THERMOCHEMISTRY Endothermic and Exothermic Reactions Exothermic Reactions Heat






- Slides: 10

THERMOCHEMISTRY Endothermic and Exothermic Reactions

Exothermic Reactions • Heat is released • AB A + B + Heat Energy released • Energy is conserved (can’t be created or destroyed), so where did the heat come from? • Which has more potential energy? 1. The products 2. The reactants

Chemical Potential Energy • What IS chemical potential energy? • BOND ENERGY! • ΔH = ∑ΔH(bonds broken) • H 2 + I 2 2 HI • H-H: 436 k. J/mol • I-I: 151 k. J/mol • H-I: 297 k. J/mol – ∑ΔH(bonds formed)

Exothermic Reactions • Combustion • Freezing water ice • Water + acids

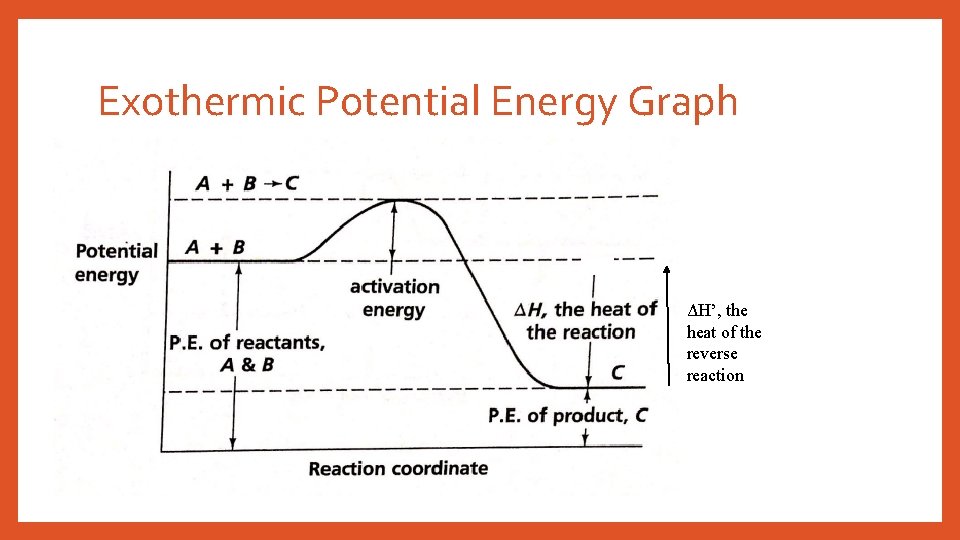
Exothermic Potential Energy Graph ΔH’, the heat of the reverse reaction

Endothermic Reactions • Heat is absorbed • Heat + A + B AB • Which has more potential energy? 1. The products 2. The reactants

Endothermic Reactions • Frying an egg • Evaporating sweat • Melting ice

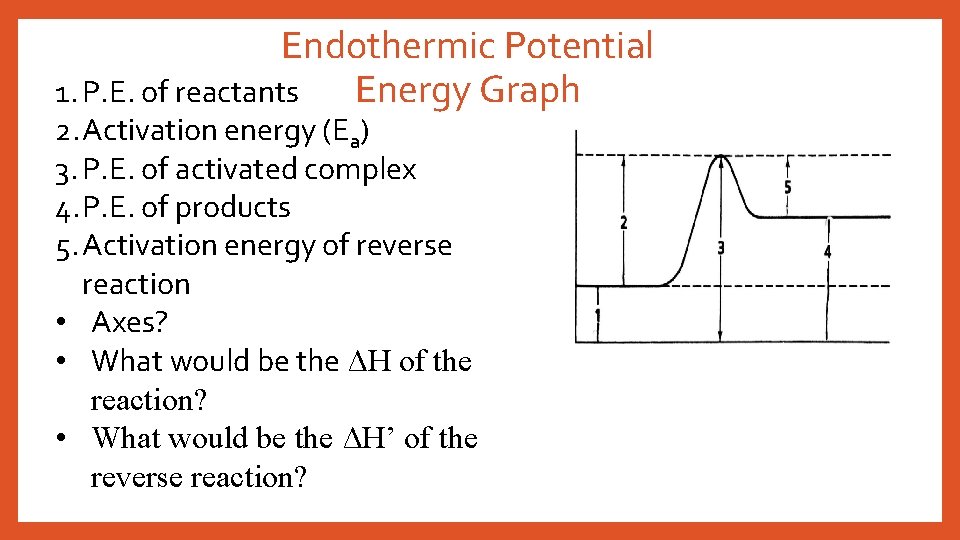
Endothermic Potential Energy Graph 1. P. E. of reactants 2. Activation energy (Ea) 3. P. E. of activated complex 4. P. E. of products 5. Activation energy of reverse reaction • Axes? • What would be the ΔH of the reaction? • What would be the ΔH’ of the reverse reaction?

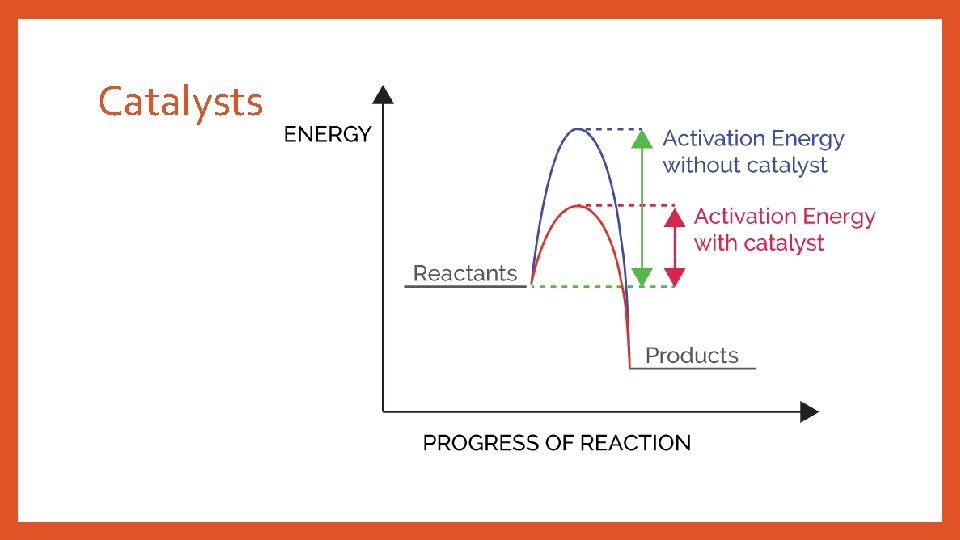
Catalysts

To Summarize… Exothermic Reactions: • Reactants have energy • Products include • ΔH is negative Both types: potential heat Endothermic Reactions: • have more potential energy • Need heat with the reactants for reaction to occur • ΔH is positive • Energy is , so it can’t be created or destroyed, but it CAN be transformed from potential heat • Require activation energy for the reaction to occur • The potential energy graph works both ways—ΔH = - ΔH’ • Catalysts reduce activation energy needed
 Thermic reactions
Thermic reactions Example of exothermic process
Example of exothermic process Endothermic and exothermic worksheet
Endothermic and exothermic worksheet Exothermic and endothermic homework
Exothermic and endothermic homework Is vaporization endothermic or exothermic
Is vaporization endothermic or exothermic Exothermic vs endothermic
Exothermic vs endothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic Heating cooling curve
Heating cooling curve Endothermic reaction examples
Endothermic reaction examples Ectotherms
Ectotherms