Energy Changes Phase Changes Heating Cooling Curves It

















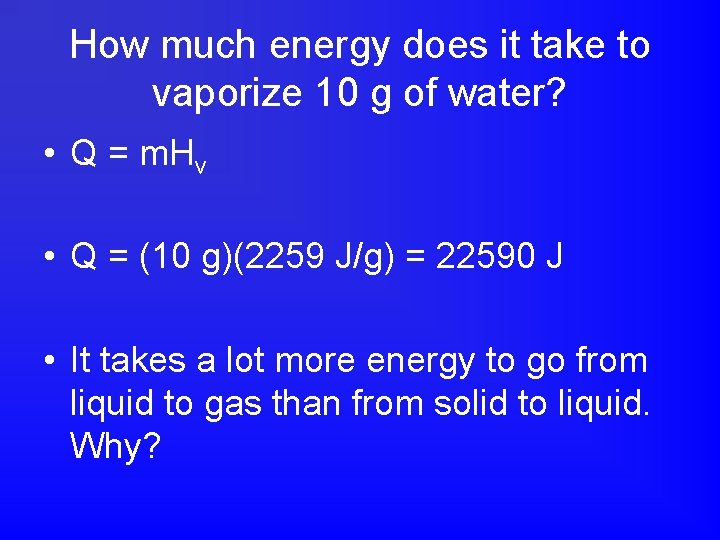

- Slides: 31

Energy Changes & Phase Changes Heating & Cooling Curves

It takes energy to heat stuff up! • For a pure substance in a single phase, we can calculate how much using Q = m. C T. – Q = energy in Joules – m = mass in grams – C = specific heat capacity – T = change in temperature = Tf - Ti • On the other hand, when something cools down, energy is released!

Q = m. C T • C = specific heat capacity = amount of heat required to raise the temperature of 1 gram of a pure substance by 1 C. • C is a physical constant. It is unique for every pure substance. • Values of C are tabulated. • CH 2 O = 4. 2 J/g

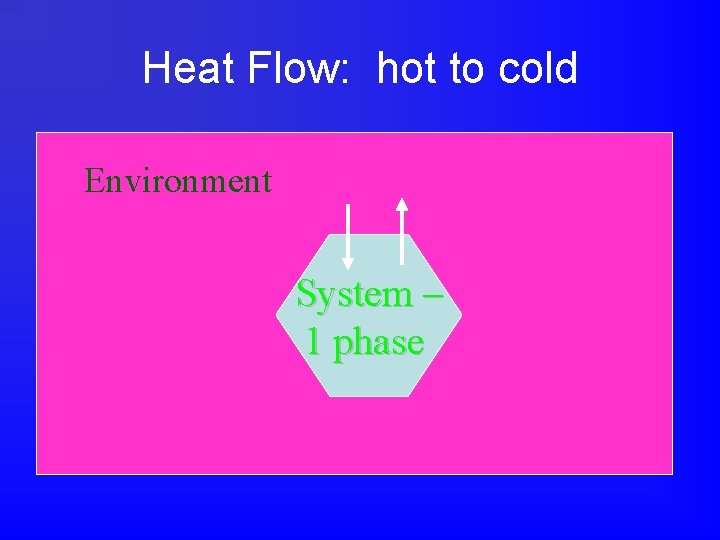
Heat Flow: hot to cold Environment System – 1 phase

But what about phase changes? • Sometimes more than one phase of a substance is present. • For example, when melting ice, both liquid water and ice are present. • Furthermore, the temperature is constant, so T = 0, even though the beaker of ice water is absorbing heat from a hot plate.

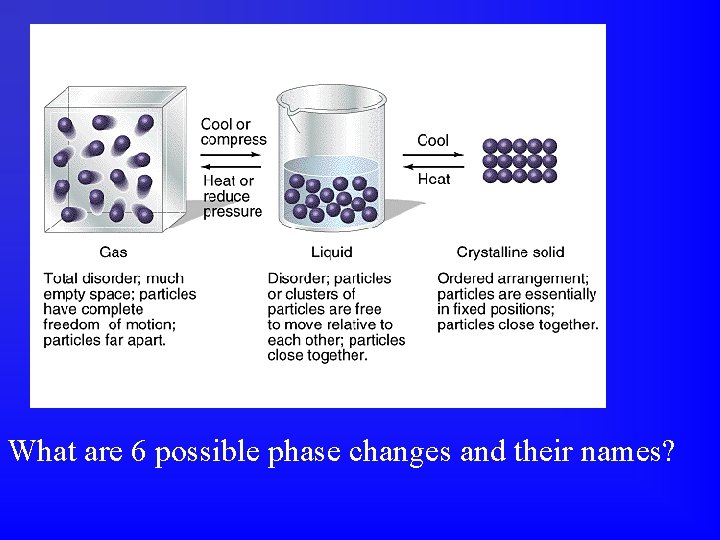
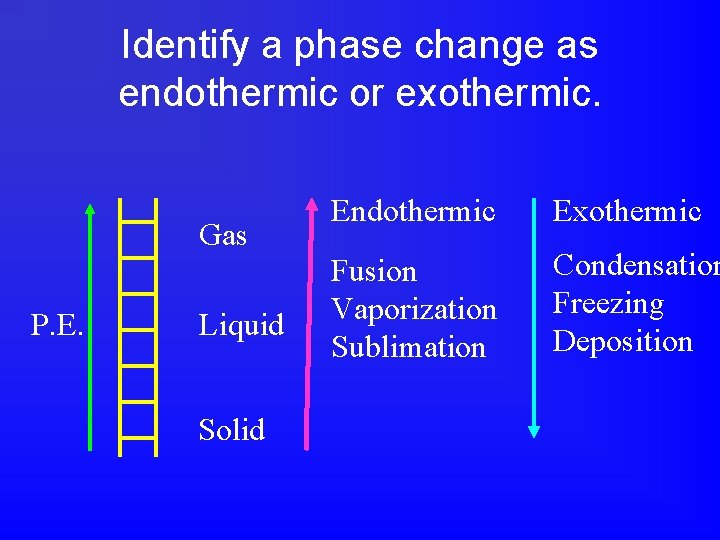
All chemical & physical changes are accompanied by energy changes. • Phase changes are physical changes. • Sometimes energy is absorbed, sometimes energy is released. • The energy change for a given phase change can be measured or calculated.

What are 6 possible phase changes and their names?

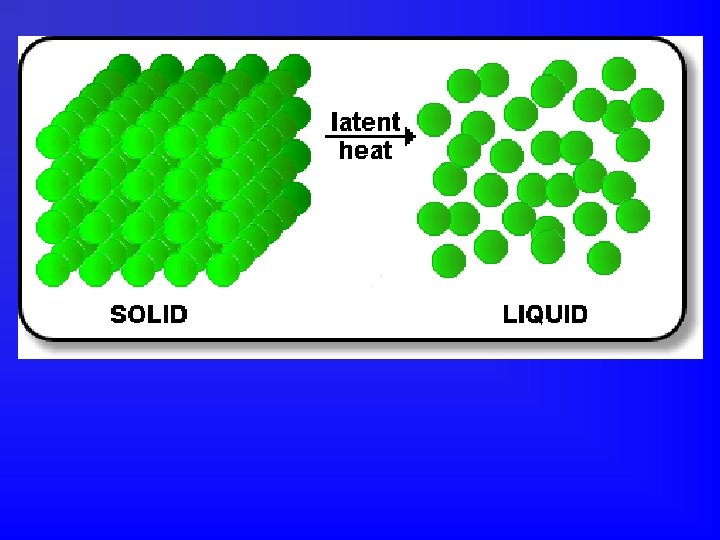
Potential Energy • Energy of relative position. • Molecules are always attracted to one another. • You have to put energy into the system to pull molecules apart from one another. • So the farther apart they are, the higher their potential energy.

Melting Ice • Ice water on hot plate: ice is melting. • The ice is absorbing heat from the hot plate and using it for the phase change. • The temperature of ice-water mix is constant -- the heat energy from the hot plate is going into the phase change or potential energy of the system. • The heat energy is not going to the kinetic energy. Remember, temperature is …

Identify a phase change as endothermic or exothermic. Gas P. E. Liquid Solid Endothermic Exothermic Fusion Vaporization Sublimation Condensation Freezing Deposition

Heating & Cooling Curves • 1 way to investigate energy changes. • Measure temperature as a function of time at a constant heating or cooling rate.

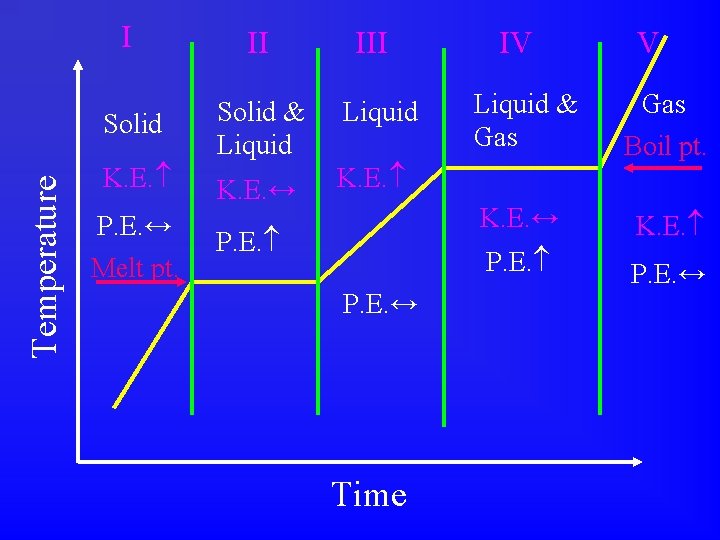
Temperature I II Solid & Liquid K. E. ↔ K. E. P. E. ↔ Melt pt. III P. E. ↔ Time IV V Liquid & Gas Boil pt. K. E. ↔ K. E. P. E. ↔

Melting & Boiling Points • Plateaus = Phase changes = Potential energy changes. • Notice that as long as 2 phases are present, the temperature is constant. • Melting point, Boiling point. Tiger

What happens to the temperature as heat is added at the boiling point? • Nothing, until only 1 phase is present!

Heating Curve • Tiger Graphic

To analyze a heating/cooling curve: • Does the curve go uphill or downhill? • Label the phases present in each region. • Describe what happens to the K. E. in each region. • Describe what happens to the P. E. in each region. • Locate the melting point and boiling point.

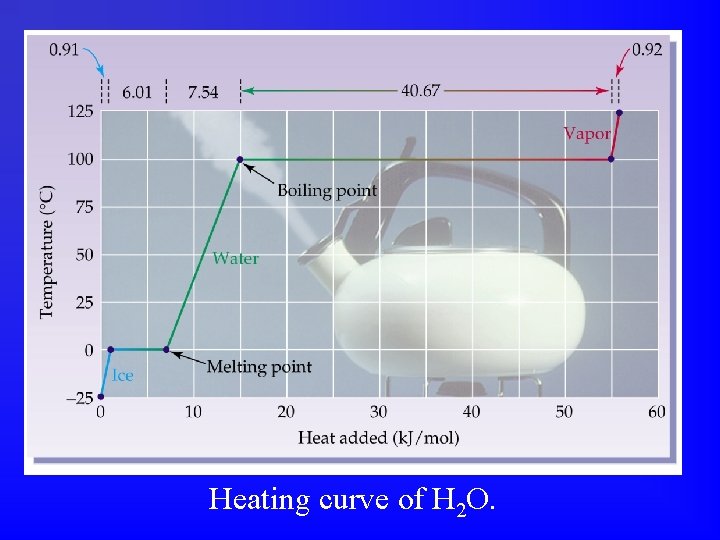
Heating curve of H 2 O.

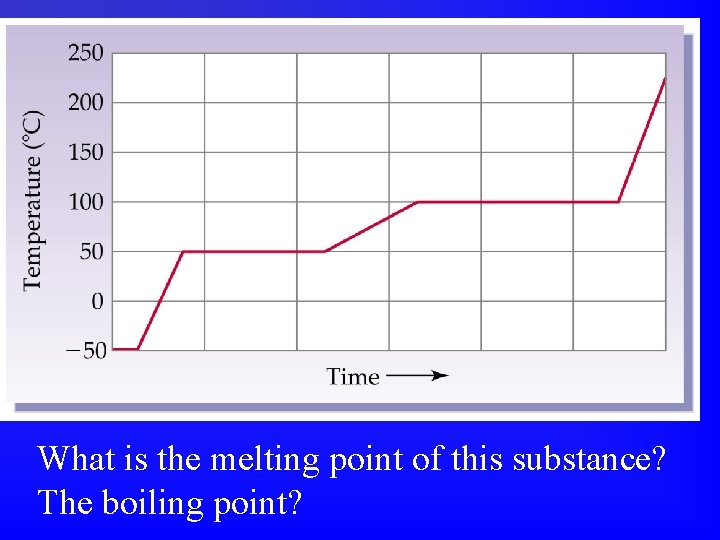
What is the melting point of this substance? The boiling point?

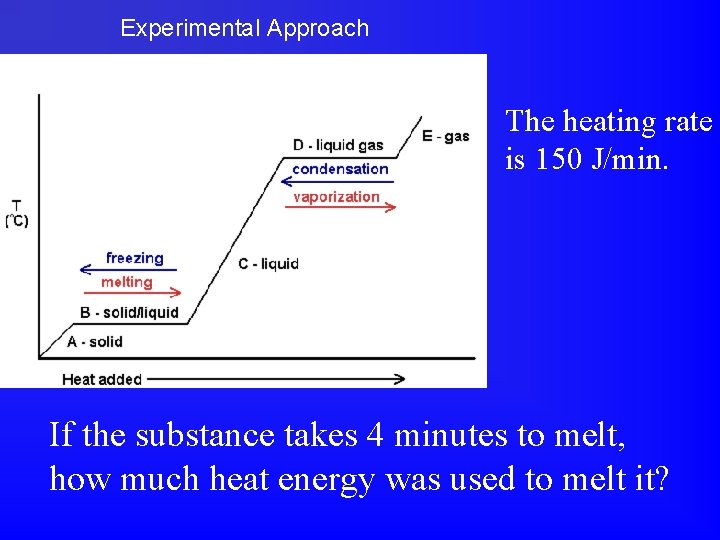
Experimental Approach The heating rate is 150 J/min. If the substance takes 4 minutes to melt, how much heat energy was used to melt it?

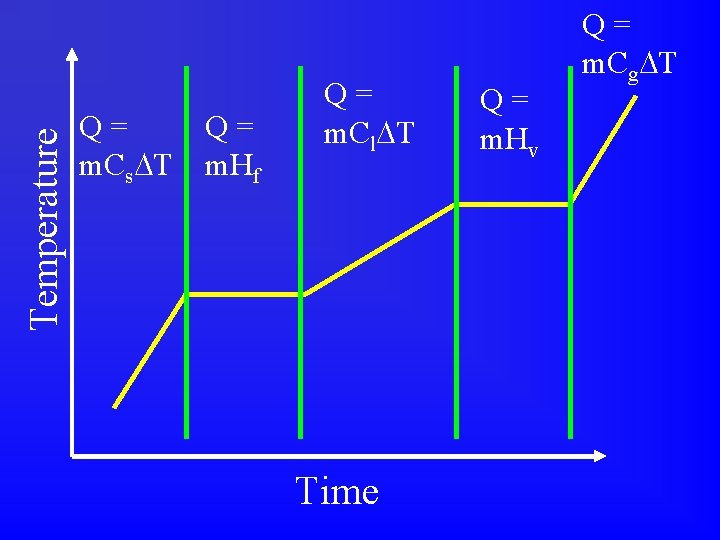
3 equations for Q • Q = m. C T • Q = m. Hf • Q = m. Hv • Have to figure out which one to use for a given problem. • Depends which section of heating curve. • Look for hints in the problem.

Temperature Q= m. Cs T Q= m. Hf Q= m. Cl T Time Q= m. Hv Q= m. Cg T

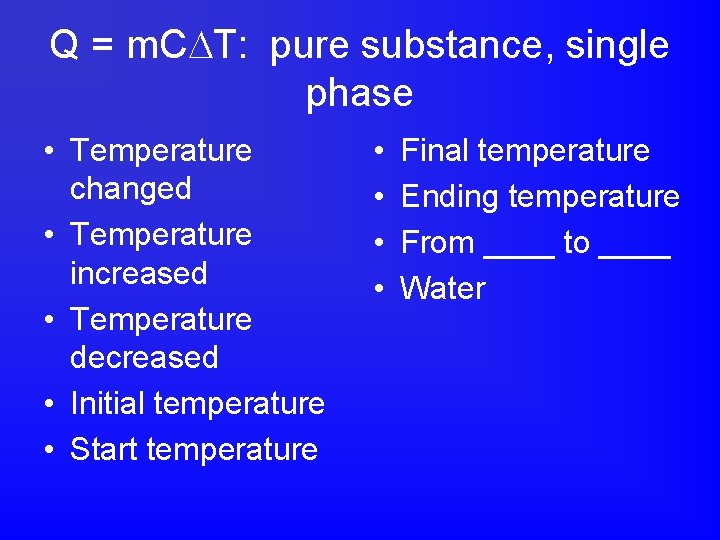
Q = m. C T: pure substance, single phase • Temperature changed • Temperature increased • Temperature decreased • Initial temperature • Start temperature • • Final temperature Ending temperature From ____ to ____ Water

Q = m. Hf : liquid and solid present • • • Ice Freezing Melting At 0 C (for H 2 O) At constant temperature

Heat of Fusion • Amount of energy required to change 1 gram of a pure substance from a solid to a liquid at its melting point. • Heat of Fusion = Hf = physical constant. • Hf for water = 333. 6 Joules per gram (Table B)


How much heat is absorbed when 10 grams of ice melts at 0 o. C? • Heat absorbed = mass of substance X heat of fusion of substance • Q = m. Hf = (10 g)(333. 6 J/g) = 3336 J • Where does that energy go? • Particles must overcome forces of attraction to move farther apart.

Q = m. Hv : liquid and gas present • • • Steam Boiling Condensation or Condensing At 100 C (for H 2 O) At constant temperature

Heat of Vaporization • Amount of energy required to convert 1 gram of a pure substance from a liquid to a gas at its boiling point. • Heat of vaporization = Hv = physical constant • Hv for water = 2259 J/g
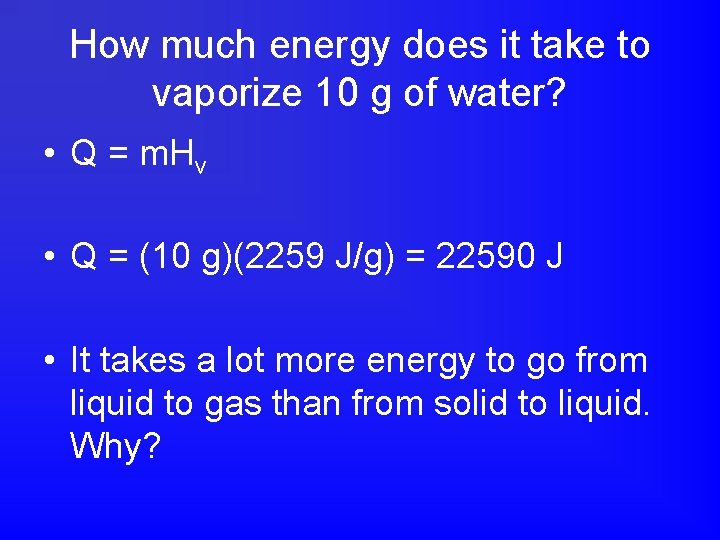
How much energy does it take to vaporize 10 g of water? • Q = m. Hv • Q = (10 g)(2259 J/g) = 22590 J • It takes a lot more energy to go from liquid to gas than from solid to liquid. Why?

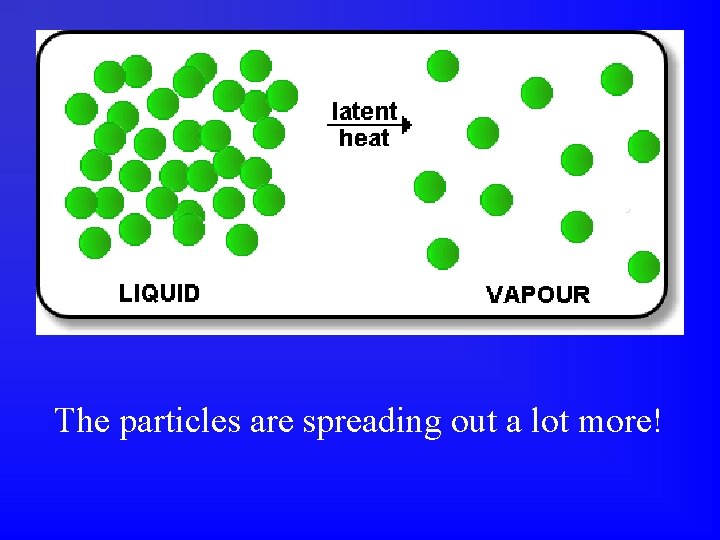
The particles are spreading out a lot more!

Heats of fusion & vaporization • Determined in calorimetry experiments.