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g) 6 moles of gas 7 moles of gas Difference of 2 k. J per mole of propane! Constant Volume Constant Pressure No PV work There is PV work Energy released is 2045 k. J per mole of propane Energy released is 2043 k. J per mole of propane E = -2045 k. J H = -2043 k. J H = E + P V -2043 = -2045 + P V = 2 k. J per mole of propane CHEM 3310 The system does 2 k. J/mole of work in the constant pressure expansion from 6 moles of gas to 7 moles of gas. 4

Enthalpy, H H = E + P V • H is a state function. This means H is path independent. • H represents the amount of heat released/absorbed at constant P for a reaction as written by the balanced chemical equation. C 3 H 8(g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g) H = -2043 k. J • If the reaction is carried out with 0. 5 mole of propane, then (0. 5)(-2043 k. J) = -1022 k. J or 1022 k. J of energy is released. Enthalpy is an extensive property. • Physical states of reactants and products must be specified as solid (s), liquid (l), gas (g), aqueous (aq) when enthalpy changes are reported. • Enthalpy change for a reaction is equal in magnitude but opposite in sign to the H for the reverse reaction. 3 CO 2 (g) + 4 H 2 O (g) C 3 H 8(g) + 5 O 2 (g) H = +2043 k. J CHEM 3310 5

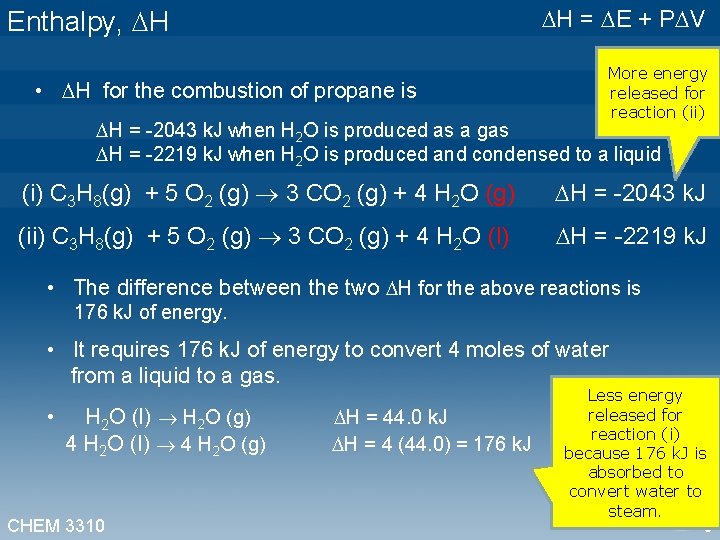
Enthalpy, H • H for the combustion of propane is H = -2043 k. J when H 2 O is produced as a gas H = E + P V More energy released for reaction (ii) H = -2219 k. J when H 2 O is produced and condensed to a liquid (i) C 3 H 8(g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g) H = -2043 k. J (ii) C 3 H 8(g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) H = -2219 k. J • The difference between the two H for the above reactions is 176 k. J of energy. • It requires 176 k. J of energy to convert 4 moles of water from a liquid to a gas. Less energy released for • H 2 O (l) H 2 O (g) H = 44. 0 k. J reaction (i) 4 H 2 O (l) 4 H 2 O (g) H = 4 (44. 0) = 176 k. J because 176 k. J is absorbed to convert water to steam. CHEM 3310 6

H = E + P V Enthalpy, H Enthalpy of Reaction: H = Hfinal – Hinitial = Hproducts – Hreactants H > 0 when Hproducts > Hreactants Endothermic CHEM 3310 H < 0 when Hproducts < Hreactants Exothermic 7

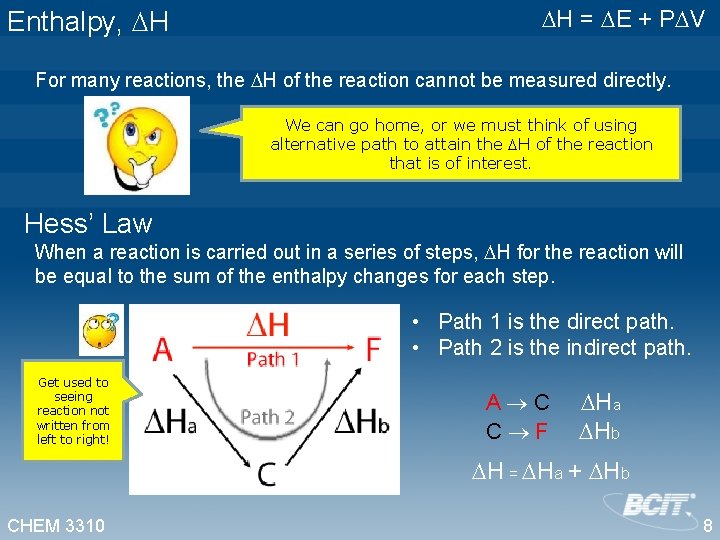
Enthalpy, H H = E + P V For many reactions, the H of the reaction cannot be measured directly. We can go home, or we must think of using alternative path to attain the H of the reaction that is of interest. Hess’ Law When a reaction is carried out in a series of steps, H for the reaction will be equal to the sum of the enthalpy changes for each step. • Path 1 is the direct path. • Path 2 is the indirect path. Get used to seeing reaction not written from left to right! A C Ha C F Hb H = Ha + Hb CHEM 3310 8

Enthalpy, H Hess’ Law in action for Experiment 7 Hhydration Na 2 CO 3 (s) + 10 H 2 O (l) Na 2 CO 3 • 10 H 2 O (s) Path 1 H’a Direct path cannot be done! Path 2 H’b Na 2 CO 3 (aq) + 10 H 2 O (l) Find an alternative path! • Path 1 is the direct path. • Path 2 is the indirect path. Na 2 CO 3 (s) + 10 H 2 O (l) Na 2 CO 3 (aq) + 10 H 2 O (l) H’a Na 2 CO 3 (aq) + 10 H 2 O (l) Na 2 CO 3 • 10 H 2 O (s) - H’b Hhydration = H’a + (- H’b) CHEM 3310 9

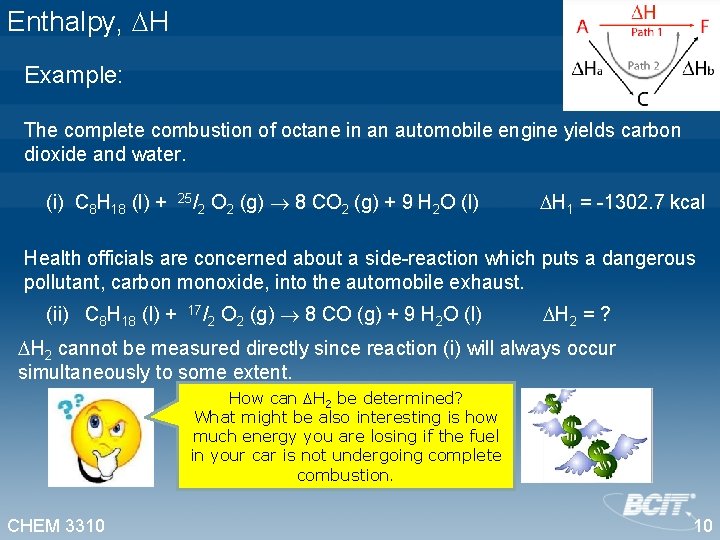
Enthalpy, H Example: The complete combustion of octane in an automobile engine yields carbon dioxide and water. (i) C 8 H 18 (l) + 25/2 O 2 (g) 8 CO 2 (g) + 9 H 2 O (l) H 1 = -1302. 7 kcal Health officials are concerned about a side-reaction which puts a dangerous pollutant, carbon monoxide, into the automobile exhaust. (ii) C 8 H 18 (l) + 17/2 O 2 (g) 8 CO (g) + 9 H 2 O (l) H 2 = ? H 2 cannot be measured directly since reaction (i) will always occur simultaneously to some extent. How can H 2 be determined? What might be also interesting is how much energy you are losing if the fuel in your car is not undergoing complete combustion. CHEM 3310 10

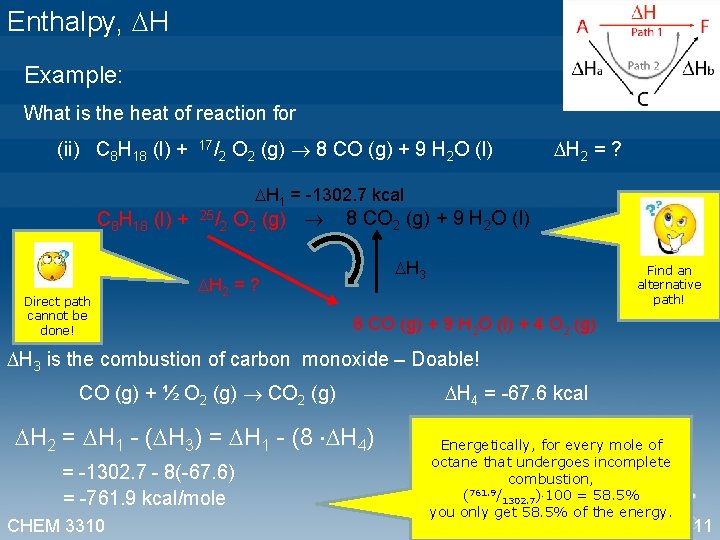
Enthalpy, H Example: What is the heat of reaction for (ii) C 8 H 18 (l) + 17/2 O 2 (g) 8 CO (g) + 9 H 2 O (l) H 2 = ? H 1 = -1302. 7 kcal C 8 H 18 (l) + 25/2 O 2 (g) 8 CO 2 (g) + 9 H 2 O (l) Direct path cannot be done! H 2 = ? H 3 Find an alternative path! 8 CO (g) + 9 H 2 O (l) + 4 O 2 (g) H 3 is the combustion of carbon monoxide – Doable! CO (g) + ½ O 2 (g) CO 2 (g) H 2 = H 1 - ( H 3) = H 1 - (8 H 4) = -1302. 7 - 8(-67. 6) = -761. 9 kcal/mole CHEM 3310 H 4 = -67. 6 kcal Energetically, for every mole of octane that undergoes incomplete combustion, (761. 9/1302. 7) 100 = 58. 5% you only get 58. 5% of the energy. 11

Example: Calculate the heat of combustion of carbon to form carbon monoxide, CO (i) C (s) + 1/2 O 2 (g) CO (g) H = ? Given that (ii) C (s) + O 2 (g) CO 2 (g) H = -393. 5 k. J (iii) CO (g) + 1/2 O 2 (g) CO 2 (g) H = -283. 0 k. J Answer: Reaction (i) is not doable, but the alternate paths are reactions (ii) and (iii). Rearrange reactions (ii) and (iii) to give reaction (i). Add reaction (ii) to the reverse of (iii) (ii) C (s) + O 2 (g) CO 2 (g) H = -393. 5 k. J + (iii) CO 2 (g) CO (g) + 1/2 O 2 (g) H = +283. 0 k. J C (s) + 1/2 O 2 (g) CO (g) H = -110. 5 k. J

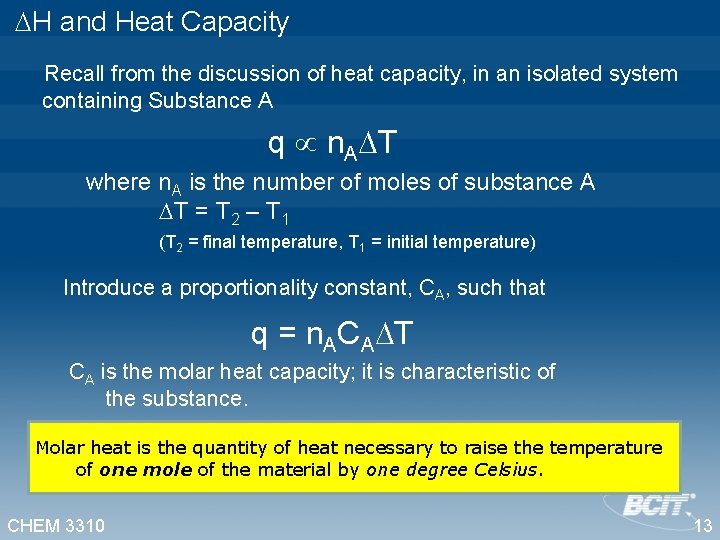
H and Heat Capacity Recall from the discussion of heat capacity, in an isolated system containing Substance A q n. A T where n. A is the number of moles of substance A T = T 2 – T 1 (T 2 = final temperature, T 1 = initial temperature) Introduce a proportionality constant, CA, such that q = n. ACA T CA is the molar heat capacity; it is characteristic of the substance. Molar heat is the quantity of heat necessary to raise the temperature of one mole of the material by one degree Celsius. CHEM 3310 13

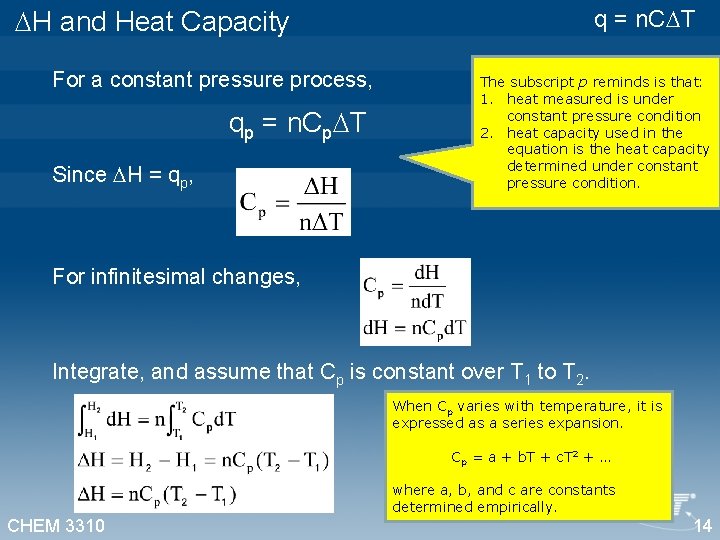
q = n. C T H and Heat Capacity For a constant pressure process, qp = n. Cp T Since H = qp, The subscript p reminds is that: 1. heat measured is under constant pressure condition 2. heat capacity used in the equation is the heat capacity determined under constant pressure condition. For infinitesimal changes, Integrate, and assume that Cp is constant over T 1 to T 2. When Cp varies with temperature, it is expressed as a series expansion. Cp = a + b. T + c. T 2 + … where a, b, and c are constants determined empirically. CHEM 3310 14

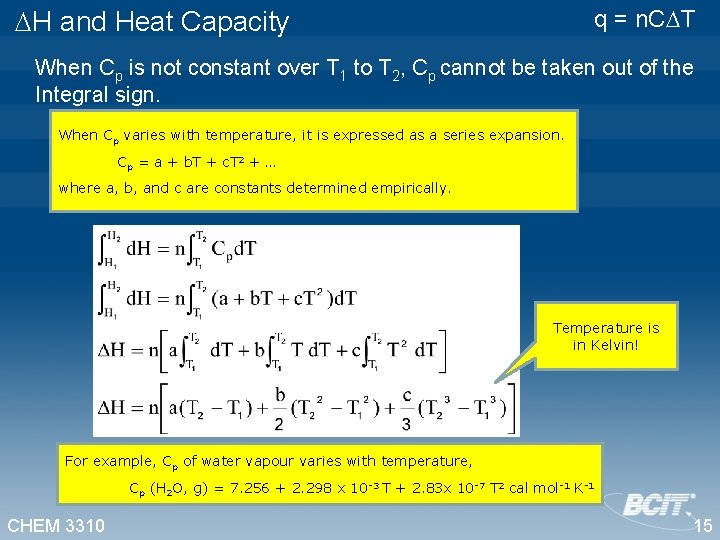
q = n. C T H and Heat Capacity When Cp is not constant over T 1 to T 2, Cp cannot be taken out of the Integral sign. When Cp varies with temperature, it is expressed as a series expansion. Cp = a + b. T + c. T 2 + … where a, b, and c are constants determined empirically. Temperature is in Kelvin! For example, Cp of water vapour varies with temperature, Cp (H 2 O, g) = 7. 256 + 2. 298 x 10 -3 T + 2. 83 x 10 -7 T 2 cal mol-1 K-1 CHEM 3310 15

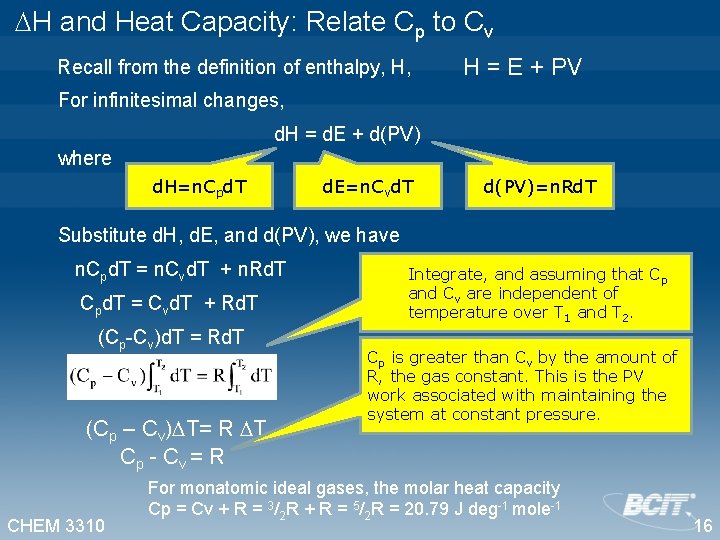
H and Heat Capacity: Relate Cp to Cv Recall from the definition of enthalpy, H, H = E + PV For infinitesimal changes, d. H = d. E + d(PV) where d. H=n. Cpd. T d. E=n. Cvd. T d(PV)=n. Rd. T Substitute d. H, d. E, and d(PV), we have n. Cpd. T = n. Cvd. T + n. Rd. T Cpd. T = Cvd. T + Rd. T (Cp-Cv)d. T = Rd. T (Cp – Cv) T= R T Cp - Cv = R CHEM 3310 Integrate, and assuming that Cp and Cv are independent of temperature over T 1 and T 2. Cp is greater than Cv by the amount of R, the gas constant. This is the PV work associated with maintaining the system at constant pressure. For monatomic ideal gases, the molar heat capacity Cp = Cv + R = 3/2 R + R = 5/2 R = 20. 79 J deg-1 mole-1 16

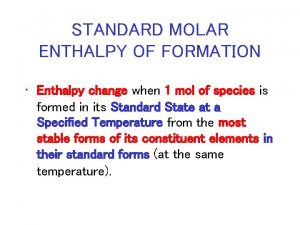
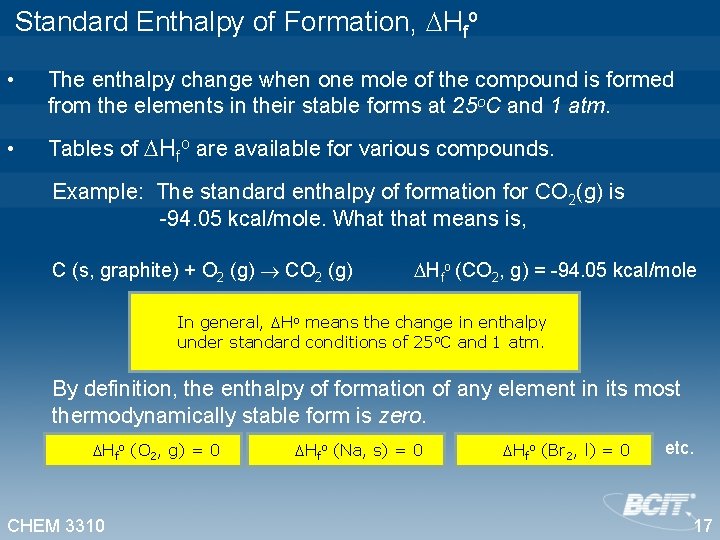
Standard Enthalpy of Formation, Hfo • The enthalpy change when one mole of the compound is formed from the elements in their stable forms at 25 o. C and 1 atm. • Tables of Hfo are available for various compounds. Example: The standard enthalpy of formation for CO 2(g) is -94. 05 kcal/mole. What that means is, C (s, graphite) + O 2 (g) CO 2 (g) Hfo (CO 2, g) = -94. 05 kcal/mole In general, Ho means the change in enthalpy under standard conditions of 25 o. C and 1 atm. By definition, the enthalpy of formation of any element in its most thermodynamically stable form is zero. Hfo (O 2, g) = 0 CHEM 3310 Hfo (Na, s) = 0 Hfo (Br 2, l) = 0 etc. 17

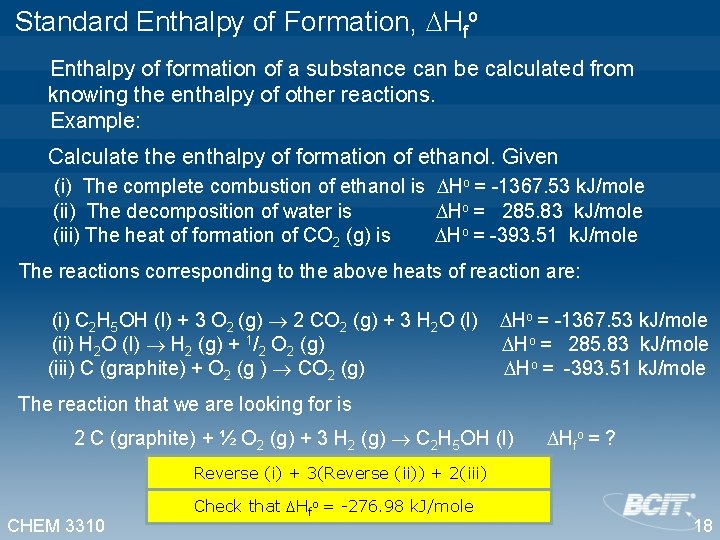
Standard Enthalpy of Formation, Hfo Enthalpy of formation of a substance can be calculated from knowing the enthalpy of other reactions. Example: Calculate the enthalpy of formation of ethanol. Given (i) The complete combustion of ethanol is Ho = -1367. 53 k. J/mole (ii) The decomposition of water is Ho = 285. 83 k. J/mole (iii) The heat of formation of CO 2 (g) is Ho = -393. 51 k. J/mole The reactions corresponding to the above heats of reaction are: (i) C 2 H 5 OH (l) + 3 O 2 (g) 2 CO 2 (g) + 3 H 2 O (l) Ho = -1367. 53 k. J/mole (ii) H 2 O (l) H 2 (g) + 1/2 O 2 (g) Ho = 285. 83 k. J/mole (iii) C (graphite) + O 2 (g ) CO 2 (g) Ho = -393. 51 k. J/mole The reaction that we are looking for is 2 C (graphite) + ½ O 2 (g) + 3 H 2 (g) C 2 H 5 OH (l) Hfo = ? Reverse (i) + 3(Reverse (ii)) + 2(iii) CHEM 3310 Check that Hfo = -276. 98 k. J/mole 18

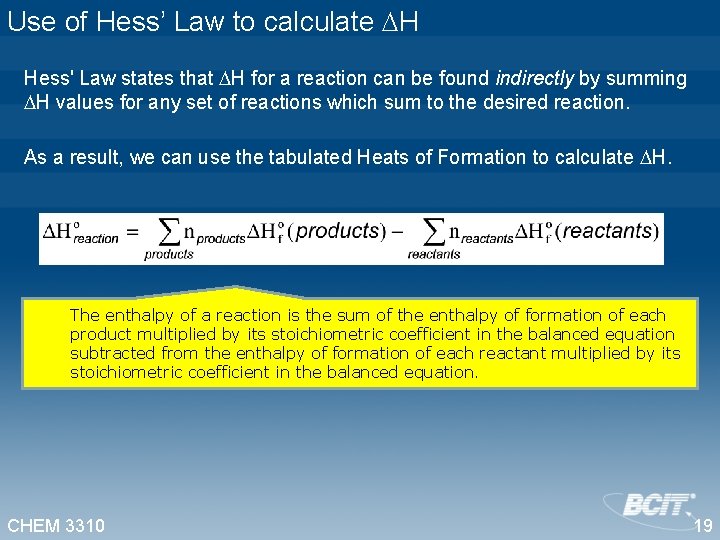
Use of Hess’ Law to calculate H Hess' Law states that H for a reaction can be found indirectly by summing H values for any set of reactions which sum to the desired reaction. As a result, we can use the tabulated Heats of Formation to calculate H. The enthalpy of a reaction is the sum of the enthalpy of formation of each product multiplied by its stoichiometric coefficient in the balanced equation subtracted from the enthalpy of formation of each reactant multiplied by its stoichiometric coefficient in the balanced equation. CHEM 3310 19

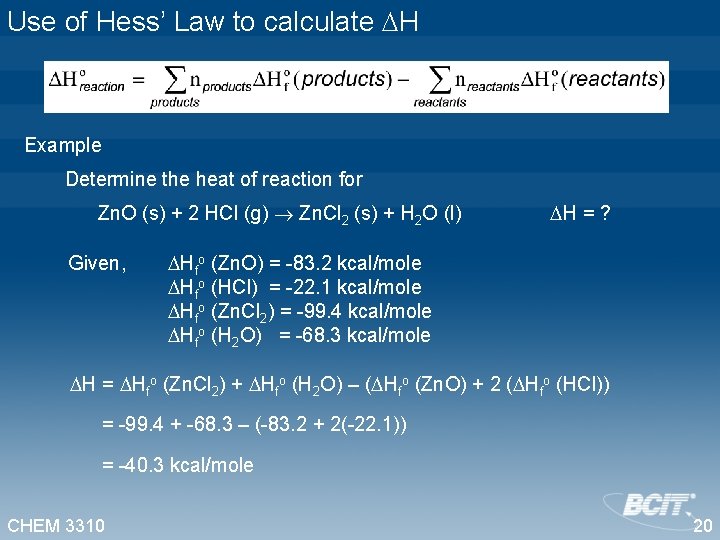
Use of Hess’ Law to calculate H Example Determine the heat of reaction for Zn. O (s) + 2 HCl (g) Zn. Cl 2 (s) + H 2 O (l) Given, H = ? Hfo (Zn. O) = -83. 2 kcal/mole Hfo (HCl) = -22. 1 kcal/mole Hfo (Zn. Cl 2) = -99. 4 kcal/mole Hfo (H 2 O) = -68. 3 kcal/mole H = Hfo (Zn. Cl 2) + Hfo (H 2 O) – ( Hfo (Zn. O) + 2 ( Hfo (HCl)) = -99. 4 + -68. 3 – (-83. 2 + 2(-22. 1)) = -40. 3 kcal/mole CHEM 3310 20

Using Bond Enthalpy to determine H The bond enthalpy (or bond energy) is the energy required to break a chemical bond. It is usually expressed in units of kcal/mole or k. J/mole, measured at 298 K. The exact bond enthalpy of a particular chemical bond depends upon the molecular environment in which the bond exists. Bond enthalpy values given in chemical data books are averaged values. CHEM 3310 21

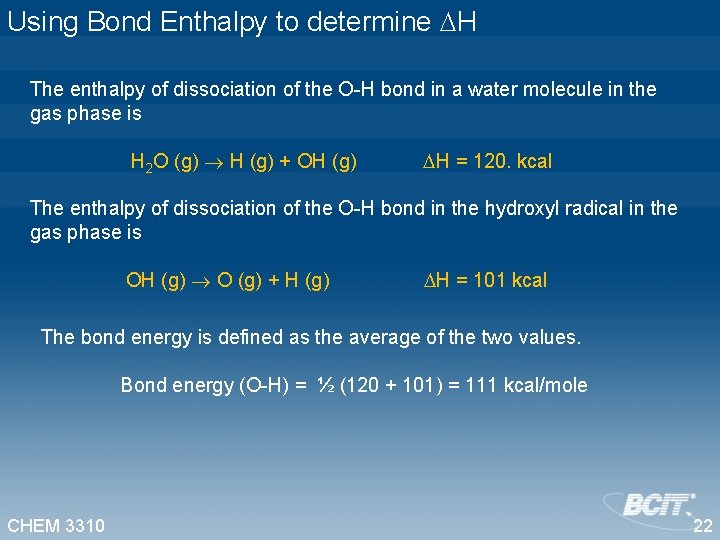
Using Bond Enthalpy to determine H The enthalpy of dissociation of the O-H bond in a water molecule in the gas phase is H 2 O (g) H (g) + OH (g) H = 120. kcal The enthalpy of dissociation of the O-H bond in the hydroxyl radical in the gas phase is OH (g) O (g) + H (g) H = 101 kcal The bond energy is defined as the average of the two values. Bond energy (O-H) = ½ (120 + 101) = 111 kcal/mole CHEM 3310 22

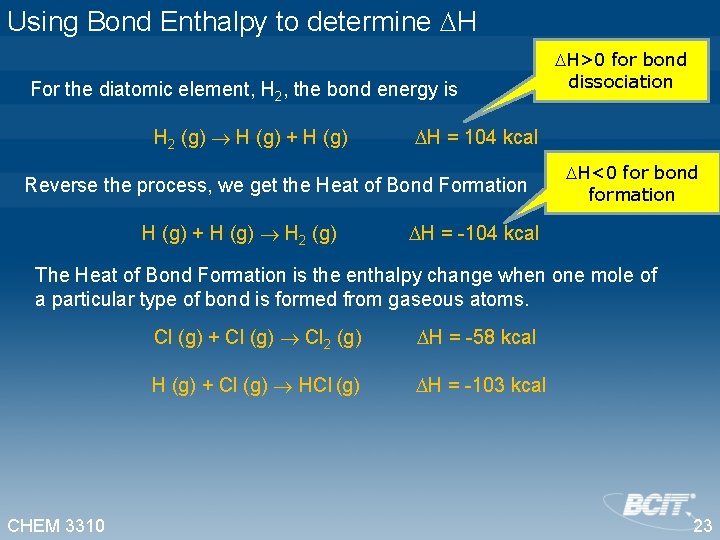
Using Bond Enthalpy to determine H For the diatomic element, H 2, the bond energy is H 2 (g) H (g) + H (g) H = 104 kcal Reverse the process, we get the Heat of Bond Formation H>0 for bond dissociation H<0 for bond formation H (g) + H (g) H 2 (g) H = -104 kcal The Heat of Bond Formation is the enthalpy change when one mole of a particular type of bond is formed from gaseous atoms. Cl (g) + Cl (g) Cl 2 (g) H = -58 kcal H (g) + Cl (g) H = -103 kcal CHEM 3310 23

Using Bond Enthalpy to determine H The reaction between hydrogen gas, H 2 (g), and chlorine gas, Cl 2 (g), forms HCl (g). The heat of reaction of H 2 (g) + Cl 2 (g) 2 HCl (g) H = -44 kcal Consider the above reaction from bond breaking and bond formation. ΔH = ∑ ΔH(bonds breaking) - ∑ ΔH(bonds forming) Bond breaking: H 2 (g) H (g) + H (g) H = 104 kcal Cl 2 (g) Cl (g) + Cl (g) H = 58 kcal Bond forming: 2 H (g) + 2 Cl (g) 2 HCl (g) H = 2 (-103) = -206 kcal ΔH = ∑ ΔH(bonds breaking) - ∑ ΔH(bonds forming) ΔH = 104 + 58 – 206 ΔH = -44 kcal CHEM 3310 24

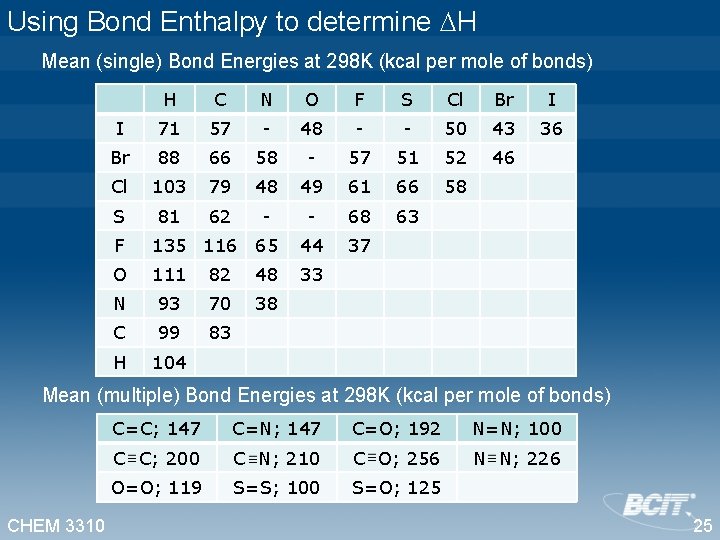
Using Bond Enthalpy to determine H Mean (single) Bond Energies at 298 K (kcal per mole of bonds) H C N O F S Cl Br I I 71 57 - 48 - - 50 43 36 Br 88 66 58 - 57 51 52 46 Cl 103 79 48 49 61 66 58 S 81 62 - - 68 63 37 F 135 116 65 44 O 111 82 48 33 N 93 70 38 C 99 83 H 104 Mean (multiple) Bond Energies at 298 K (kcal per mole of bonds) CHEM 3310 C=C; 147 C=N; 147 C=O; 192 N=N; 100 C C; 200 C N; 210 C O; 256 N N; 226 O=O; 119 S=S; 100 S=O; 125 25

Using Bond Enthalpy to determine H Bond enthalpy values: 1. Single Bonds: generally, H < 100 kcal/mole eg – single carbon-carbon bond H = 83 kcal/mole 2. Double Bonds: generally, H < 200 kcal/mole eg – double carbon-carbon bond H = 147 kcal/mole A double bond is usually not as strong as 2 single bonds. 3. Triple Bonds: generally, H < 300 kcal/mole eg – triple carbon-carbon bond H = 200 kcal/mole A triple bond is usually not as strong as 3 single bonds. 4. Usually single bonds with different atoms are stronger than those between like atoms. eg – X-Y bond is stronger than X-X and Y-Y bonds CHEM 3310 26

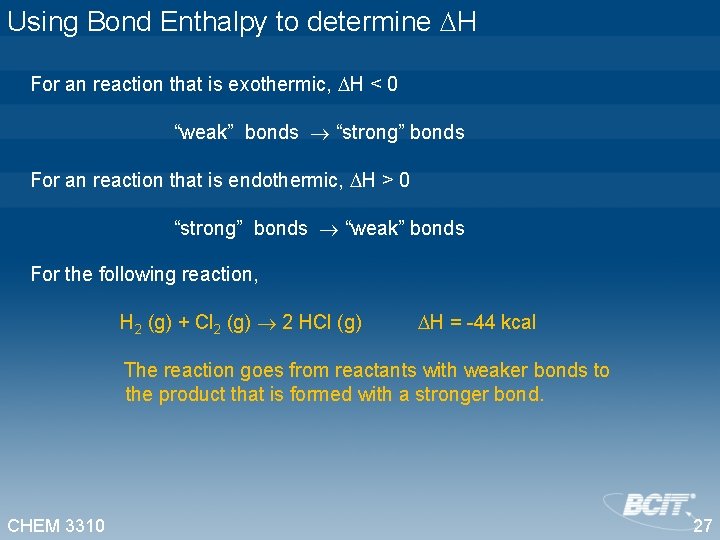
Using Bond Enthalpy to determine H For an reaction that is exothermic, H < 0 “weak” bonds “strong” bonds For an reaction that is endothermic, H > 0 “strong” bonds “weak” bonds For the following reaction, H 2 (g) + Cl 2 (g) 2 HCl (g) H = -44 kcal The reaction goes from reactants with weaker bonds to the product that is formed with a stronger bond. CHEM 3310 27

Using Bond Enthalpy to determine H ΔH = ∑ ΔH(bonds breaking) - ∑ ΔH(bonds forming) Example Find H for the following reaction from bond energies 2 H 2 (g) + O 2 (g) 2 H 2 O (g) H = ? Bond breaking: 2 H-H H = 2 (104) = 208 kcal 1 O=O H = 119 kcal Bond forming: 4 O-H H = 4 (-111) = -444 kcal ΔH = ∑ ΔH(bonds breaking) - ∑ ΔH(bonds forming) ΔH = 208 + 119 – 444 ΔH = -117 kcal Or, Keep the signs associated with bond breaking (+) and bond forming (-) and simply add them up to get ΔH. Eg: ΔH= (208+119)+(-444)=-117 kcal CHEM 3310 If you use this formula, remove the signs associated with bond breaking and bond forming. 28

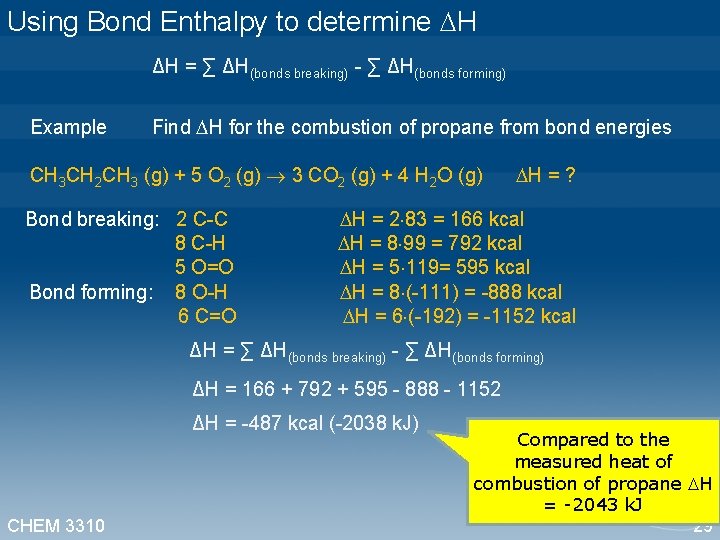
Using Bond Enthalpy to determine H ΔH = ∑ ΔH(bonds breaking) - ∑ ΔH(bonds forming) Example Find H for the combustion of propane from bond energies CH 3 CH 2 CH 3 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g) H = ? Bond breaking: 2 C-C H = 2 83 = 166 kcal 8 C-H H = 8 99 = 792 kcal 5 O=O H = 5 119= 595 kcal Bond forming: 8 O-H H = 8 (-111) = -888 kcal 6 C=O H = 6 (-192) = -1152 kcal ΔH = ∑ ΔH(bonds breaking) - ∑ ΔH(bonds forming) ΔH = 166 + 792 + 595 - 888 - 1152 ΔH = -487 kcal (-2038 k. J) CHEM 3310 Compared to the measured heat of combustion of propane H = -2043 k. J 29

Using Bond Enthalpy to determine H ΔH = ∑ ΔH(bonds breaking) - ∑ ΔH(bonds forming) Example Show, by bond energies, that C (graphite) + ½ O 2 (g) CO (g) H = -26. 42 kcal Heat of vaporization of carbon is 171. 70 kcal/mole Bond forming: 1 C≡O H = -256 kcal Bond breaking: C (graphite) C (g) H = 171. 70 kcal ½ O=O H = ½ 119= 59. 5 kcal ΔH = ∑ ΔH(bonds breaking) - ∑ ΔH(bonds forming) ΔH = 171. 7 + 59. 5 - 256 ΔH = -25 kcal CHEM 3310 Bond enthalpy calculation of the reaction is close to the reported H value. 30

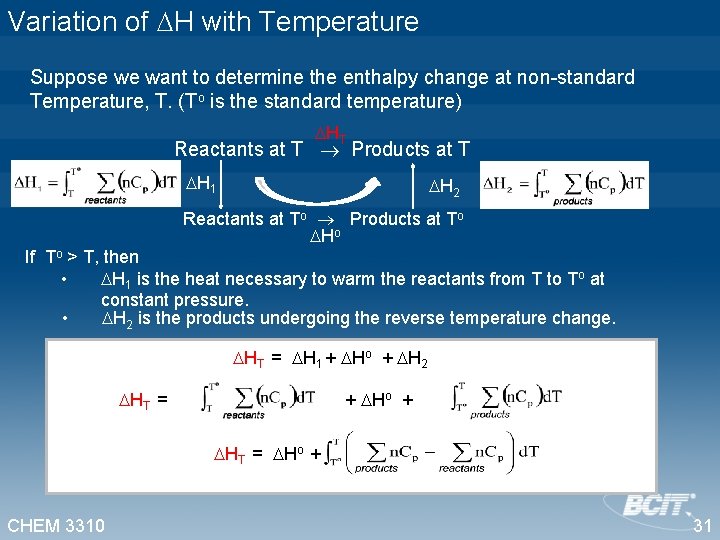
Variation of H with Temperature Suppose we want to determine the enthalpy change at non-standard Temperature, T. (To is the standard temperature) HT Reactants at T Products at T H 1 H 2 Reactants at To Products at To Ho If To > T, then • H 1 is the heat necessary to warm the reactants from T to To at constant pressure. • H 2 is the products undergoing the reverse temperature change. HT = H 1 + Ho + H 2 HT = + Ho + HT = Ho + CHEM 3310 31

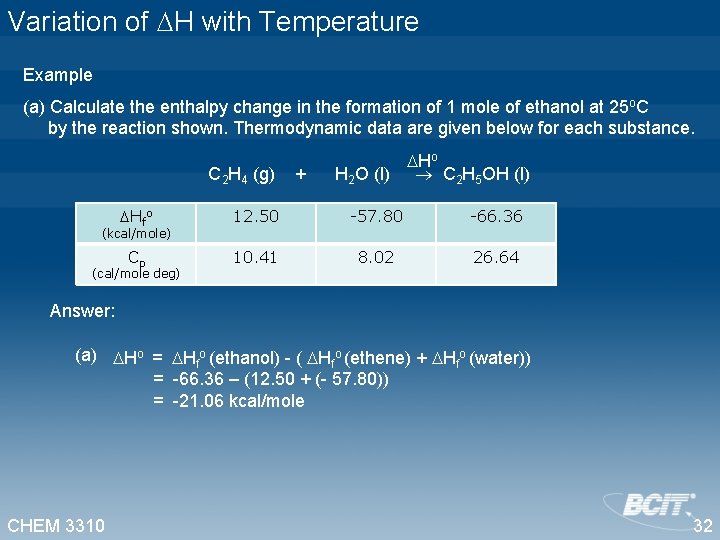
Variation of H with Temperature Example (a) Calculate the enthalpy change in the formation of 1 mole of ethanol at 25 o. C by the reaction shown. Thermodynamic data are given below for each substance. Ho C 2 H 4 (g) + H 2 O (l) C 2 H 5 OH (l) Hfo 12. 50 -57. 80 -66. 36 Cp 10. 41 8. 02 26. 64 (kcal/mole) (cal/mole deg) Answer: (a) Ho = Hfo (ethanol) - ( Hfo (ethene) + Hfo (water)) = -66. 36 – (12. 50 + (- 57. 80)) = -21. 06 kcal/mole CHEM 3310 32

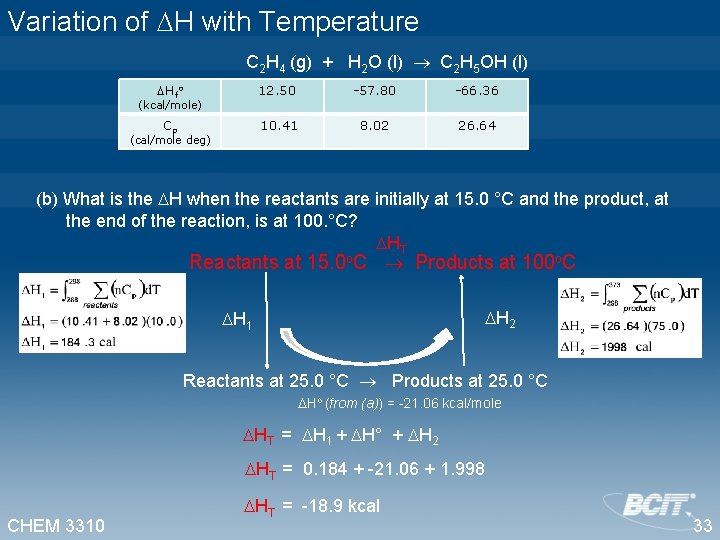
Variation of H with Temperature C 2 H 4 (g) + H 2 O (l) C 2 H 5 OH (l) Hfo 12. 50 -57. 80 -66. 36 Cp 10. 41 8. 02 26. 64 (kcal/mole) (cal/mole deg) (b) What is the H when the reactants are initially at 15. 0 °C and the product, at the end of the reaction, is at 100. °C? HT Reactants at 15. 0 o. C Products at 100 o. C H 2 H 1 Reactants at 25. 0 °C Products at 25. 0 °C Ho (from (a)) = -21. 06 kcal/mole HT = H 1 + H° + H 2 HT = 0. 184 + -21. 06 + 1. 998 CHEM 3310 HT = -18. 9 kcal 33

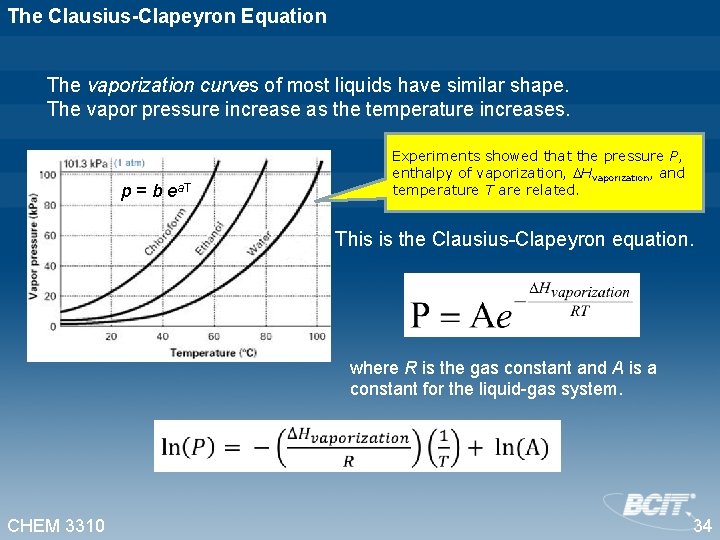
The Clausius-Clapeyron Equation The vaporization curves of most liquids have similar shape. The vapor pressure increase as the temperature increases. p = b ea. T Experiments showed that the pressure P, enthalpy of vaporization, Hvaporization, and temperature T are related. This is the Clausius-Clapeyron equation. where R is the gas constant and A is a constant for the liquid-gas system. CHEM 3310 34

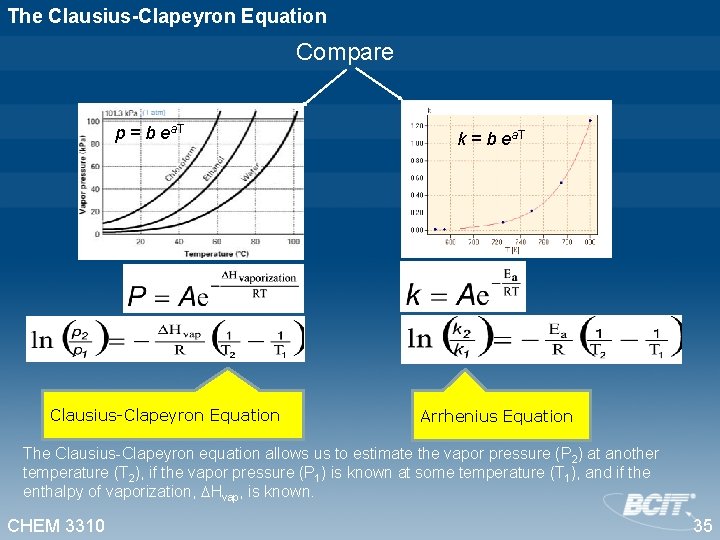
The Clausius-Clapeyron Equation Compare p = b ea. T Clausius-Clapeyron Equation k = b ea. T Arrhenius Equation The Clausius-Clapeyron equation allows us to estimate the vapor pressure (P 2) at another temperature (T 2), if the vapor pressure (P 1) is known at some temperature (T 1), and if the enthalpy of vaporization, Hvap, is known. CHEM 3310 35

The Clausius-Clapeyron Equation p = b ea. T The boiling points of a pure compound was measured at two different pressures. Estimate the heat of vaporization of the compound. T (K) P (k. Pa) 260 3. 3 300 325 Check your answer: Hvap = 74. 4 k. J/mole If the phase transition is from solid to gas, the enthalpy of sublimation. ( Hsub) is the change in enthalpy when one mole of solid vaporizes to form one mole of gas. CHEM 3310 36

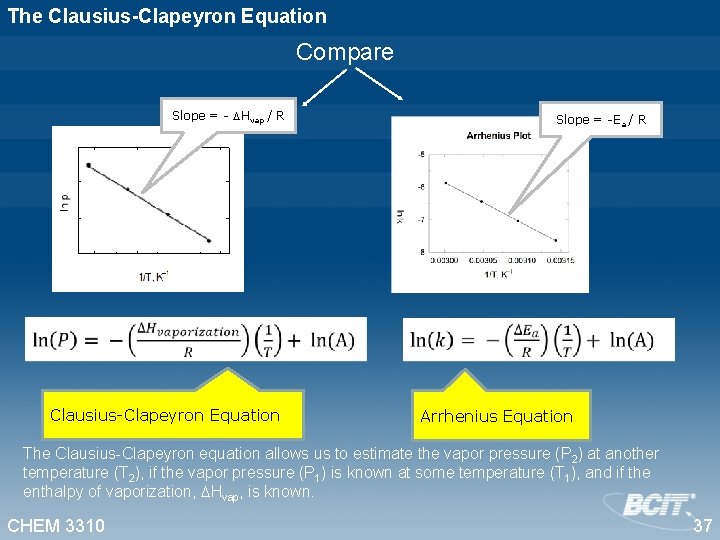
The Clausius-Clapeyron Equation Compare Slope = - Hvap / R Slope = -Ea / R Clausius-Clapeyron Equation Arrhenius Equation The Clausius-Clapeyron equation allows us to estimate the vapor pressure (P 2) at another temperature (T 2), if the vapor pressure (P 1) is known at some temperature (T 1), and if the enthalpy of vaporization, Hvap, is known. CHEM 3310 37

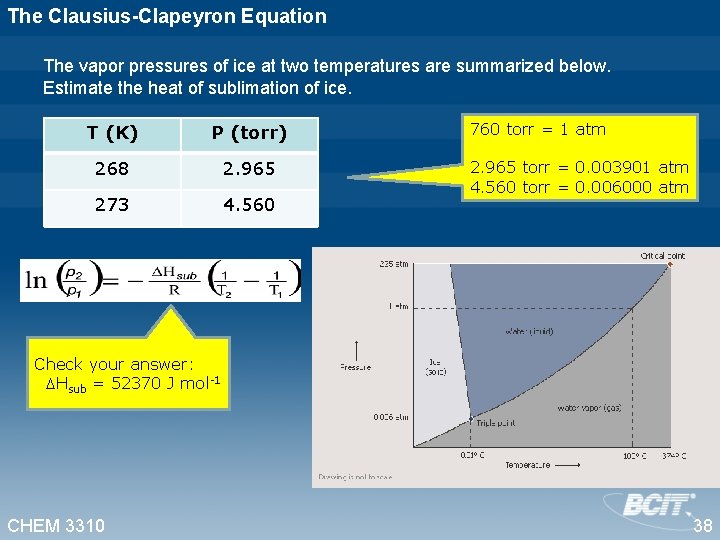
The Clausius-Clapeyron Equation The vapor pressures of ice at two temperatures are summarized below. Estimate the heat of sublimation of ice. T (K) P (torr) 268 2. 965 273 4. 560 760 torr = 1 atm 2. 965 torr = 0. 003901 atm 4. 560 torr = 0. 006000 atm Check your answer: Hsub = 52370 J mol-1 CHEM 3310 38

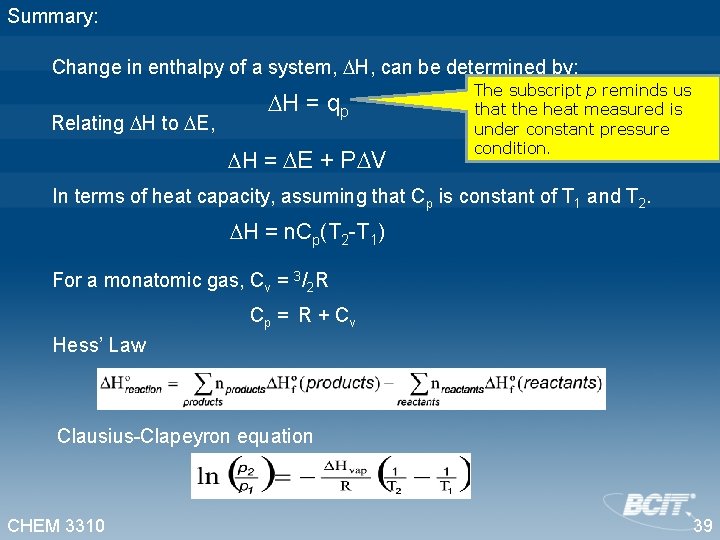
Summary: Change in enthalpy of a system, H, can be determined by: Relating H to E, H = qp H = E + P V The subscript p reminds us that the heat measured is under constant pressure condition. In terms of heat capacity, assuming that Cp is constant of T 1 and T 2. H = n. Cp(T 2 -T 1) For a monatomic gas, Cv = 3/2 R Cp = R + Cv Hess’ Law Clausius-Clapeyron equation CHEM 3310 39
 Change in enthalpy formula thermodynamics
Change in enthalpy formula thermodynamics 3310
3310 District 3310
District 3310 Ns 3310
Ns 3310 Rfc 3310
Rfc 3310 Fin 3310
Fin 3310 Difference between internal energy and enthalpy
Difference between internal energy and enthalpy Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Q mc ∆ t
Q mc ∆ t Enthalpy change in a potential energy diagram
Enthalpy change in a potential energy diagram Enthalpy change of combustion
Enthalpy change of combustion Qp=qv+nrt
Qp=qv+nrt Enthalpy change unit
Enthalpy change unit Knockhardy
Knockhardy Enthalpy of a reaction
Enthalpy of a reaction Enthalpy change definition
Enthalpy change definition Identify
Identify Unit of enthalpy change
Unit of enthalpy change Enthalpy definition
Enthalpy definition Zelinsky reaction
Zelinsky reaction Chem 30 data booklet
Chem 30 data booklet Cpa chem
Cpa chem Chem quiz.net
Chem quiz.net Lacl3
Lacl3 Ka and kb
Ka and kb Dipole dipole interaction
Dipole dipole interaction Sugar ior
Sugar ior Chem 109
Chem 109 Chem
Chem Chem
Chem Imf chem
Imf chem Molecular polarity
Molecular polarity Equilibrium in chemistry
Equilibrium in chemistry Types of equations chem worksheet 10-3
Types of equations chem worksheet 10-3 Ap chem equilibrium problems
Ap chem equilibrium problems Photoelectron spectroscopy pogil
Photoelectron spectroscopy pogil Ap chemistry entropy and free energy
Ap chemistry entropy and free energy Organometallic
Organometallic Metylocyklopentan
Metylocyklopentan Environics chempro x
Environics chempro x