Enthalpies of Formation The enthalpy of formation DHf










- Slides: 29

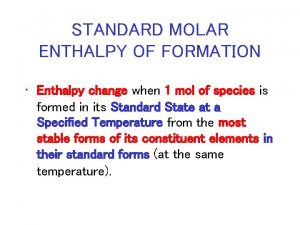
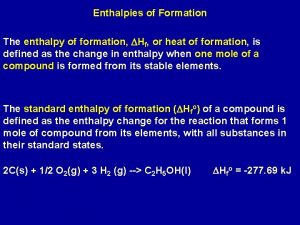
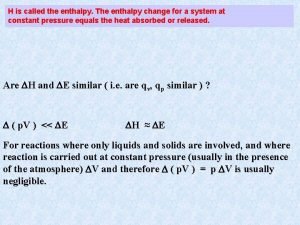
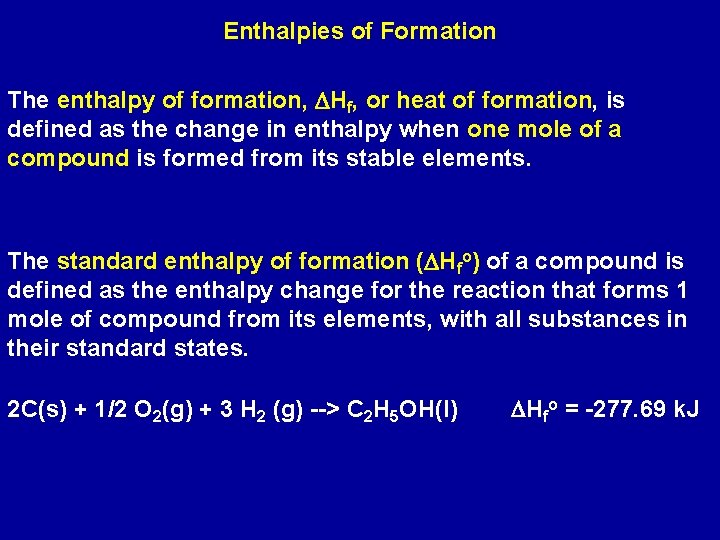
Enthalpies of Formation The enthalpy of formation, DHf, or heat of formation, is defined as the change in enthalpy when one mole of a compound is formed from its stable elements. The standard enthalpy of formation (DHfo) of a compound is defined as the enthalpy change for the reaction that forms 1 mole of compound from its elements, with all substances in their standard states. 2 C(s) + 1/2 O 2(g) + 3 H 2 (g) --> C 2 H 5 OH(l) DHfo = -277. 69 k. J

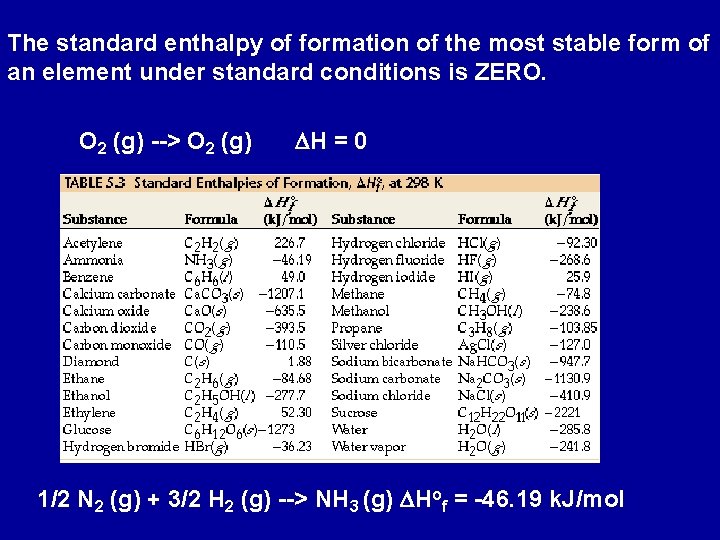
The standard enthalpy of formation of the most stable form of an element under standard conditions is ZERO. O 2 (g) --> O 2 (g) DH = 0 1/2 N 2 (g) + 3/2 H 2 (g) --> NH 3 (g) DHof = -46. 19 k. J/mol

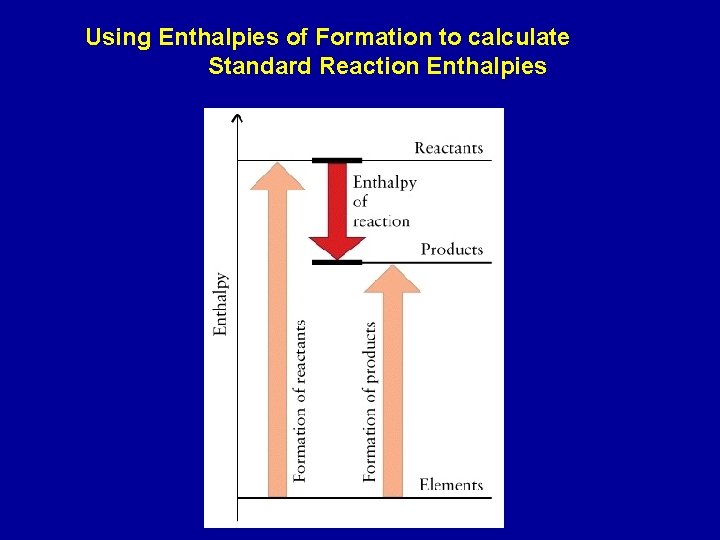
Using Enthalpies of Formation to calculate Standard Reaction Enthalpies

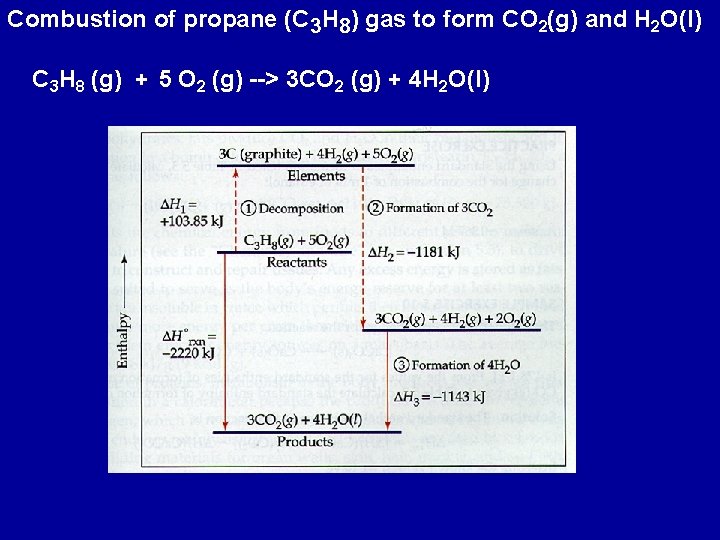
Combustion of propane (C 3 H 8) gas to form CO 2(g) and H 2 O(l) C 3 H 8 (g) + 5 O 2 (g) --> 3 CO 2 (g) + 4 H 2 O(l)

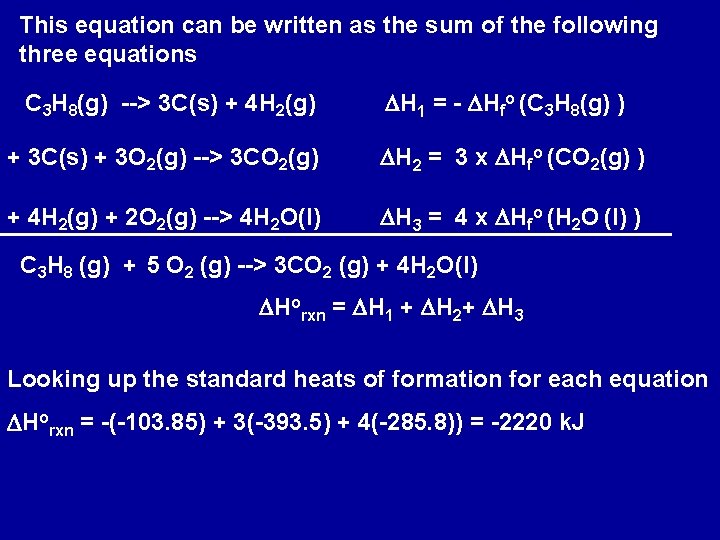
This equation can be written as the sum of the following three equations C 3 H 8(g) --> 3 C(s) + 4 H 2(g) DH 1 = - DHfo (C 3 H 8(g) ) + 3 C(s) + 3 O 2(g) --> 3 CO 2(g) DH 2 = 3 x DHfo (CO 2(g) ) + 4 H 2(g) + 2 O 2(g) --> 4 H 2 O(l) DH 3 = 4 x DHfo (H 2 O (l) ) C 3 H 8 (g) + 5 O 2 (g) --> 3 CO 2 (g) + 4 H 2 O(l) DHorxn = DH 1 + DH 2+ DH 3 Looking up the standard heats of formation for each equation DHorxn = -(-103. 85) + 3(-393. 5) + 4(-285. 8)) = -2220 k. J

In general, DHorxn = S n DHfo (products) - S n DHfo (reactants) n is the stoichiometric coefficients in the reaction

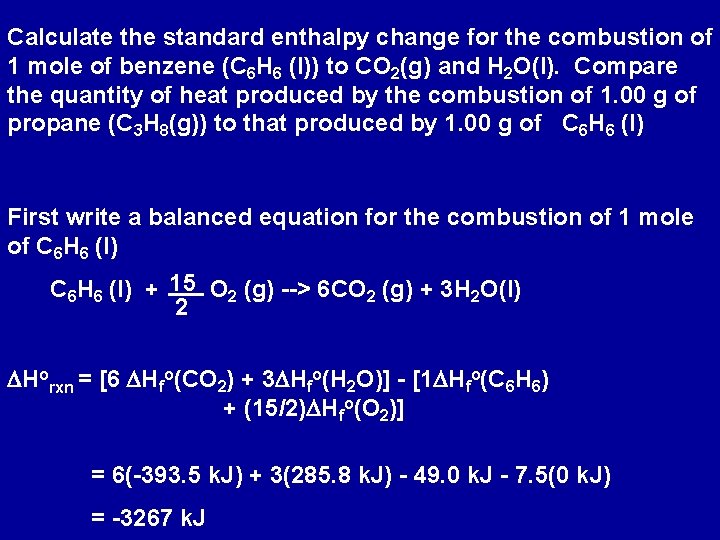
Calculate the standard enthalpy change for the combustion of 1 mole of benzene (C 6 H 6 (l)) to CO 2(g) and H 2 O(l). Compare the quantity of heat produced by the combustion of 1. 00 g of propane (C 3 H 8(g)) to that produced by 1. 00 g of C 6 H 6 (l) First write a balanced equation for the combustion of 1 mole of C 6 H 6 (l) + 15 O 2 (g) --> 6 CO 2 (g) + 3 H 2 O(l) 2 DHorxn = [6 DHfo(CO 2) + 3 DHfo(H 2 O)] - [1 DHfo(C 6 H 6) + (15/2)DHfo(O 2)] = 6(-393. 5 k. J) + 3(285. 8 k. J) - 49. 0 k. J - 7. 5(0 k. J) = -3267 k. J

For the combustion of 1 mole of propane DHorxn = -2220 k. J Hence for 1. 00 g propane, which corresponds to 0. 0227 mol propane, DHorxn = 0. 0227 mol x -2220 k. J/mol = - 50. 3 k. J/g For C 6 H 6 (l) => DHorxn = - 41. 8 k. J/g

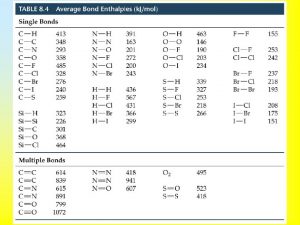
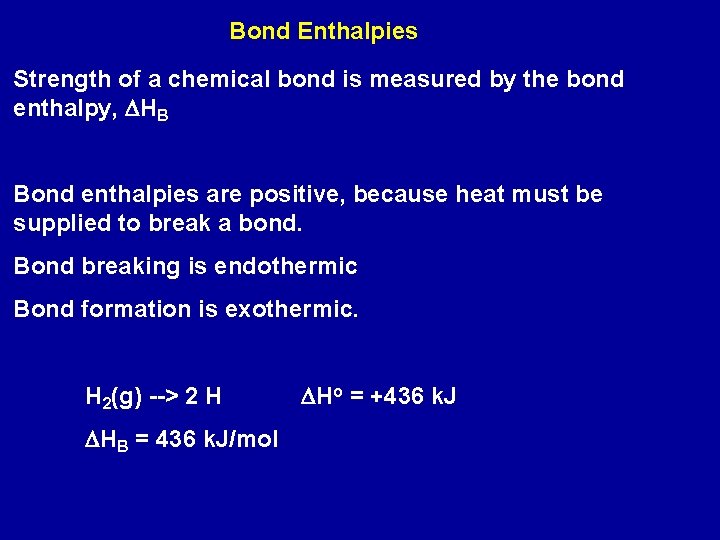
Bond Enthalpies Strength of a chemical bond is measured by the bond enthalpy, DHB Bond enthalpies are positive, because heat must be supplied to break a bond. Bond breaking is endothermic Bond formation is exothermic. H 2(g) --> 2 H DHB = 436 k. J/mol DHo = +436 k. J


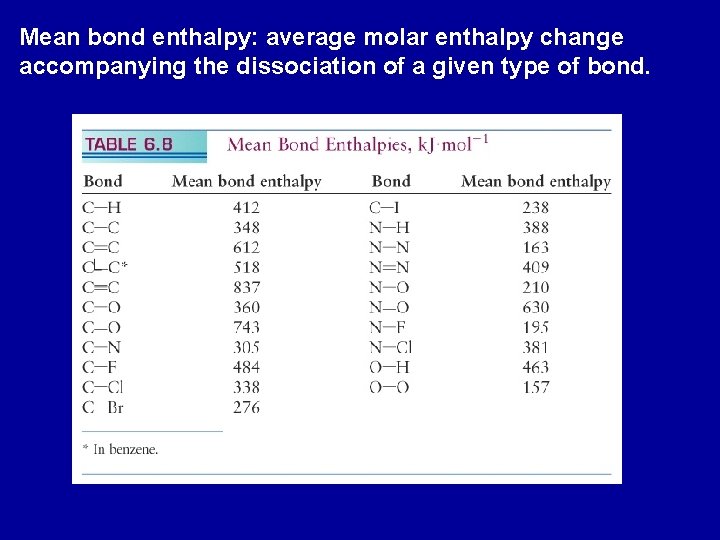
Mean bond enthalpy: average molar enthalpy change accompanying the dissociation of a given type of bond.

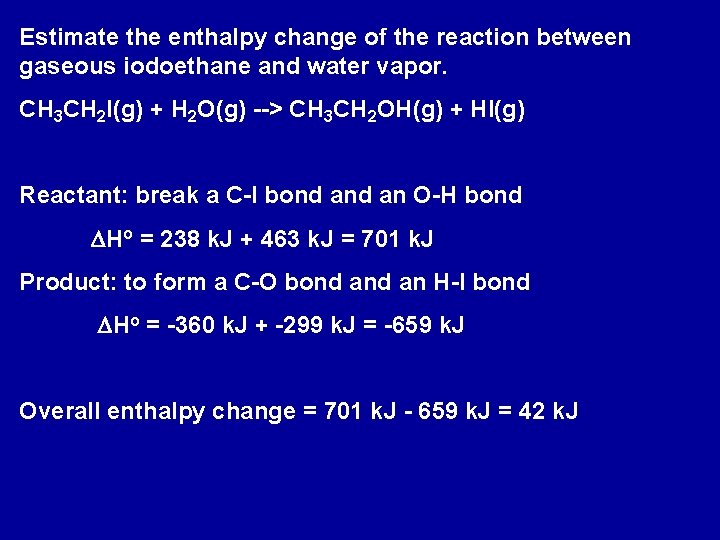
Estimate the enthalpy change of the reaction between gaseous iodoethane and water vapor. CH 3 CH 2 I(g) + H 2 O(g) --> CH 3 CH 2 OH(g) + HI(g) Reactant: break a C-I bond an O-H bond DHo = 238 k. J + 463 k. J = 701 k. J Product: to form a C-O bond an H-I bond DHo = -360 k. J + -299 k. J = -659 k. J Overall enthalpy change = 701 k. J - 659 k. J = 42 k. J

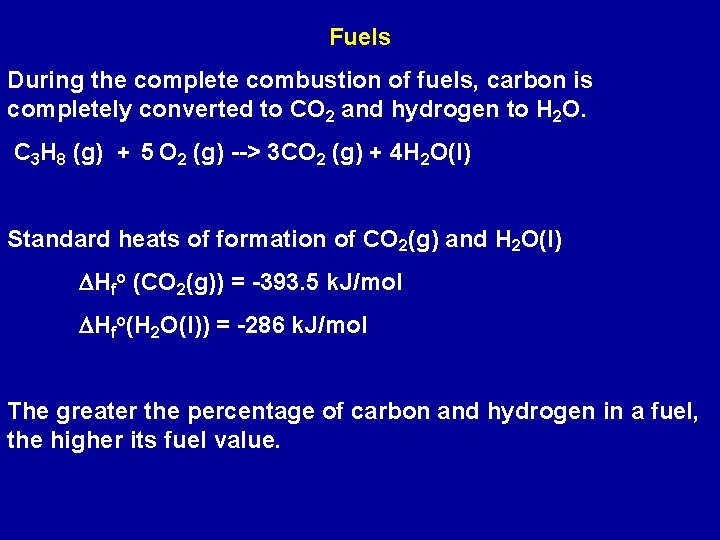
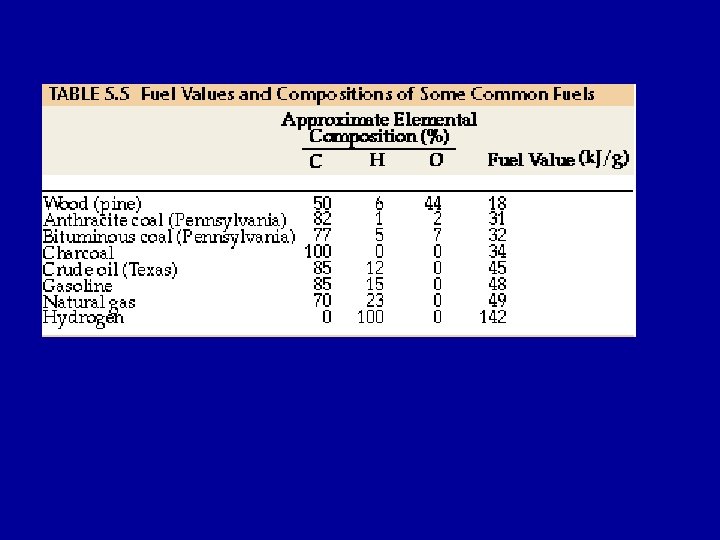
Fuels During the complete combustion of fuels, carbon is completely converted to CO 2 and hydrogen to H 2 O. C 3 H 8 (g) + 5 O 2 (g) --> 3 CO 2 (g) + 4 H 2 O(l) Standard heats of formation of CO 2(g) and H 2 O(l) DHfo (CO 2(g)) = -393. 5 k. J/mol DHfo(H 2 O(l)) = -286 k. J/mol The greater the percentage of carbon and hydrogen in a fuel, the higher its fuel value.


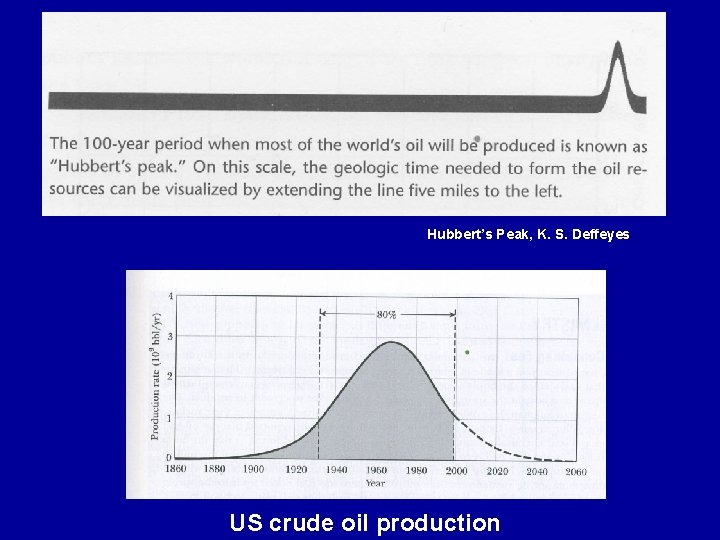
Hubbert’s Peak, K. S. Deffeyes US crude oil production

Global Energy Reserves (1988) (units of Q = 1021 J) Fuel Type Proven Reserves Est. Reserves Coal 25 Q 118 Q Oil 5 Q 9 Q Natural Gas 4 Q 10 Q Total amount of commercially energy currently consumed by humans ~ 0. 5 Q annually “Non-renewable” sources of energy

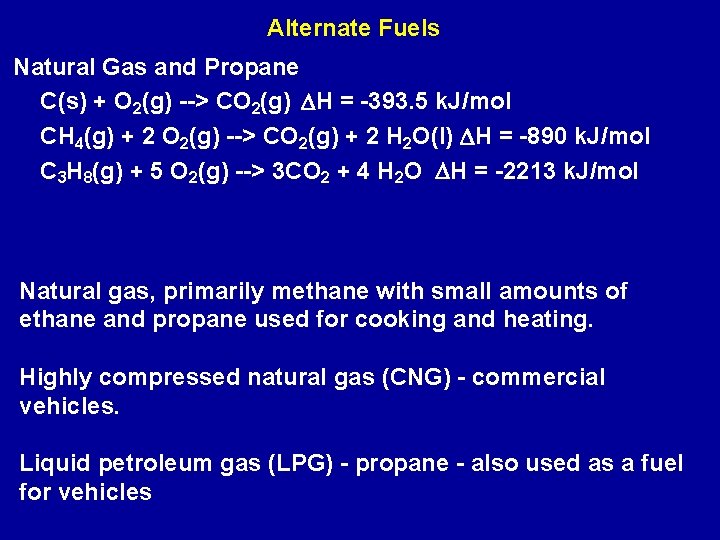
Alternate Fuels Natural Gas and Propane C(s) + O 2(g) --> CO 2(g) DH = -393. 5 k. J/mol CH 4(g) + 2 O 2(g) --> CO 2(g) + 2 H 2 O(l) DH = -890 k. J/mol C 3 H 8(g) + 5 O 2(g) --> 3 CO 2 + 4 H 2 O DH = -2213 k. J/mol Natural gas, primarily methane with small amounts of ethane and propane used for cooking and heating. Highly compressed natural gas (CNG) - commercial vehicles. Liquid petroleum gas (LPG) - propane - also used as a fuel for vehicles

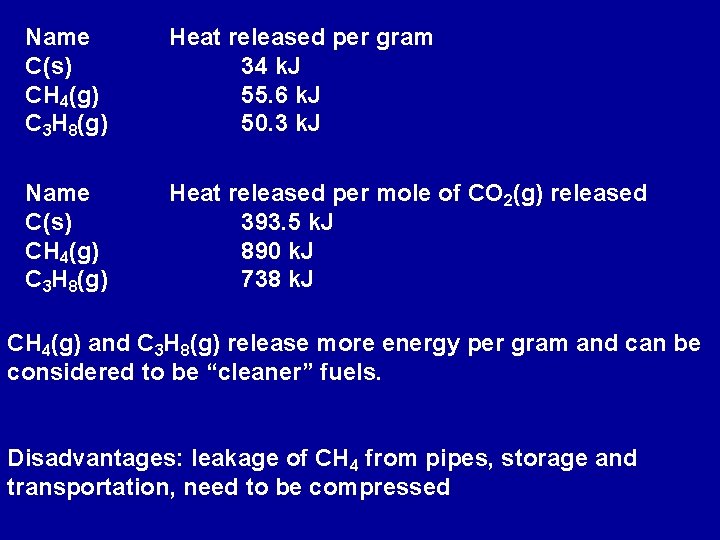
Name C(s) CH 4(g) C 3 H 8(g) Heat released per gram 34 k. J 55. 6 k. J 50. 3 k. J Name C(s) CH 4(g) C 3 H 8(g) Heat released per mole of CO 2(g) released 393. 5 k. J 890 k. J 738 k. J CH 4(g) and C 3 H 8(g) release more energy per gram and can be considered to be “cleaner” fuels. Disadvantages: leakage of CH 4 from pipes, storage and transportation, need to be compressed

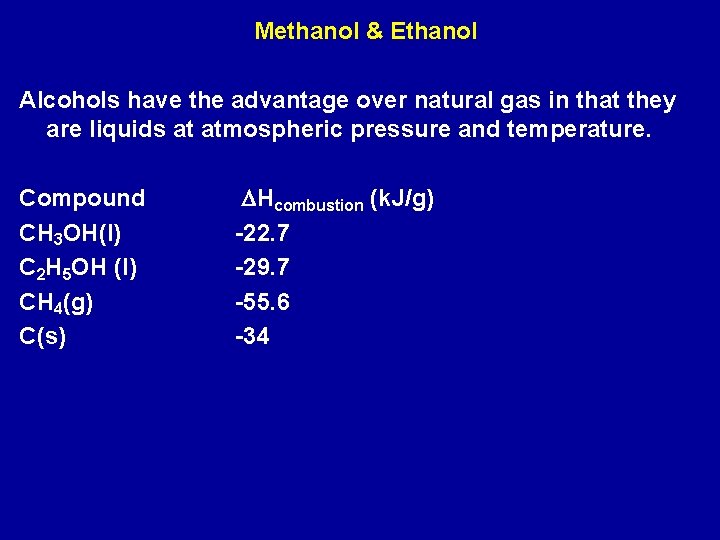
Methanol & Ethanol Alcohols have the advantage over natural gas in that they are liquids at atmospheric pressure and temperature. Compound CH 3 OH(l) C 2 H 5 OH (l) CH 4(g) C(s) DHcombustion (k. J/g) -22. 7 -29. 7 -55. 6 -34

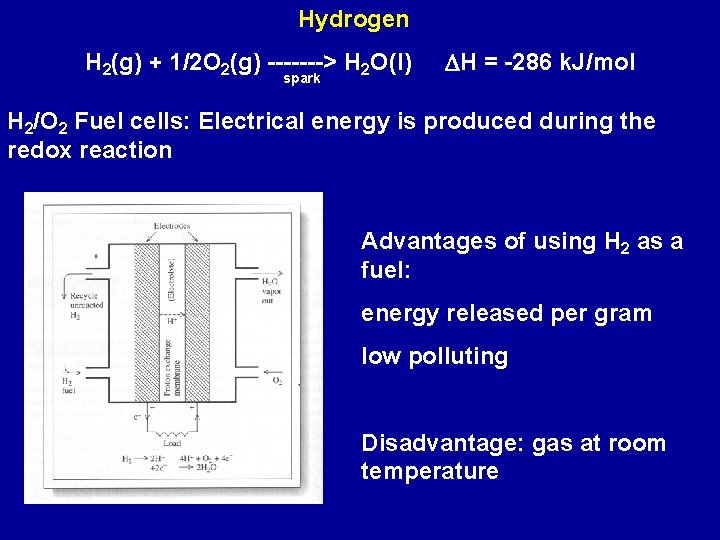
Hydrogen H 2(g) + 1/2 O 2(g) -------> H 2 O(l) spark DH = -286 k. J/mol H 2/O 2 Fuel cells: Electrical energy is produced during the redox reaction Advantages of using H 2 as a fuel: energy released per gram low polluting Disadvantage: gas at room temperature

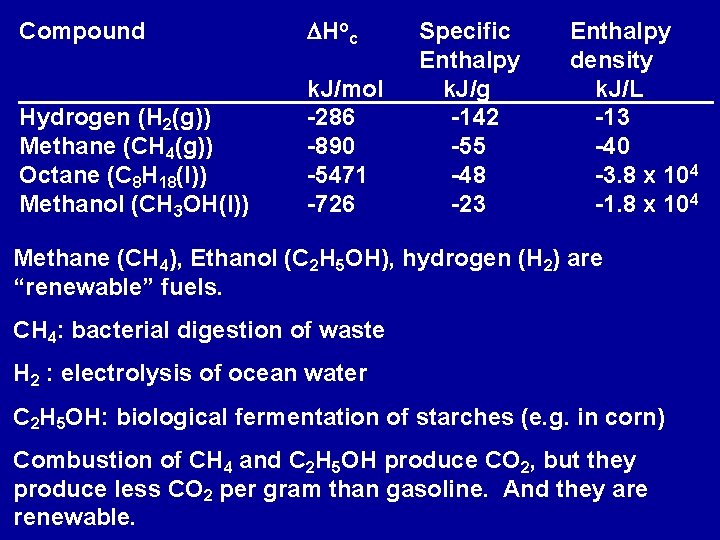
Compound DHoc Hydrogen (H 2(g)) Methane (CH 4(g)) Octane (C 8 H 18(l)) Methanol (CH 3 OH(l)) k. J/mol -286 -890 -5471 -726 Specific Enthalpy k. J/g -142 -55 -48 -23 Enthalpy density k. J/L -13 -40 -3. 8 x 104 -1. 8 x 104 Methane (CH 4), Ethanol (C 2 H 5 OH), hydrogen (H 2) are “renewable” fuels. CH 4: bacterial digestion of waste H 2 : electrolysis of ocean water C 2 H 5 OH: biological fermentation of starches (e. g. in corn) Combustion of CH 4 and C 2 H 5 OH produce CO 2, but they produce less CO 2 per gram than gasoline. And they are renewable.

Spontaneous Change A spontaneous change is one that occurs without external intervention and has definite direction. Spontaneous for T > 0 o. C Spontaneous for T < 0 o. C

A spontaneous process need not be fast

The change in enthalpy during a reaction is an important factor in determining whether a reaction is favored in the forward or reverse direction. Are exothermic reaction more likely to be spontaneous than an endothermic reaction? Not necessarily. The endothermic dissolution of ammonium nitrate, NH 4 NO 3, occurs spontaneously.

Entropy Both endothermic and exothermic reactions can be spontaneous Are there additional factors which determine spontaneity? Energy and matter tend to become more disordered. A measure of disorder is ENTROPY.


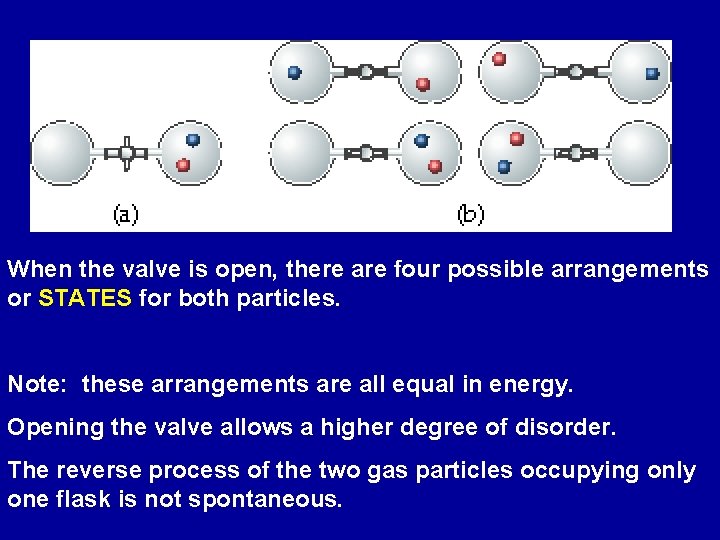
When the valve is open, there are four possible arrangements or STATES for both particles. Note: these arrangements are all equal in energy. Opening the valve allows a higher degree of disorder. The reverse process of the two gas particles occupying only one flask is not spontaneous.

As the number of particles increases in the system, the number of possible arrangements that the system can be in increases Processes in which the disorder of the system increases tend to occur spontaneously.

Ice melts spontaneously at T>0 o. C even though it is an endothermic process. The molecules of water that make up the ice crystal lattice are held rigidly in place. When the ice melts the water molecules are free to move around, and hence more disordered than in the solid lattice. Melting increases the disorder of the system.
 Limitations of average bond enthalpies
Limitations of average bond enthalpies Reactants minus products
Reactants minus products Sap dhf pada anak
Sap dhf pada anak Gambar neutrofil batang
Gambar neutrofil batang Molar heat of formation
Molar heat of formation How to find delta h
How to find delta h Enthalpy of formation hess law
Enthalpy of formation hess law Enthalpy of formation table
Enthalpy of formation table Definition of enthalpy change
Definition of enthalpy change Application of hess law
Application of hess law Formation initiale vs formation continue
Formation initiale vs formation continue Lời thề hippocrates
Lời thề hippocrates Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ điện thế nghỉ
điện thế nghỉ Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Chó sói
Chó sói Lp html
Lp html Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Số nguyên là gì
Số nguyên là gì Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người Tư thế ngồi viết
Tư thế ngồi viết