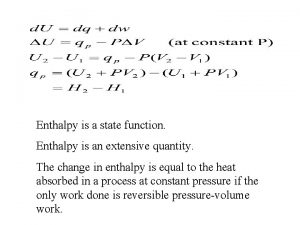
12 Thermodynamics 12 1 Types of Enthalpy Change























- Slides: 62

12 Thermodynamics 12. 1 Types of Enthalpy Change 12. 2 Born-Haber Cycles 12. 3 Enthalpy Changes – Enthalpy of Solution 12. 4 Mean Bond Enthalpy 12. 5 Entropy

12. 1 Enthalpy Change – Ionic Compounds Learning Objectives: 1. Describe what is meant by the term enthalpy change. 2. Describe the different types of enthalpy changes (formation, atomisation, ionisation energy, electron affinity, lattice formation, hydration, solution, bond enthalpy). 3. Calculate the enthalpy changes on forming ionic compounds.

Enthalpy Review • Enthalpy change is the heat change at constant pressure. • Standard conditions: 100 k. Pa, 298 K (starting temperature) • Remember that heat and temperature are not the same. • Heat is a type of energy and is measured in joules and heat changes lead to temperature changes, which is measure in Kelvins.

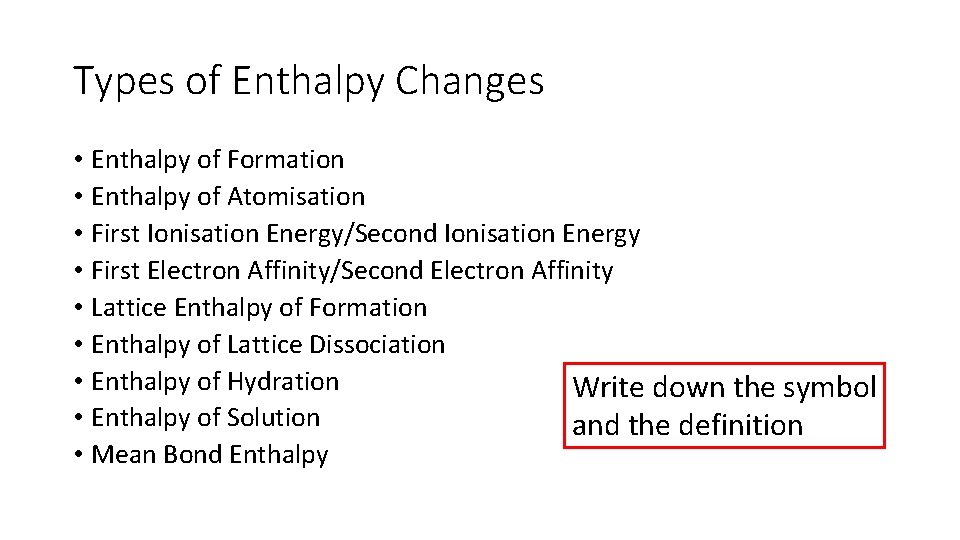
Types of Enthalpy Changes • Enthalpy of Formation • Enthalpy of Atomisation • First Ionisation Energy/Second Ionisation Energy • First Electron Affinity/Second Electron Affinity • Lattice Enthalpy of Formation • Enthalpy of Lattice Dissociation • Enthalpy of Hydration Write down the symbol • Enthalpy of Solution and the definition • Mean Bond Enthalpy

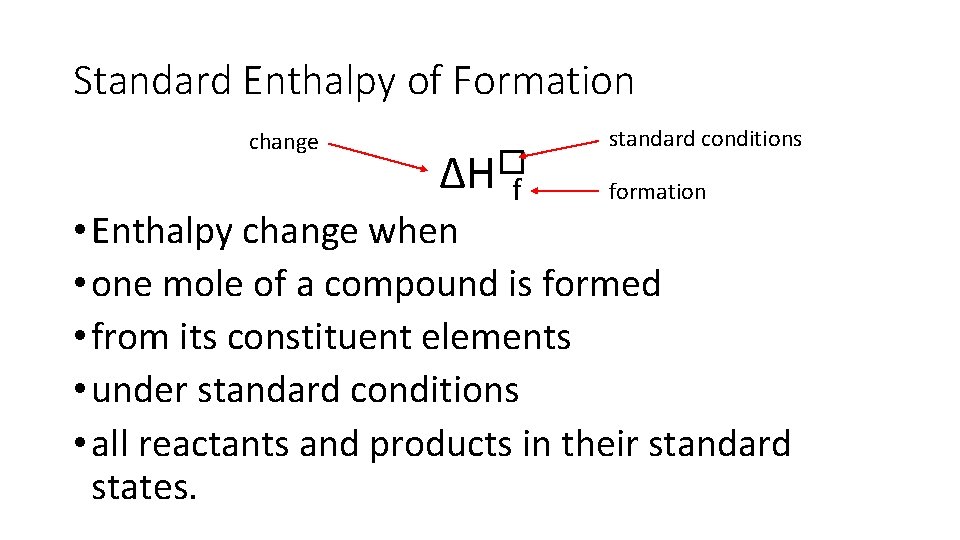
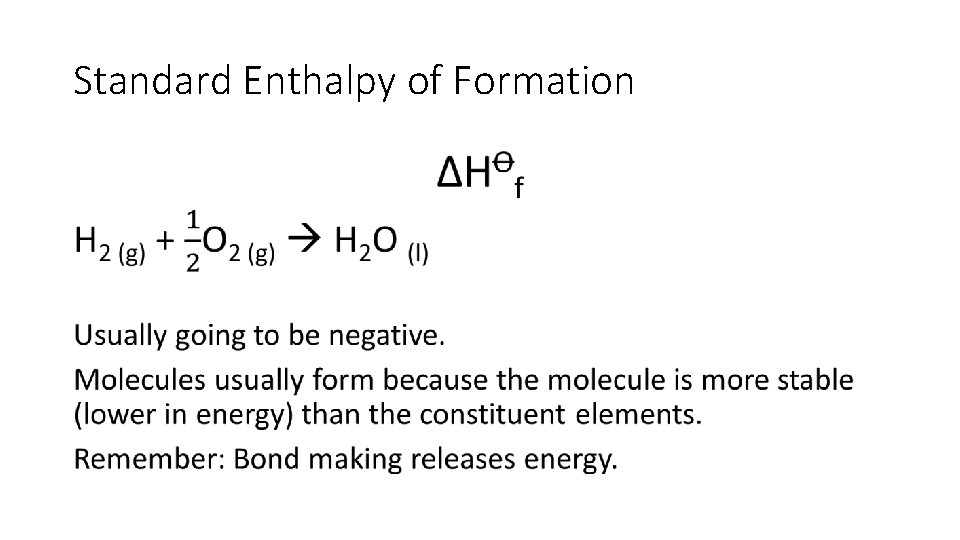
Standard Enthalpy of Formation change ∆H�f standard conditions formation • Enthalpy change when • one mole of a compound is formed • from its constituent elements • under standard conditions • all reactants and products in their standard states.

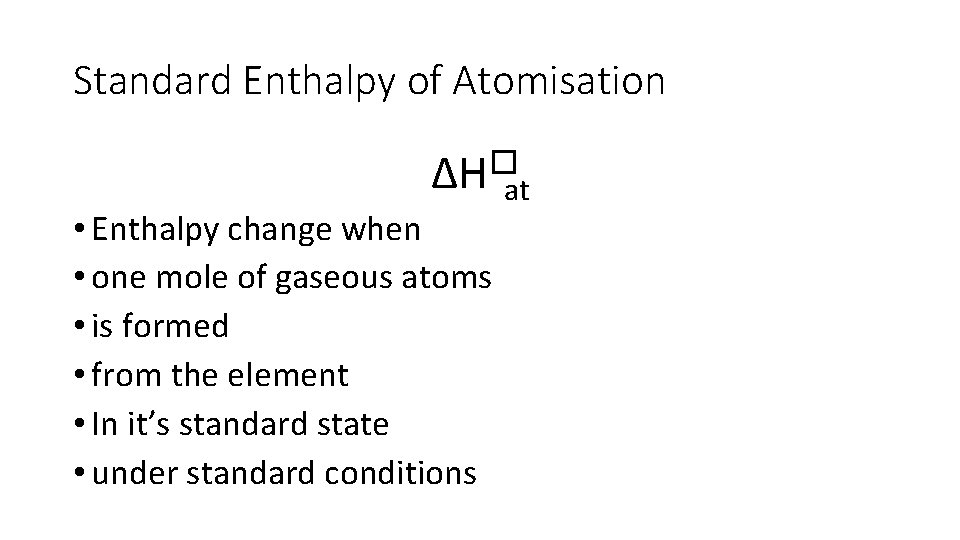
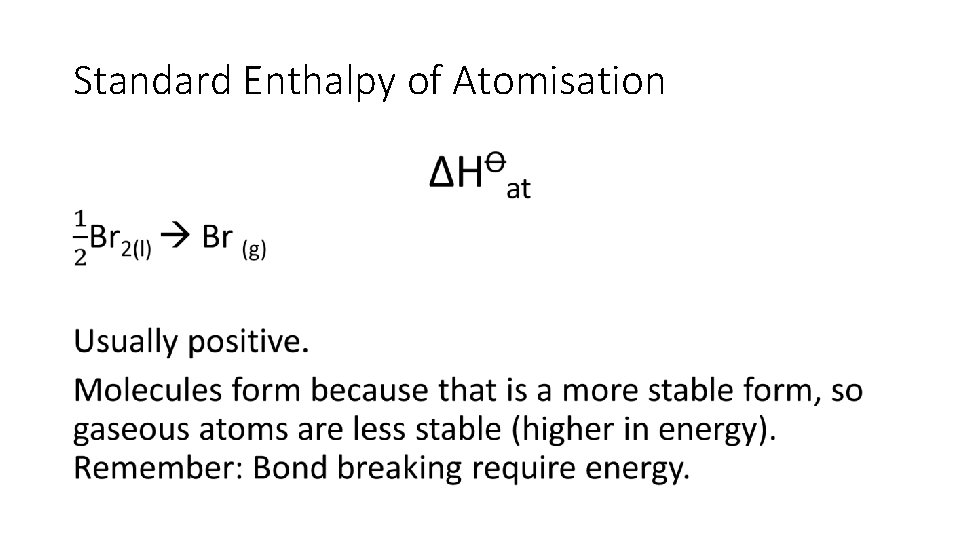
Standard Enthalpy of Atomisation ∆H�at • Enthalpy change when • one mole of gaseous atoms • is formed • from the element • In it’s standard state • under standard conditions

First Ionisation Energy First IE • Enthalpy change when • one mole of gaseous atoms • is converted into • one mole of gaseous +1 ions • under standard conditions

Second Ionisation Energy Second IE • Enthalpy change when • one mole of gaseous +1 ions • is converted into • one mole of gaseous +2 ions • under standard conditions

First Electron Affinity First ∆H�ea • Enthalpy change when • one mole of gaseous atoms • is converted into • one mole of gaseous -1 ions • under standard conditions

Second Electron Affinity Second ∆H�ea • Enthalpy change when • one mole of gaseous -1 ions • is converted into • one mole of gaseous -2 ions • under standard conditions

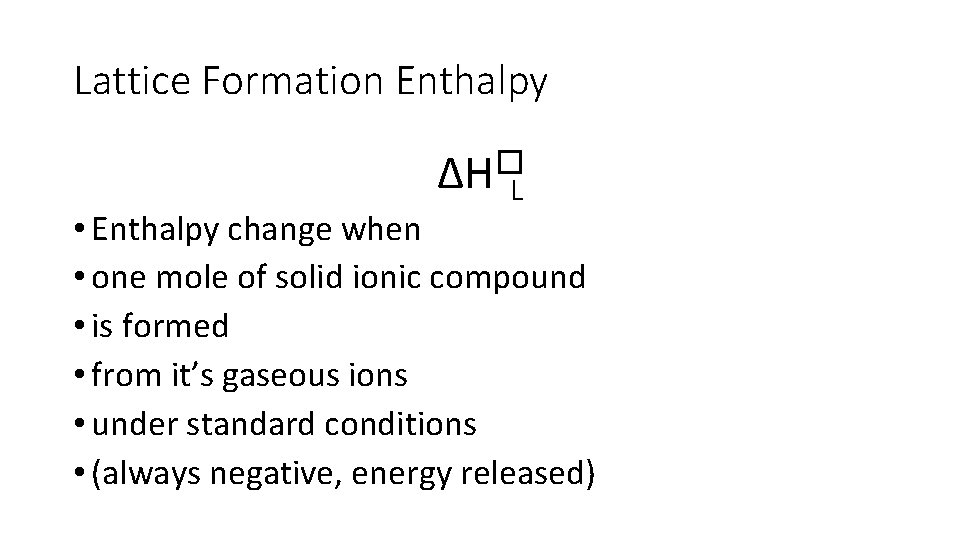
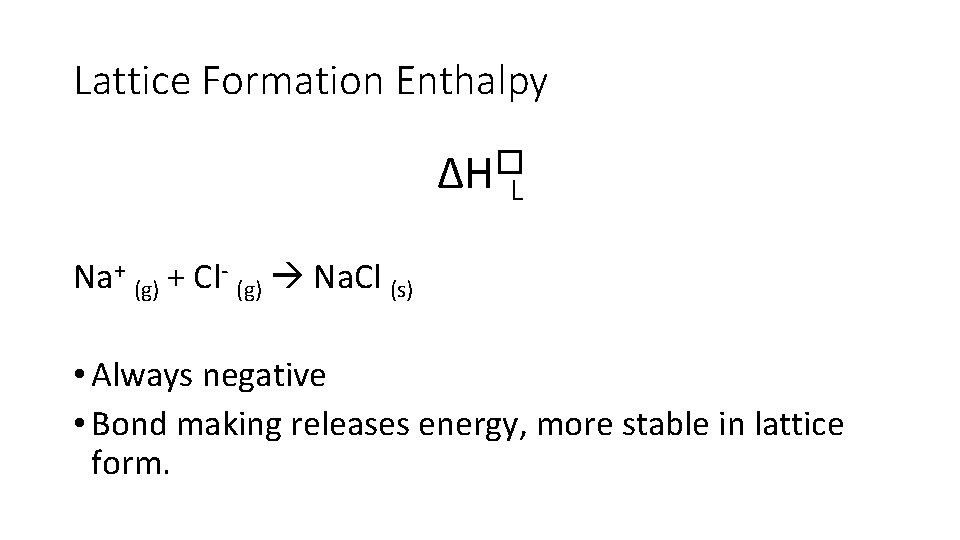
Lattice Formation Enthalpy ∆H�L • Enthalpy change when • one mole of solid ionic compound • is formed • from it’s gaseous ions • under standard conditions • (always negative, energy released)

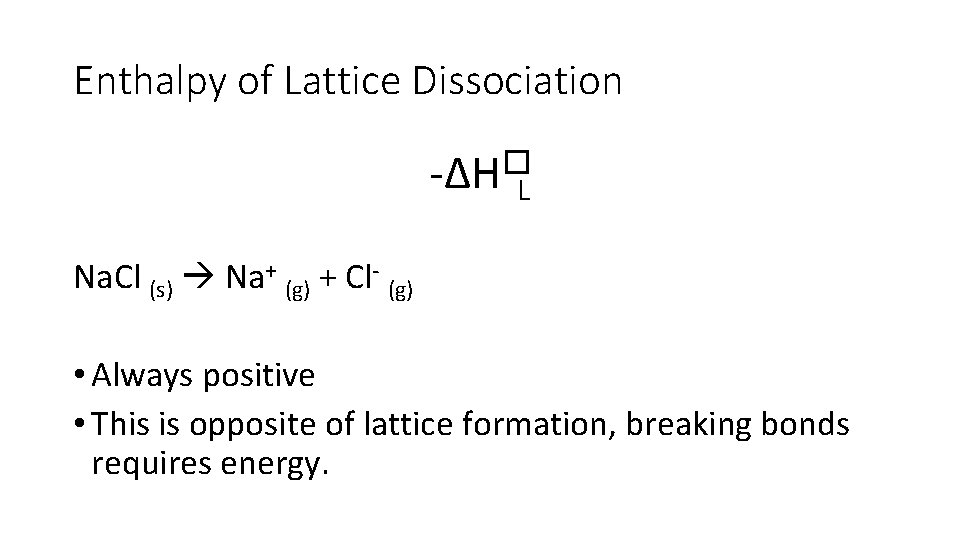
Enthalpy of Lattice Dissociation -∆H�L • Enthalpy change when • one mole of solid ionic compound • dissociates into • it’s gaseous ions • under standard conditions • (always positive, energy is absorbed)

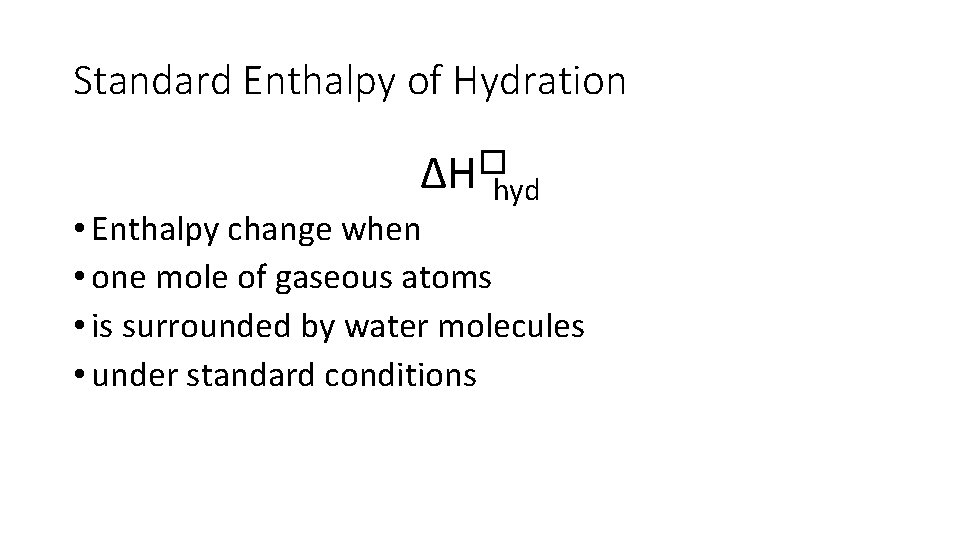
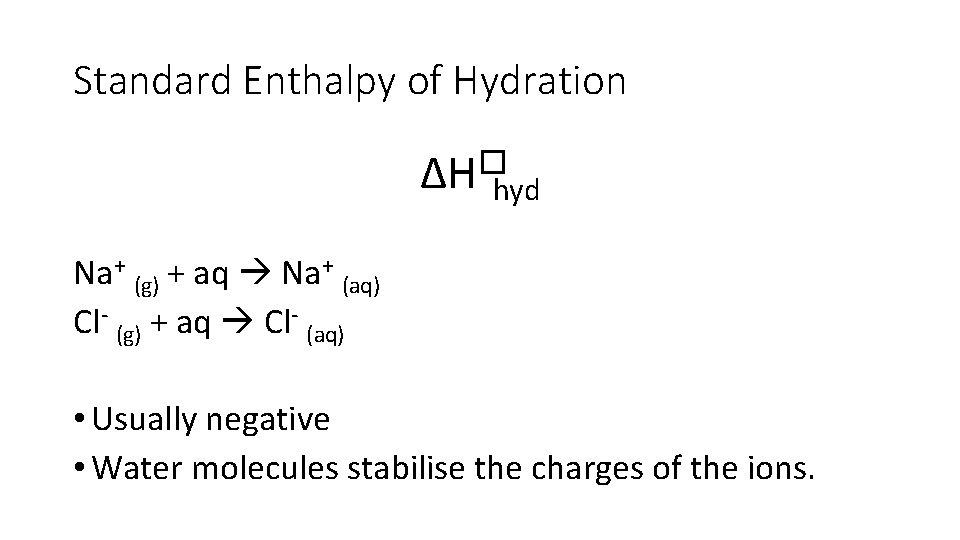
Standard Enthalpy of Hydration ∆H�hyd • Enthalpy change when • one mole of gaseous atoms • is surrounded by water molecules • under standard conditions

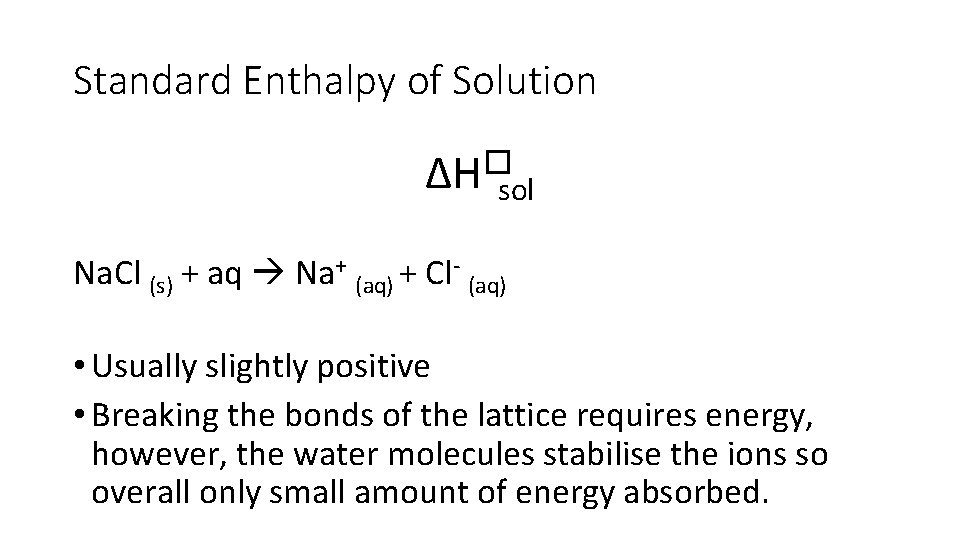
Standard Enthalpy of Solution ∆H�sol • Enthalpy change when • one mole of solute • completely dissolves • in sufficient solvent to form a solution in which the molecules are ions do not interact • under standard conditions

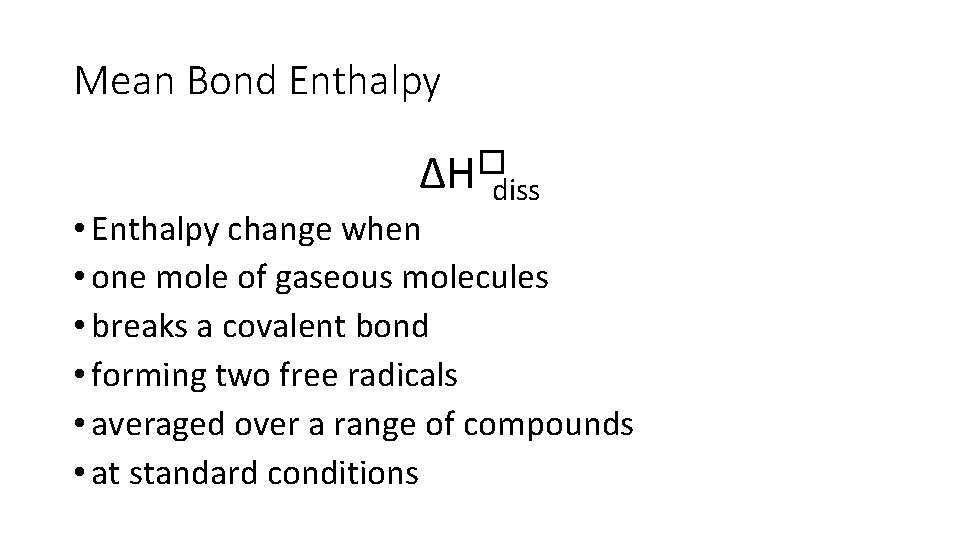
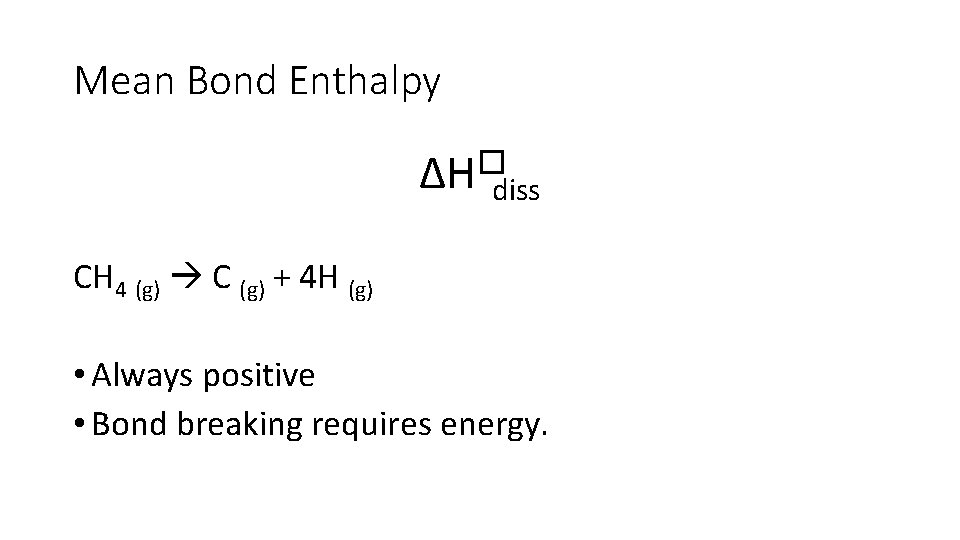
Mean Bond Enthalpy ∆H�diss • Enthalpy change when • one mole of gaseous molecules • breaks a covalent bond • forming two free radicals • averaged over a range of compounds • at standard conditions

For each type… a) Write an equation to represent the chemical reaction being described b) Tell me if the process is likely to be positive or negative c) Explain why.

Standard Enthalpy of Formation •

Standard Enthalpy of Atomisation •

First Ionisation Energy First IE Na (g) Na+(g) + e- • Positive • Removing an electron takes energy

Second Ionisation Energy Second IE Na+ (g) Na 2+(g) + e- • Very positive • Removing electron from positive ion require a lot of energy.

First Electron Affinity First ∆H�ea O (g) + e- O- (g) • Usually Negative • Energy is gained when electrons are added.

Second Electron Affinity O- (g) + e- O 2 - (g) Second ∆H�ea • Usually Positive • Because of repulsion, adding the second electron requires more energy than is gained.

Lattice Formation Enthalpy ∆H�L Na+ (g) + Cl- (g) Na. Cl (s) • Always negative • Bond making releases energy, more stable in lattice form.

Enthalpy of Lattice Dissociation -∆H�L Na. Cl (s) Na+ (g) + Cl- (g) • Always positive • This is opposite of lattice formation, breaking bonds requires energy.

Standard Enthalpy of Hydration ∆H�hyd Na+ (g) + aq Na+ (aq) Cl- (g) + aq Cl- (aq) • Usually negative • Water molecules stabilise the charges of the ions.

Standard Enthalpy of Solution ∆H�sol Na. Cl (s) + aq Na+ (aq) + Cl- (aq) • Usually slightly positive • Breaking the bonds of the lattice requires energy, however, the water molecules stabilise the ions so overall only small amount of energy absorbed.

Mean Bond Enthalpy ∆H�diss CH 4 (g) C (g) + 4 H (g) • Always positive • Bond breaking requires energy.

12. 2 Born-Haber Cycles Learning Objectives: 1. Describe Hess’ Law. 2. Use Born-Haber Cycles to calculate enthalpy changes

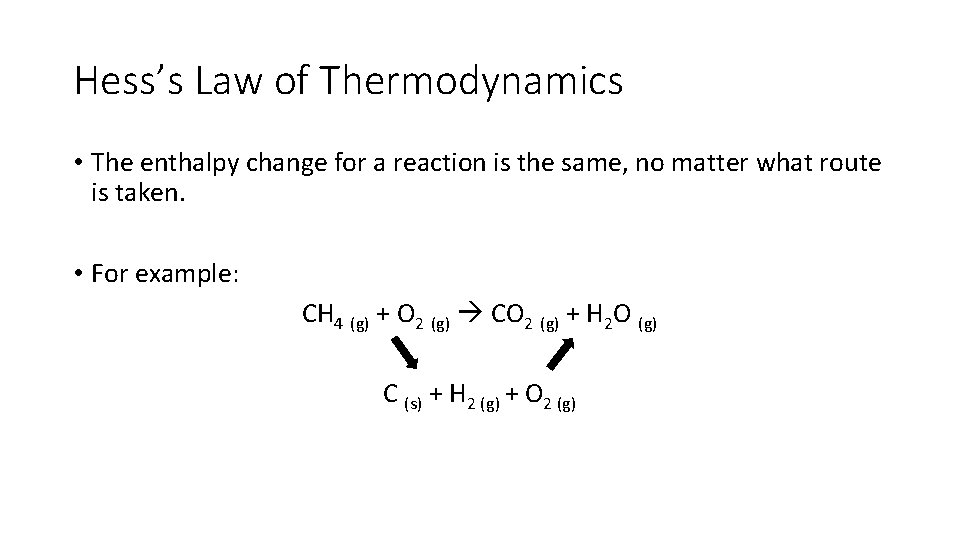
Hess’s Law of Thermodynamics • The enthalpy change for a reaction is the same, no matter what route is taken. • For example: CH 4 (g) + O 2 (g) CO 2 (g) + H 2 O (g) C (s) + H 2 (g) + O 2 (g)

Born-Haber Cycles • Born-Haber Cycles are just another method to solve for the unknown enthalpy change of a chemical reaction by using enthalpy changes that we DO know. • It uses a diagram to represent the enthalpy changes on a vertical scale. Increases in energy are UP ( ) arrows, decreases in energy are DOWN ( )arrows.

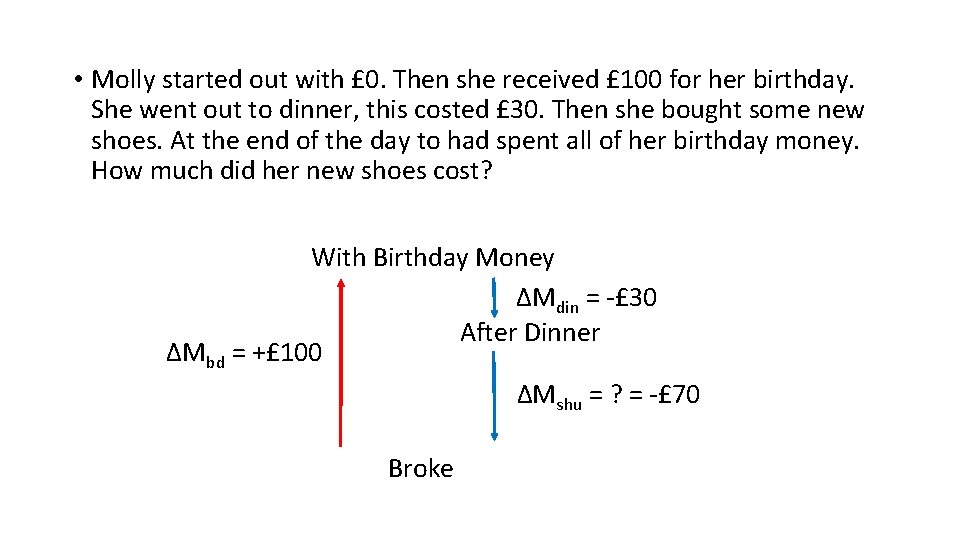
• Molly started out with £ 0. Then she received £ 100 for her birthday. She went out to dinner, this costed £ 30. Then she bought some new shoes. At the end of the day to had spent all of her birthday money. How much did her new shoes cost? With Birthday Money ∆Mdin = -£ 30 After Dinner ∆Mbd = +£ 100 ∆Mshu = ? = -£ 70 Broke

Formation of an Ionic Compound • Electrons are transferred to atoms to form ions. • Ions then attract and are arranged into an ionic lattice. • This is how ionic lattices are formed.

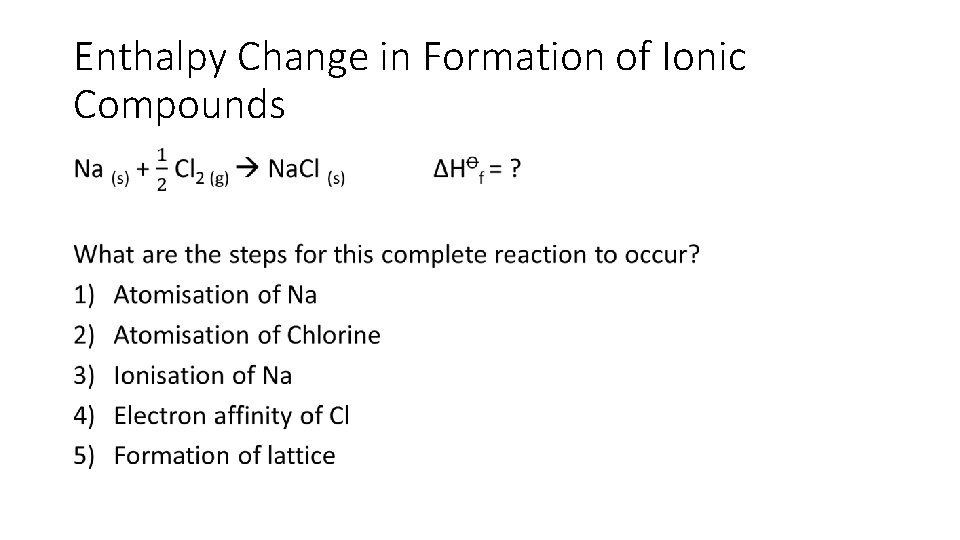
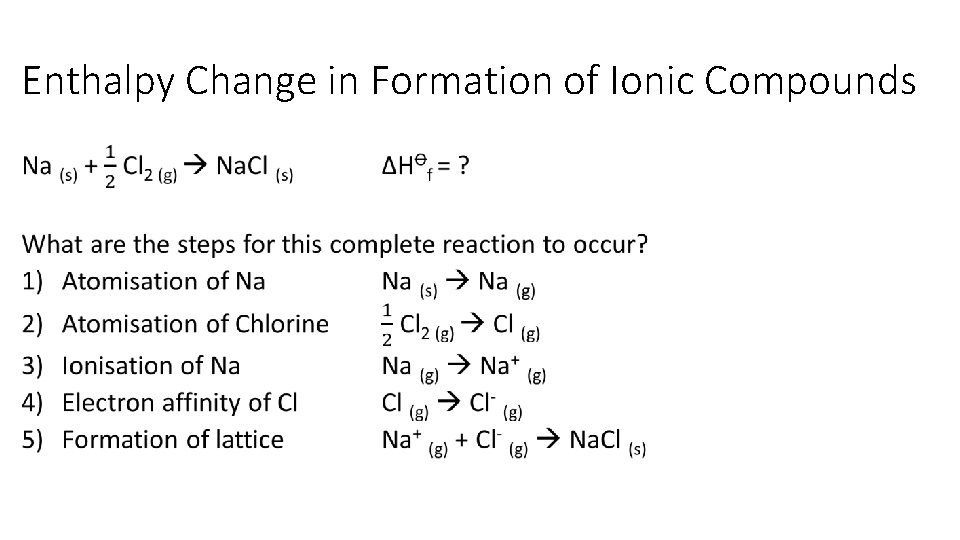
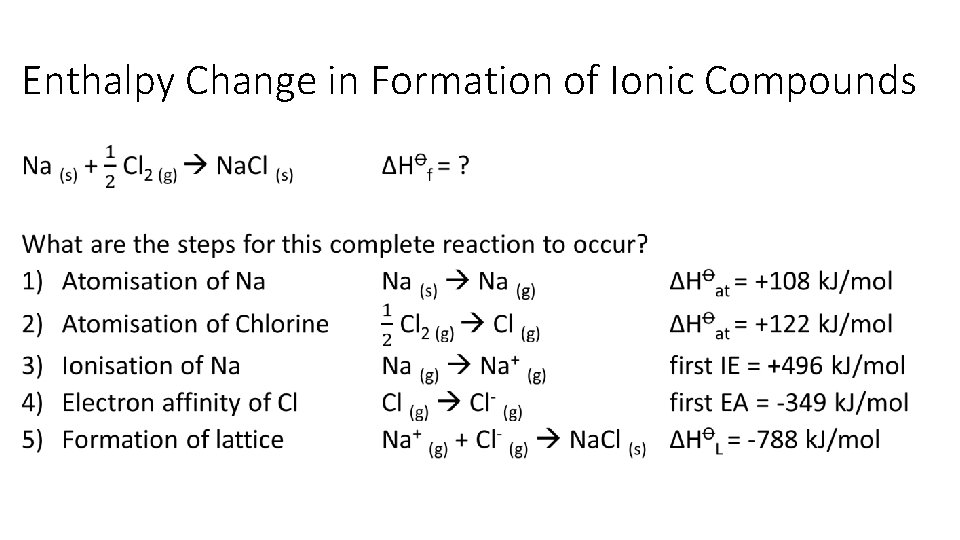
Enthalpy Change in Formation of Ionic Compounds •

Enthalpy Change in Formation of Ionic Compounds •

Enthalpy Change in Formation of Ionic Compounds •

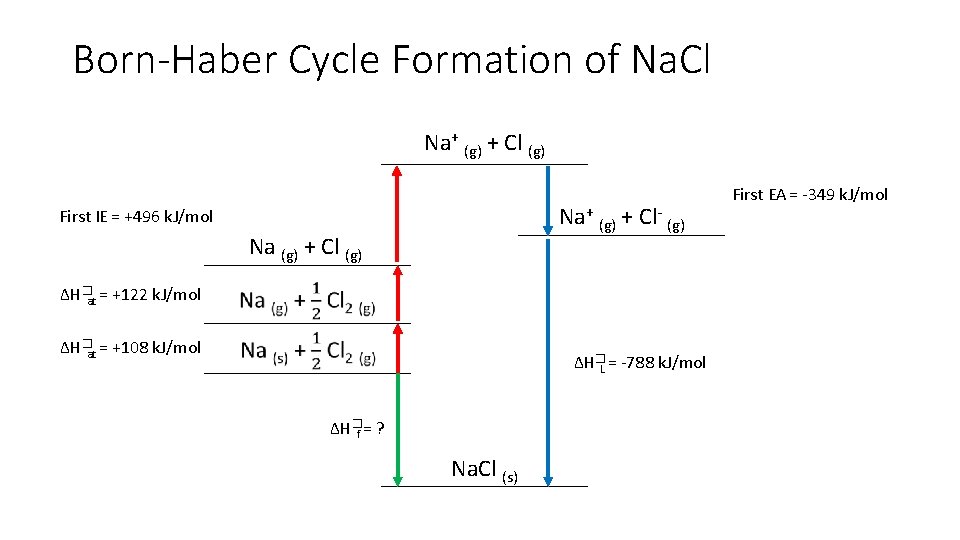
Born-Haber Cycle Formation of Na. Cl Na+ (g) + Cl (g) Na+ (g) + Cl- (g) First IE = +496 k. J/mol Na (g) + Cl (g) ∆H�at = +122 k. J/mol ∆H�at = +108 k. J/mol ∆H�L = -788 k. J/mol ∆H�f = ? Na. Cl (s) First EA = -349 k. J/mol

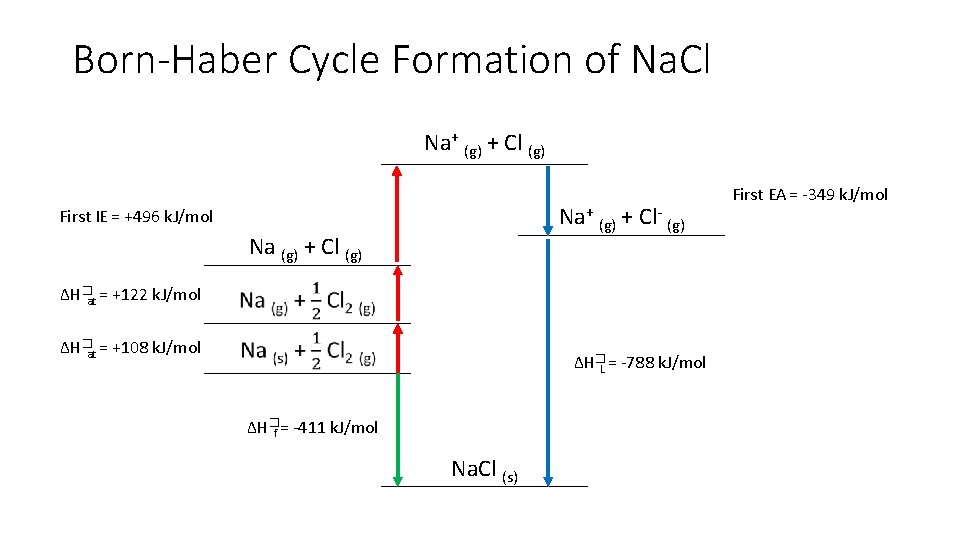
Born-Haber Cycle Formation of Na. Cl Na+ (g) + Cl (g) Na+ (g) + Cl- (g) First IE = +496 k. J/mol Na (g) + Cl (g) ∆H�at = +122 k. J/mol ∆H�at = +108 k. J/mol ∆H�L = -788 k. J/mol ∆H�f = -411 k. J/mol Na. Cl (s) First EA = -349 k. J/mol

Example: Lattice Formation Enthalpy of Mg. Cl 2 • Write out the overall equation for the formation of magnesium chloride. • Write equations for all of the steps in the formation of magnesium chloride. • HINT: there are six steps

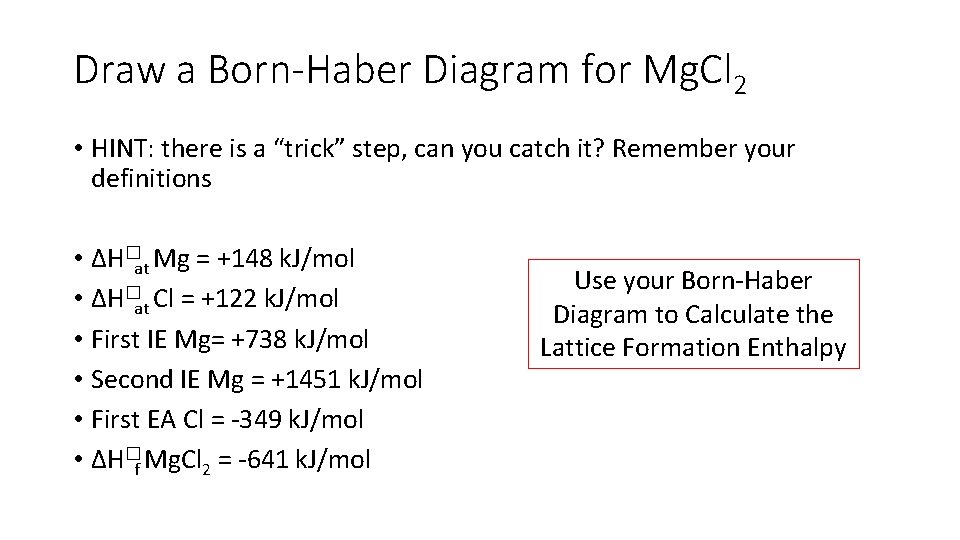
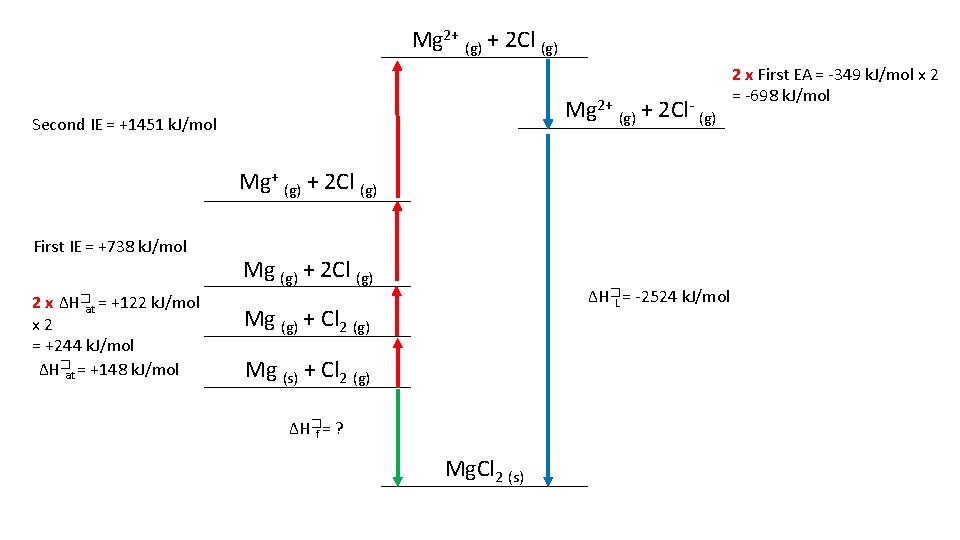
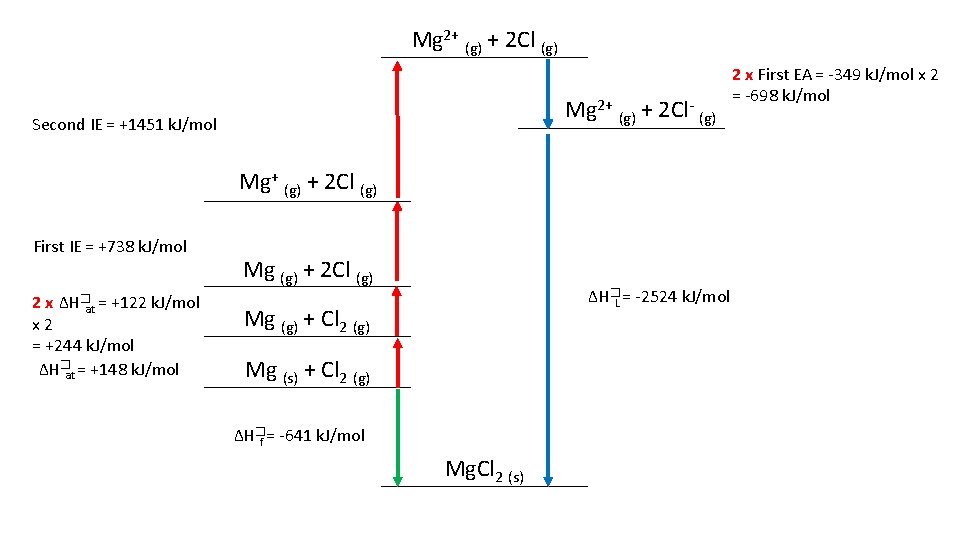
Draw a Born-Haber Diagram for Mg. Cl 2 • HINT: there is a “trick” step, can you catch it? Remember your definitions • ∆H�at Mg = +148 k. J/mol • ∆H�at Cl = +122 k. J/mol • First IE Mg= +738 k. J/mol • Second IE Mg = +1451 k. J/mol • First EA Cl = -349 k. J/mol • ∆H�f Mg. Cl 2 = -641 k. J/mol Use your Born-Haber Diagram to Calculate the Lattice Formation Enthalpy

Mg 2+ (g) + 2 Cl (g) Mg 2+ (g) + 2 Cl- (g) Second IE = +1451 k. J/mol Mg+ (g) + 2 Cl (g) First IE = +738 k. J/mol 2 x ∆H�at = +122 k. J/mol x 2 = +244 k. J/mol ∆H�at = +148 k. J/mol Mg (g) + 2 Cl (g) ∆H�L = -2524 k. J/mol Mg (g) + Cl 2 (g) Mg (s) + Cl 2 (g) ∆H�f = ? Mg. Cl 2 (s) 2 x First EA = -349 k. J/mol x 2 = -698 k. J/mol

Mg 2+ (g) + 2 Cl (g) Mg 2+ (g) + 2 Cl- (g) Second IE = +1451 k. J/mol Mg+ (g) + 2 Cl (g) First IE = +738 k. J/mol 2 x ∆H�at = +122 k. J/mol x 2 = +244 k. J/mol ∆H�at = +148 k. J/mol Mg (g) + 2 Cl (g) ∆H�L = -2524 k. J/mol Mg (g) + Cl 2 (g) Mg (s) + Cl 2 (g) ∆H�f = -641 k. J/mol Mg. Cl 2 (s) 2 x First EA = -349 k. J/mol x 2 = -698 k. J/mol

12. 3 More Enthalpy Changes Learning Objectives: 1. Calculate enthalpy change of solution. 2. Describe how lattice enthalpy calculations support models for ionic bonding. 3. Explain how ions can become polarised.

Enthalpy of Solution • Ionic solids can dissolve in polar solvents. • This is called hydration if the solvent is water. • Hydration is when the water molecules surround ions. • What are the steps for process of forming a solution? 1. Breaking the ionic lattice (enthalpy of lattice dissociation). 2. Hydrating the positive ions (enthalpy of hydration). 3. Hydrating the negative ions (enthalpy of hydration).
Example: Na. Cl

Ionic Bonding Models • For most ionic compounds theoretical values calculated from Born-Haber cycles agrees with experimental values. • This proves that the model for ionic bonding (lattice) is correct. • However, some ionic compounds have theoretical and experimental values that DO NOT agree. • Another model needed to be found to explain these discrepancies.

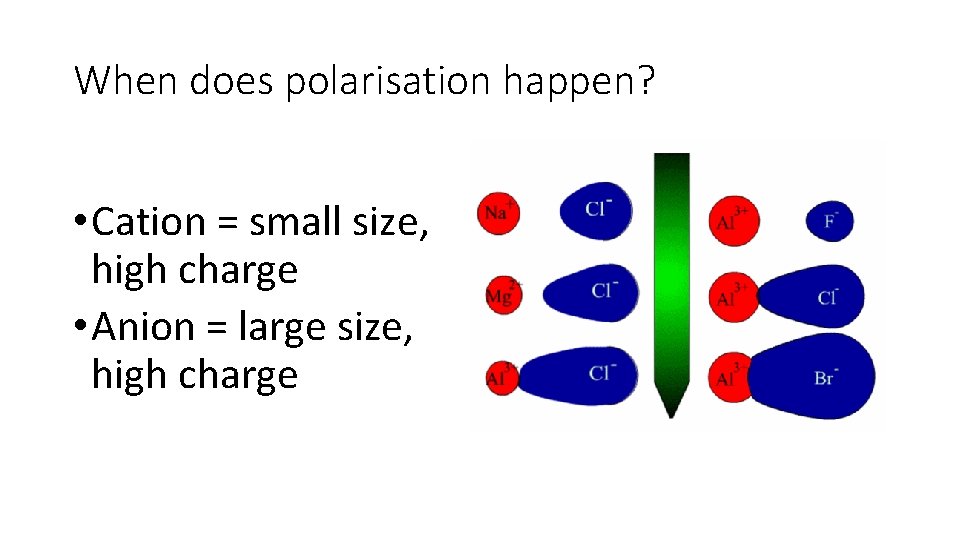
Polarisation • Zn. Se • experimental lattice formation enthalpy = -3611 k. J/mol • theoretical lattice formation enthalpy = -3305 k. J/mol WHY? • Zn 2+ is very small and has a high + charge • Se 2 - is very large and has a high – charge • Zn 2+ moves closely to electron density of Se 2 - and attracts the e • Since Se 2 - is large, the e- are far from the nucleus and easily pulled away • This distorts the electron cloud surrounding Se 2 -

Polarisation • The distortion causes their to be some electron density shared between the two ions (slightly covalent nature). • The Se 2 - ion is said to be polarised. • This causes the enthalpy change to be greater than expected.

When does polarisation happen? • Cation = small size, high charge • Anion = large size, high charge

12. 4 Mean Bond Enthalpy Learning Objectives: 1. Explain the term mean bond enthalpy. 2. Calculate enthalpy changes using mean bond enthalpy. 3. Explain why this method is not as accurate.

Mean Bond Enthalpy • The average bond enthalpy term is the average amount of energy needed to break a specific covalent bond, measured over a wide variety of different molecules. • A measure of strength of a covalent bond. • In comparison, lattice enthalpy is a measure of the strength of an ionic bond.

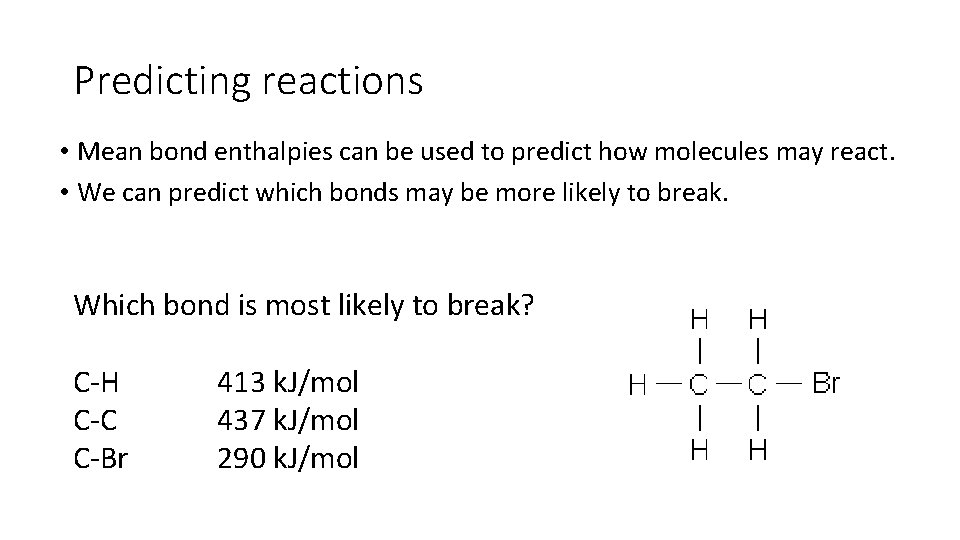
Predicting reactions • Mean bond enthalpies can be used to predict how molecules may react. • We can predict which bonds may be more likely to break. Which bond is most likely to break? C-H C-C C-Br 413 k. J/mol 437 k. J/mol 290 k. J/mol

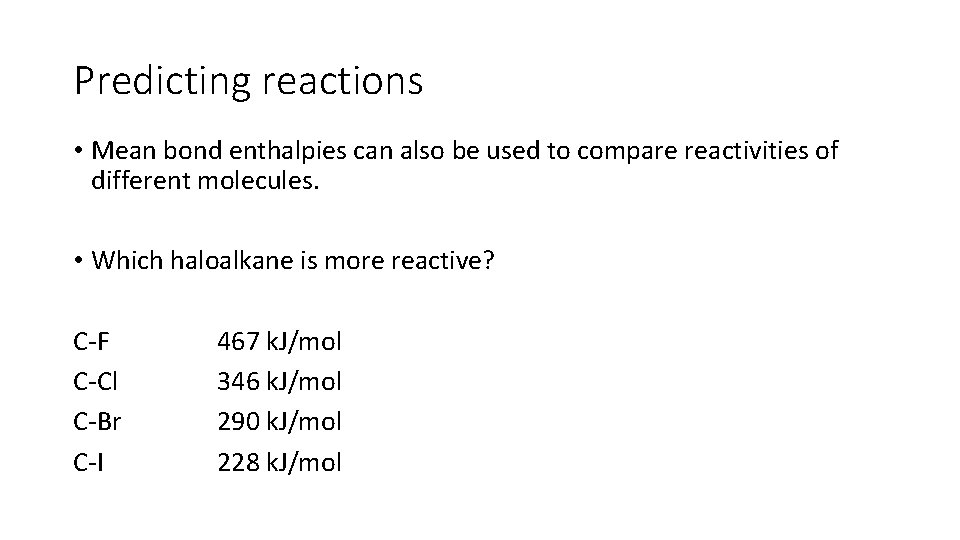
Predicting reactions • Mean bond enthalpies can also be used to compare reactivities of different molecules. • Which haloalkane is more reactive? C-F C-Cl C-Br C-I 467 k. J/mol 346 k. J/mol 290 k. J/mol 228 k. J/mol

Calculating Approximate Enthalpy Changes • Hess’s Law can be applied. • One possible route to products would be to break all bonds in the reactants and then form all of the bonds for the products. • The enthalpies for these two processes can then be summed up to find the total enthalpy change. • Remember: bond breaking requires energy (+ value) bond formation releases energy (- value)



12. 5 Why do chemical reactions take place? Learning Objectives: 1. Explain the concept of entropy. 2. Calculate using enthalpy and entropy whether a reaction will spontaneously occur. 3. Analyse the effects of temperature on feasibility of a reaction.

Is a reaction feasible or spontaneous? • Reactions that will take place on their own are called spontaneous. • If it is possible for a reaction to take place on their own, the reaction is feasible. • What determines if a reaction is feasible? • If ΔH (enthalpy) is negative, the reaction is exothermic • If ΔS (entropy) is positive, the reaction increases in randomness

Entropy • Entropy is a mathematical measure of the randomness of a system. • Change in entropy is represented as ΔS. • The universe prefers randomness (higher entropy) and is always moving towards disorder. • Values for entropy of different substances are determined mathematically, you will not be expected to calculate these, only how to use them. (see pg. 179)

Calculating Entropy Changes • Calculate the difference in entropy from reactants to products to find the ΔS of a reaction. ΔS = Sproducts – Sreactants • If ΔS is positive, entropy is increasing, disorder is increasing. The products are more disordered than the reactants. • If ΔS is negative, entropy is decreasing, disorder is decreasing. The products are less disordered than the reactants.

Gibbs Free Energy • ΔG represents the Gibbs free energy and combines both enthalpy and entropy. • It is used to determine whether or not a reaction is feasible. ΔG = ΔH – TΔS • If ΔG is negative (-) the reaction is feasible. • If ΔG is positive (+) the reaction is NOT feasible.

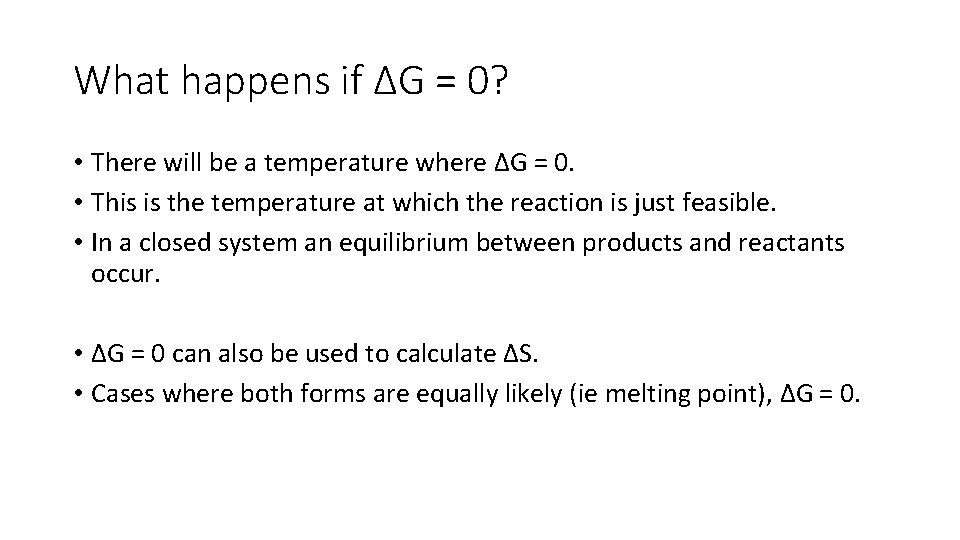
What happens if ΔG = 0? • There will be a temperature where ΔG = 0. • This is the temperature at which the reaction is just feasible. • In a closed system an equilibrium between products and reactants occur. • ΔG = 0 can also be used to calculate ΔS. • Cases where both forms are equally likely (ie melting point), ΔG = 0.

Thermodynamics does not predict the rate of a reaction • Thermodynamics = Kinetics • Thermodynamics only predicts whether a reaction is feasible. • It DOES NOT predict how quickly the reaction may take place. • Kinetics is the branch of chemistry dealing with rate of reaction.
 Enthalpy of atomisation
Enthalpy of atomisation Enthalpy vs internal energy
Enthalpy vs internal energy Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Unit of enthalpy change
Unit of enthalpy change Enthalpy change in a potential energy diagram
Enthalpy change in a potential energy diagram How to do enthalpy change
How to do enthalpy change Enthalpy change definition
Enthalpy change definition Enthalpy change definition
Enthalpy change definition Enthalpy change definition
Enthalpy change definition How to calculate change in h
How to calculate change in h Enthalpy change of combustion definition a level
Enthalpy change of combustion definition a level Communicating enthalpy changes
Communicating enthalpy changes Q = mc∆t
Q = mc∆t What is the overall enthalpy change dhrxn for the system?
What is the overall enthalpy change dhrxn for the system? Examples for physical change
Examples for physical change Physical changes of matter
Physical changes of matter Absolute change and relative change formula
Absolute change and relative change formula Integer number definition
Integer number definition Difference between chemical and physical changes
Difference between chemical and physical changes Quantity supplied vs supply
Quantity supplied vs supply Change in supply and change in quantity supplied
Change in supply and change in quantity supplied Enagic founder
Enagic founder Proactive vs reactive change
Proactive vs reactive change Physical change and chemical change
Physical change and chemical change Spare change physical versus chemical change
Spare change physical versus chemical change Rocks change due to temperature and pressure change
Rocks change due to temperature and pressure change Whats the difference between chemical and physical change
Whats the difference between chemical and physical change How does a physical change differ from a chemical change
How does a physical change differ from a chemical change How does a physical change differ from a chemical change?
How does a physical change differ from a chemical change? First order vs second order change
First order vs second order change Chopping wood physical or chemical
Chopping wood physical or chemical Climate change 2014 mitigation of climate change
Climate change 2014 mitigation of climate change Bond energy worksheet
Bond energy worksheet Sensible enthalpy formula
Sensible enthalpy formula Measuring and expressing enthalpy changes
Measuring and expressing enthalpy changes Enthalpy of formation hess law
Enthalpy of formation hess law Enthalpy of melting of ice
Enthalpy of melting of ice Economy of a multiple effect evaporator depends upon the
Economy of a multiple effect evaporator depends upon the Enthalpy state function
Enthalpy state function What is enthalpy and entropy
What is enthalpy and entropy Enthalpy entropy free energy
Enthalpy entropy free energy Enthalpy of reactants and products
Enthalpy of reactants and products Enthalpy and heat equation
Enthalpy and heat equation Minimum enthalpy and maximum entropy
Minimum enthalpy and maximum entropy Satuan enthalpy
Satuan enthalpy Thermochemistry calorimetry
Thermochemistry calorimetry Enthalpy exo or endo
Enthalpy exo or endo Does a catalyst affect enthalpy
Does a catalyst affect enthalpy Enthalpy vs internal energy
Enthalpy vs internal energy First law of thermodynamics control mass
First law of thermodynamics control mass Relationship between entropy and free energy
Relationship between entropy and free energy Entropy vs enthalpy
Entropy vs enthalpy Enthalpy entropy free energy
Enthalpy entropy free energy Enthalpy of melting of ice
Enthalpy of melting of ice Bond enthalpy definition ib
Bond enthalpy definition ib Enthalpy vs entropy
Enthalpy vs entropy Lesson 2: measuring and expressing enthalpy changes
Lesson 2: measuring and expressing enthalpy changes Application of hess law
Application of hess law Enthalpy of combustion formula ib
Enthalpy of combustion formula ib Absolute entropy
Absolute entropy Molar enthalpy units
Molar enthalpy units What is molar enthalpy of fusion
What is molar enthalpy of fusion Enthalpy of formation table
Enthalpy of formation table