Endothermic Exothermic Reactions Enthalpy H Heat Enthalpy Change





- Slides: 11

Endothermic & Exothermic Reactions • Enthalpy- (H) Heat • Enthalpy Change- (ΔH) The change in heat for a reaction

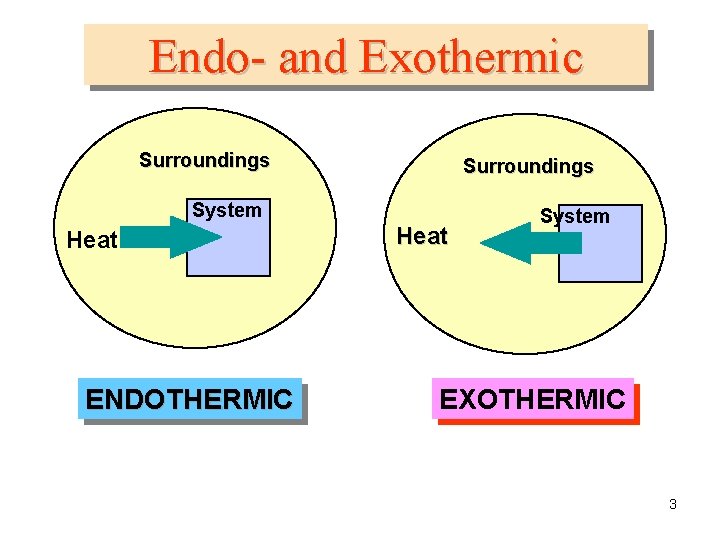
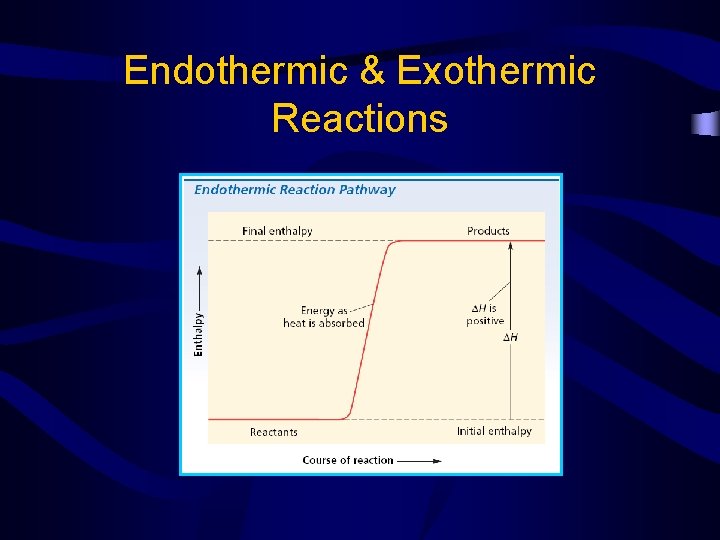
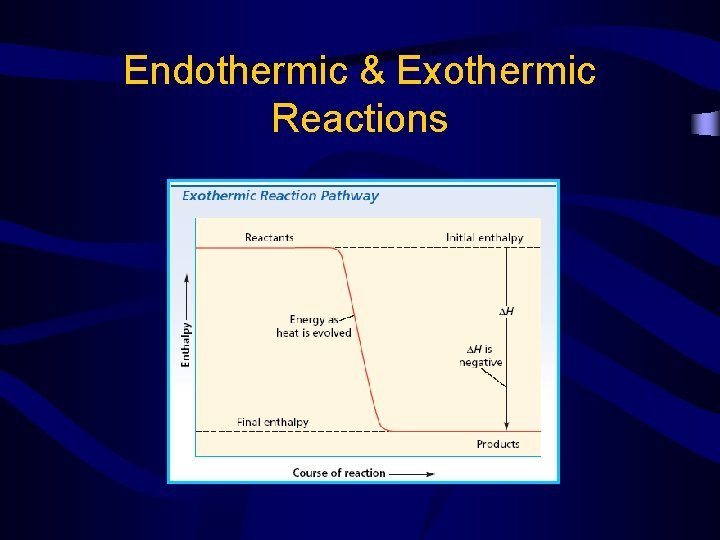
• Endothermic reactions – – – Energy is absorbed Heat goes into the system from the surroundings will feel colder Temperature of surroundings goes down +ΔH • Exothermic reactions – – energy is released Heat goes out of the system into the surroundings Temperature of the surrounding will increase -∆H

Endo- and Exothermic Surroundings System Heat ENDOTHERMIC Heat System EXOTHERMIC 3

Endothermic & Exothermic Reactions

Endothermic & Exothermic Reactions

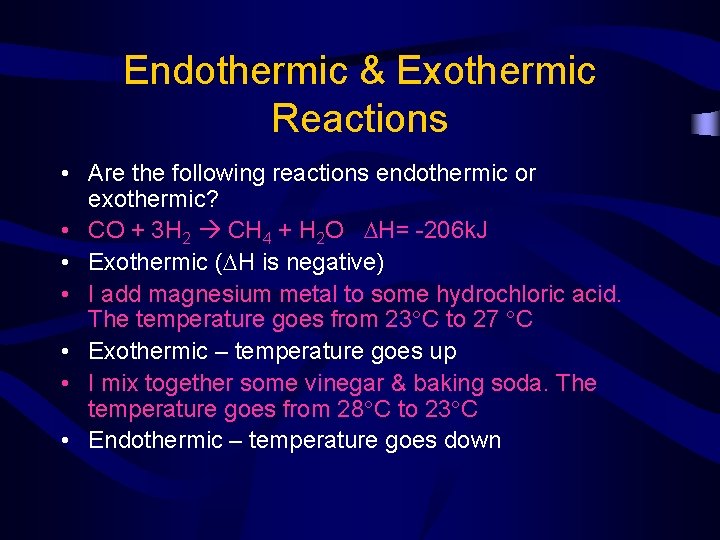
Endothermic & Exothermic Reactions • Are the following reactions endothermic or exothermic? • CO + 3 H 2 CH 4 + H 2 O H= -206 k. J • Exothermic ( H is negative) • I add magnesium metal to some hydrochloric acid. The temperature goes from 23 C to 27 C • Exothermic – temperature goes up • I mix together some vinegar & baking soda. The temperature goes from 28 C to 23 C • Endothermic – temperature goes down

Thermochemical equations • An equation that gives us information about H … S + O 2 SO 2 H = – 296. 9 k. J • If we change the equation, then the H also changes … SO 2 S + O 2 H = + 296. 9 k. J • If the reaction is reversed the sign is reversed • Also, if the coefficients in the equation change, so will the amount of energy produced/absorbed: 2 S + 2 O 2 2 SO 2 H = – 593. 8 k. J

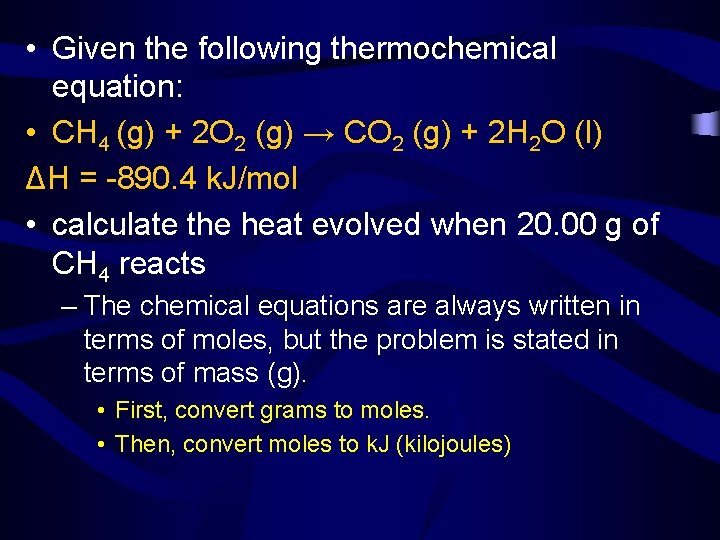
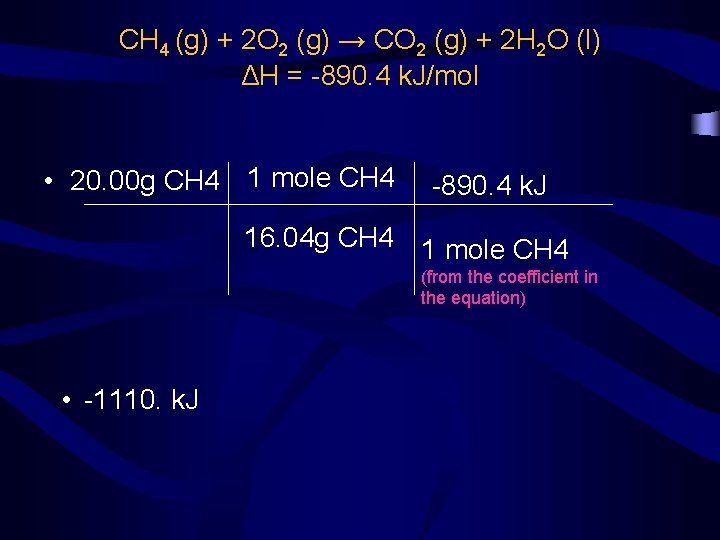
• Given the following thermochemical equation: • CH 4 (g) + 2 O 2 (g) → CO 2 (g) + 2 H 2 O (l) ΔH = -890. 4 k. J/mol • calculate the heat evolved when 20. 00 g of CH 4 reacts – The chemical equations are always written in terms of moles, but the problem is stated in terms of mass (g). • First, convert grams to moles. • Then, convert moles to k. J (kilojoules)

CH 4 (g) + 2 O 2 (g) → CO 2 (g) + 2 H 2 O (l) ΔH = -890. 4 k. J/mol • 20. 00 g CH 4 1 mole CH 4 -890. 4 k. J 16. 04 g CH 4 1 mole CH 4 (from the coefficient in the equation) • -1110. k. J

CH 4 (g) + 2 O 2 (g) � → CO 2 (g) + 2 H 2 O (l) ∆H = - 890. 4 k. J • How much energy is given off when 2. 00 mol of CH 4 are burned? • How much energy is released when 22. 4 g of CH 4 are burned? • If you were to attempt to make 45. 0 g of methane from CO 2 and H 2 O , how much energy would be required?

• -1780 k. J • -1240 k. J • 2. 50 x 103 k. J
 Thermic reaction
Thermic reaction Exothermic process
Exothermic process Phase change diagram endothermic exothermic
Phase change diagram endothermic exothermic Is vaporization endothermic or exothermic
Is vaporization endothermic or exothermic Exothermic vs endothermic
Exothermic vs endothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic Heating cooling curve
Heating cooling curve How to determine exothermic or endothermic
How to determine exothermic or endothermic Ectotherms
Ectotherms Endothermic and exothermic worksheet
Endothermic and exothermic worksheet Exothermic and endothermic homework
Exothermic and endothermic homework