Endothermic and Exothermic Reactions Exothermic Endothermic Exothermic Reactions


- Slides: 10

Endothermic and Exothermic Reactions

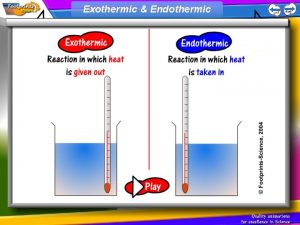
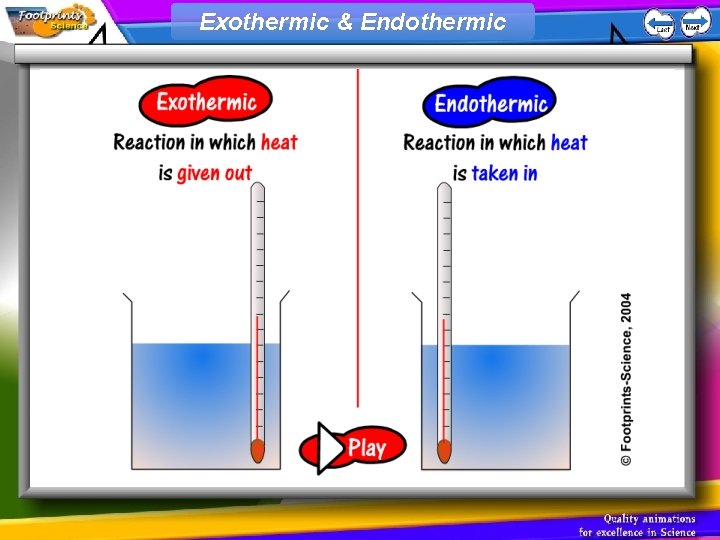
Exothermic & Endothermic

Exothermic Reactions 1. An exothermic reaction is one which releases heat energy to the surroundings 2. Temperature increases Hot © Boardworks Ltd 2003

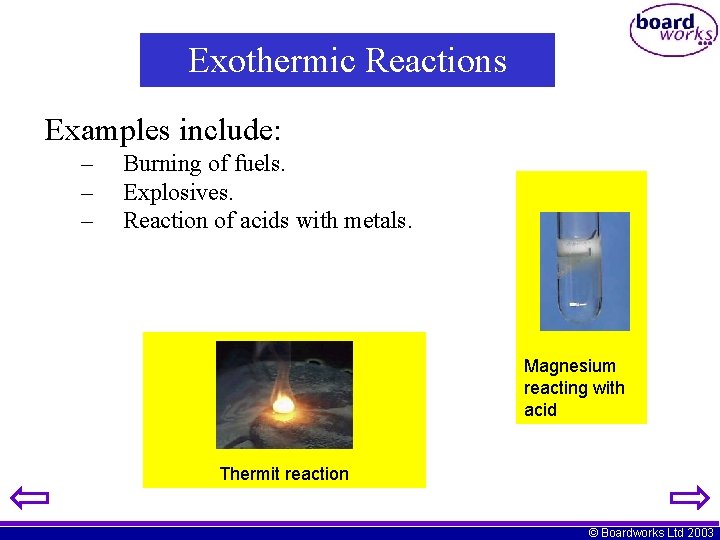
Exothermic Reactions Examples include: – – – Burning of fuels. Explosives. Reaction of acids with metals. Magnesium reacting with acid Thermit reaction © Boardworks Ltd 2003

Activity Say whether these processes are exothermic. 1. 2. 3. 4. 5. Charcoal burning A candle burning. A kettle boiling Ice melting A firework exploding yes no no yes You have to put heat in for boiling and melting. You get heat out from all the other processes © Boardworks Ltd 2003

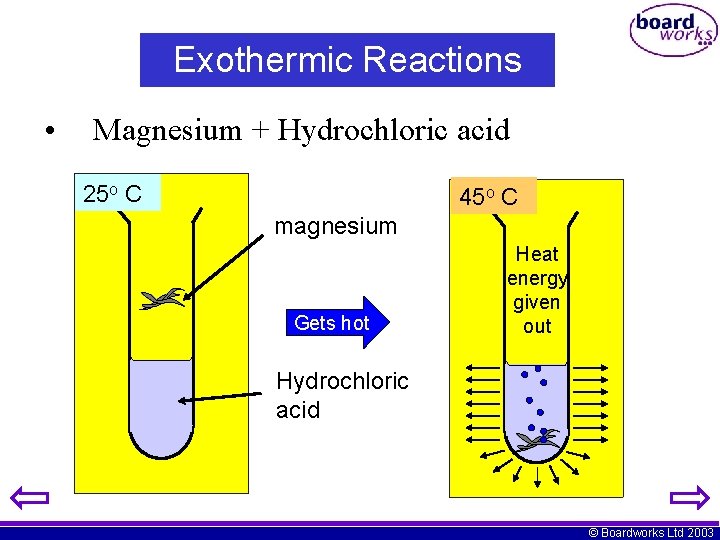
Exothermic Reactions • Magnesium + Hydrochloric acid 25 o C 45 o C magnesium Gets hot Heat energy given out Hydrochloric acid © Boardworks Ltd 2003

Endothermic Reactions 1. An endothermic reaction is one which takes in heat energy from the surroundings 2. Temperature decreases Cold © Boardworks Ltd 2003

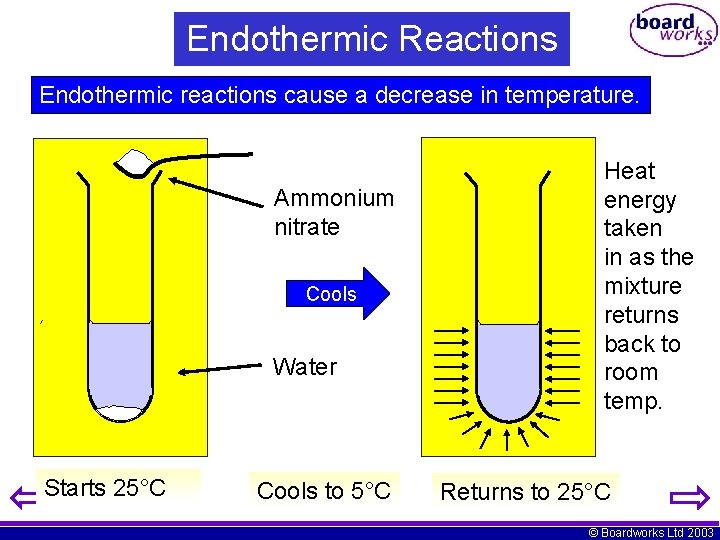
Endothermic Reactions Endothermic reactions cause a decrease in temperature. • • • Endothermic chemical reactions are relatively rare. A few reactions that give off gases are highly endothermic - get very cold. Dissolving salts in water is another process that is often endothermic. © Boardworks Ltd 2003

Endothermic Reactions Endothermic reactions cause a decrease in temperature. Ammonium nitrate Cools Water Starts 25°C Cools to 5°C Heat energy taken in as the mixture returns back to room temp. Returns to 25°C © Boardworks Ltd 2003

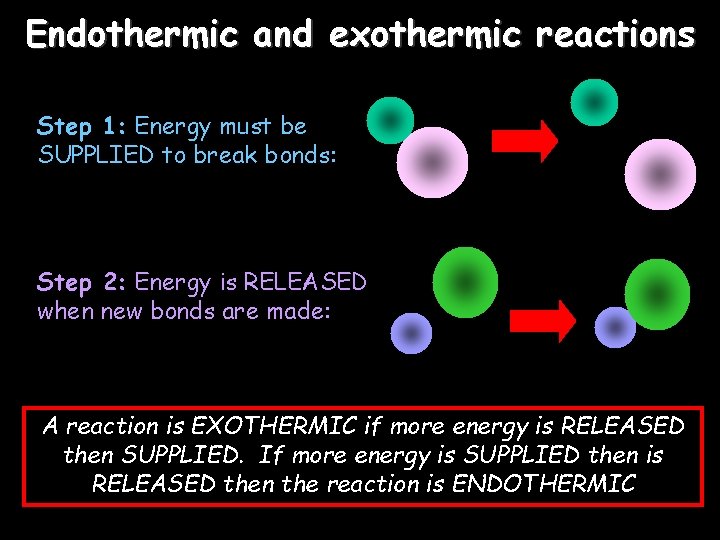
Endothermic and exothermic reactions Step 1: Energy must be SUPPLIED to break bonds: Step 2: Energy is RELEASED when new bonds are made: A reaction is EXOTHERMIC if more energy is RELEASED then SUPPLIED. If more energy is SUPPLIED then is RELEASED then the reaction is ENDOTHERMIC