Exothermic and Endothermic Reactions Aims We are learning






- Slides: 12

Exothermic and Endothermic Reactions Aims We are learning to: • Know that energy changes can be measured in chemical reactions. Keywords Exothermic, endothermic, energy.

Learning Outcomes Must: State a definition for exothermic and endothermic. Should: Recognise an exothermic and endothermic reaction from a practical. Could : Explain the difference between endothermic and exothermic.

Reaction Videos Thermite Reaction. • After adding one or more catalysts, iron oxide (rust) and aluminum react to produce elemental iron and aluminum oxide. • So much heat is produced in this reaction that the iron becomes a liquid. The heat is so intense that the molten iron can be used to weld railroad tracks together. www. middleschoolchemistry. com/multimedia/chapter 6/lesson 7

Reaction Videos Nitrogen Triiodide Reaction • This is a decomposition reaction where nitrogen triiodide decomposes into nitrogen gas and purple iodine vapor. • Nitrogen triiodide crystals are so unstable that just a light touch will cause them to rapidly decompose generating a great deal of heat. www. middleschoolchemistry. com/multimedia/chapter 6/lesson 7

Reaction Videos White Phosphorous Reaction. White phosphorous is dissolved in a solvent and spread on a piece of paper. When the solvent evaporates, the phosphorous reacts with oxygen in the air in a combustion reaction. www. middleschoolchemistry. com/multimedia/chapter 6/lesson 7

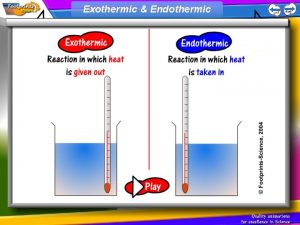
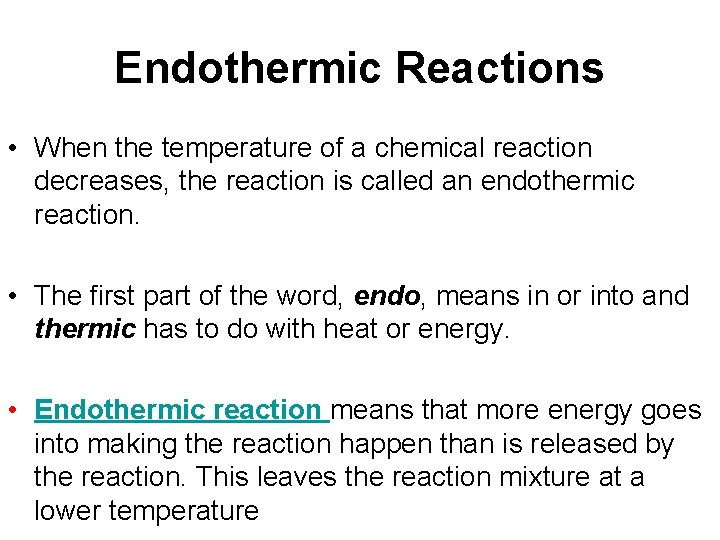
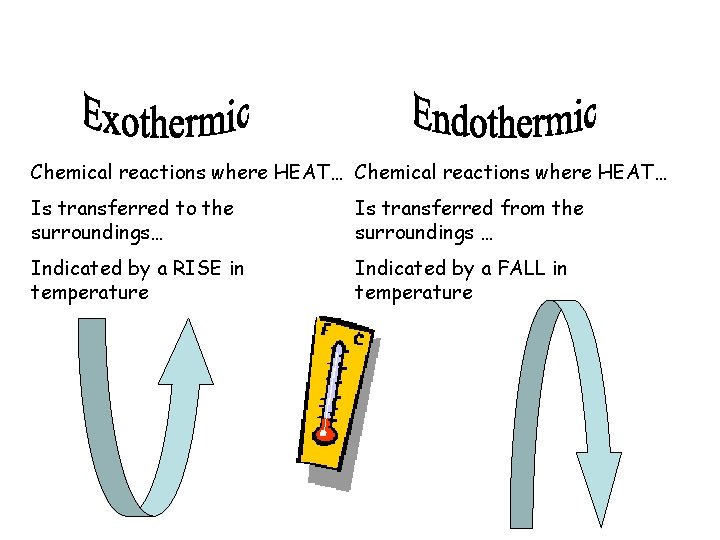
Endothermic Reactions • When the temperature of a chemical reaction decreases, the reaction is called an endothermic reaction. • The first part of the word, endo, means in or into and thermic has to do with heat or energy. • Endothermic reaction means that more energy goes into making the reaction happen than is released by the reaction. This leaves the reaction mixture at a lower temperature

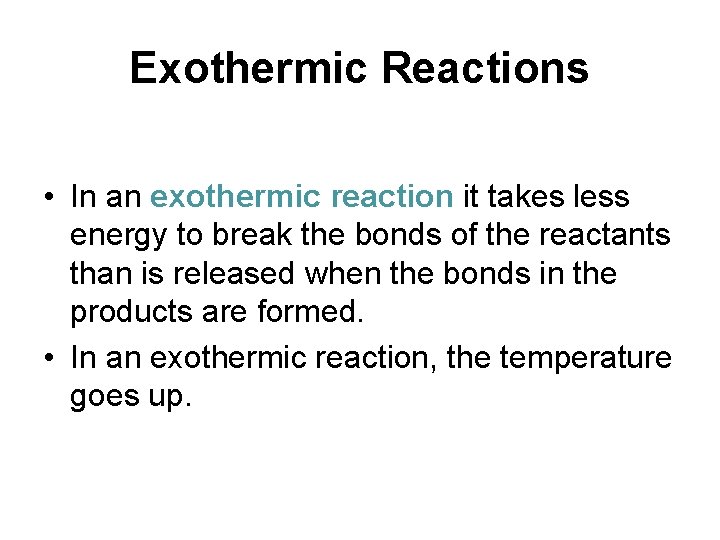
Exothermic Reactions • In an exothermic reaction it takes less energy to break the bonds of the reactants than is released when the bonds in the products are formed. • In an exothermic reaction, the temperature goes up.

Temperature Experiments Carry out the experiments on the Weebly to investigate whether they are Exothermic or Endothermic

Chemical reactions where HEAT… Is transferred to the surroundings… Is transferred from the surroundings … Indicated by a RISE in temperature Indicated by a FALL in temperature

Conclusion Write in a sentence which reactions are endothermic and which are exothermic.

Break bonds which releases heat energy. Need to make bonds so take in energy, reducing the temperature.

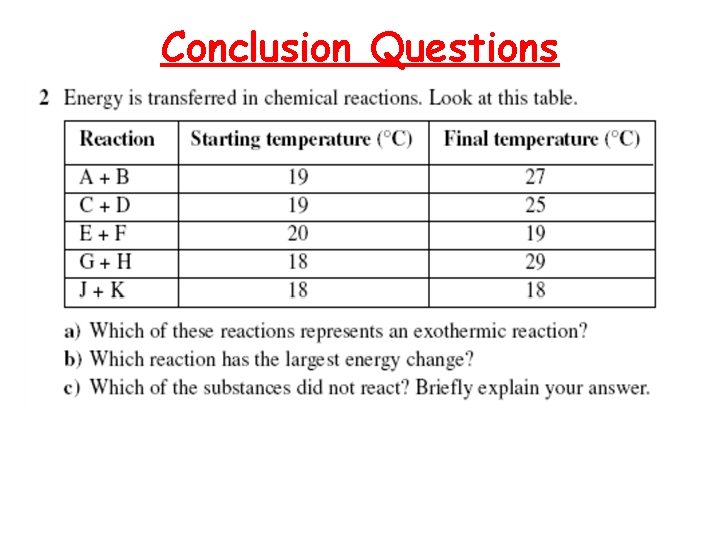
Conclusion Questions
 Thermic reaction
Thermic reaction Exothermic reaction examples
Exothermic reaction examples Insidan region jh
Insidan region jh Endothermic and exothermic worksheet
Endothermic and exothermic worksheet Exothermic and endothermic homework
Exothermic and endothermic homework Is vaporization endothermic or exothermic
Is vaporization endothermic or exothermic Endothermic vs exothermic
Endothermic vs exothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic Fusion chemistry phase change
Fusion chemistry phase change Exo vs endothermic
Exo vs endothermic Examples of ectotherms
Examples of ectotherms Cellular respiration endothermic or exothermic
Cellular respiration endothermic or exothermic