Module 3 Lesson 6 Hess Law and enthalpy








- Slides: 26

Module 3 Lesson 6 – Hess’ Law and enthalpy cycles part 2

Objectives Must Recall Hess’ law and construct an enthalpy cycle based on given data for enthalpy changes of combustion Should Extend the principle to calculate enthalpy changes of reaction from enthalpy changes of formation. Could Construct and use an unfamiliar enthalpy cycle to determine an enthalpy change of reaction.

Starter 1. Define ΔHf 2. What are standard conditions? 3. What is the value of ΔHf for any element?

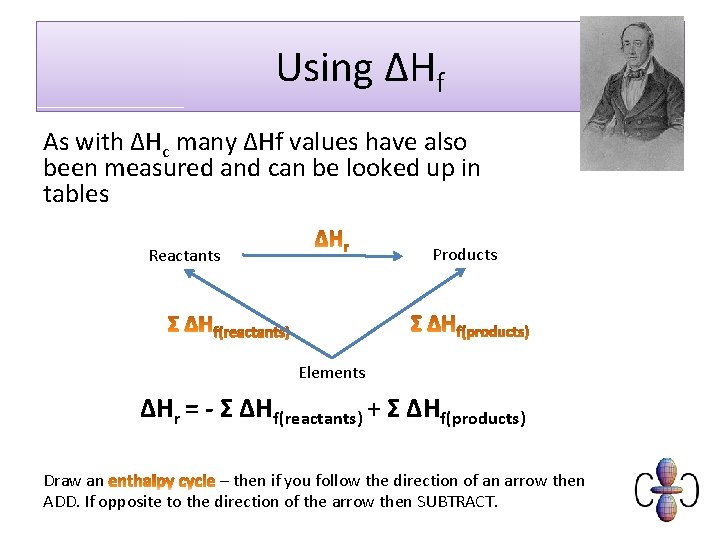
Using ΔHf As with ΔHc many ΔHf values have also been measured and can be looked up in tables Products Reactants Elements ΔHr = - Σ ΔHf(reactants) + Σ ΔHf(products) Draw an – then if you follow the direction of an arrow then ADD. If opposite to the direction of the arrow then SUBTRACT.

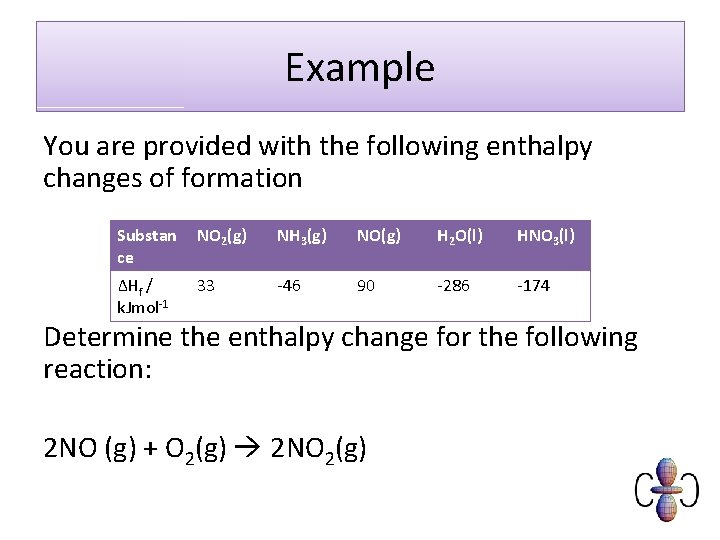
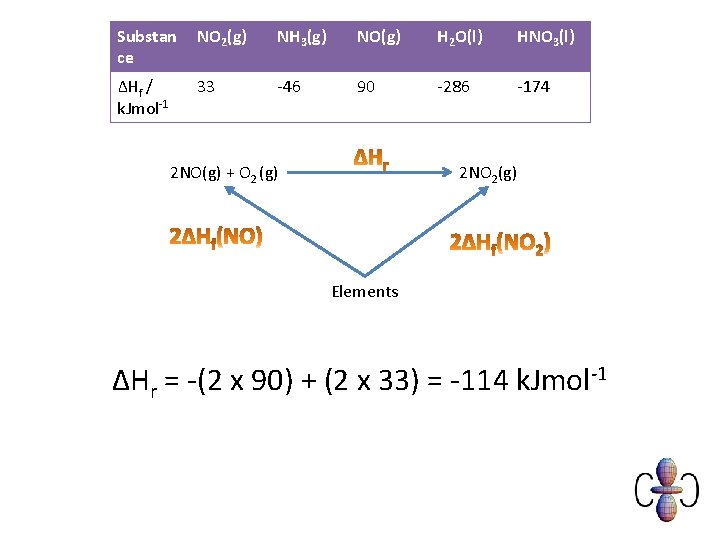
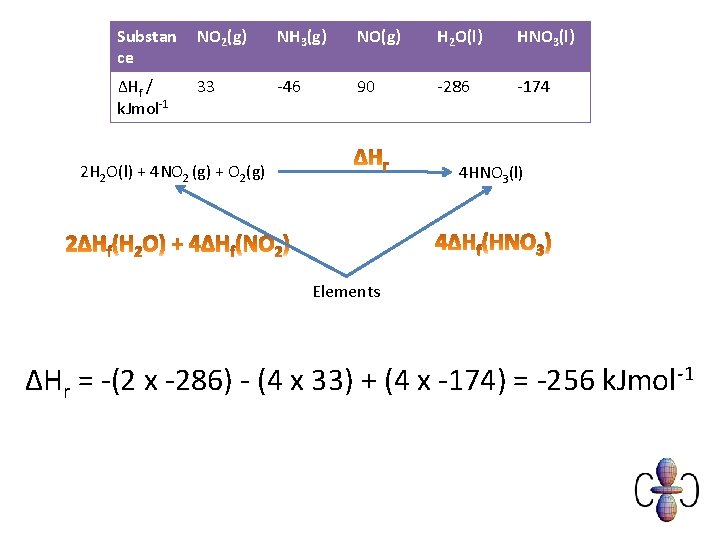
Example You are provided with the following enthalpy changes of formation Substan ce NO 2(g) NH 3(g) NO(g) H 2 O(l) HNO 3(l) ΔHf / k. Jmol-1 33 -46 90 -286 -174 Determine the enthalpy change for the following reaction: 2 NO (g) + O 2(g) 2 NO 2(g)

Substan ce NO 2(g) NH 3(g) NO(g) H 2 O(l) HNO 3(l) ΔHf / k. Jmol-1 33 -46 90 -286 -174 2 NO(g) + O 2 (g) 2 NO 2(g) Elements ΔHr = -(2 x 90) + (2 x 33) = -114 k. Jmol-1

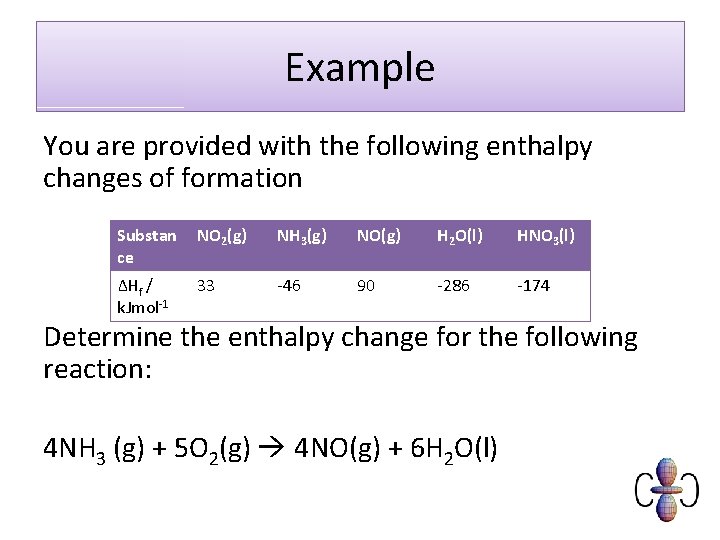
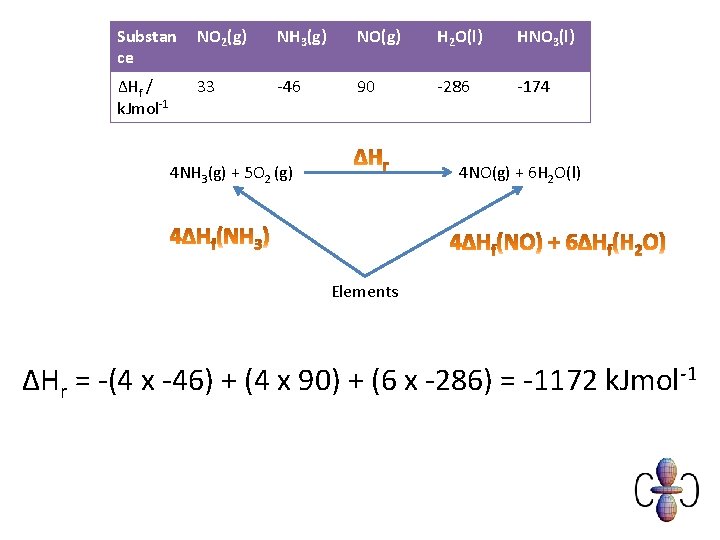
Example You are provided with the following enthalpy changes of formation Substan ce NO 2(g) NH 3(g) NO(g) H 2 O(l) HNO 3(l) ΔHf / k. Jmol-1 33 -46 90 -286 -174 Determine the enthalpy change for the following reaction: 4 NH 3 (g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(l)

Substan ce NO 2(g) NH 3(g) NO(g) H 2 O(l) HNO 3(l) ΔHf / k. Jmol-1 33 -46 90 -286 -174 4 NH 3(g) + 5 O 2 (g) 4 NO(g) + 6 H 2 O(l) Elements ΔHr = -(4 x -46) + (4 x 90) + (6 x -286) = -1172 k. Jmol-1

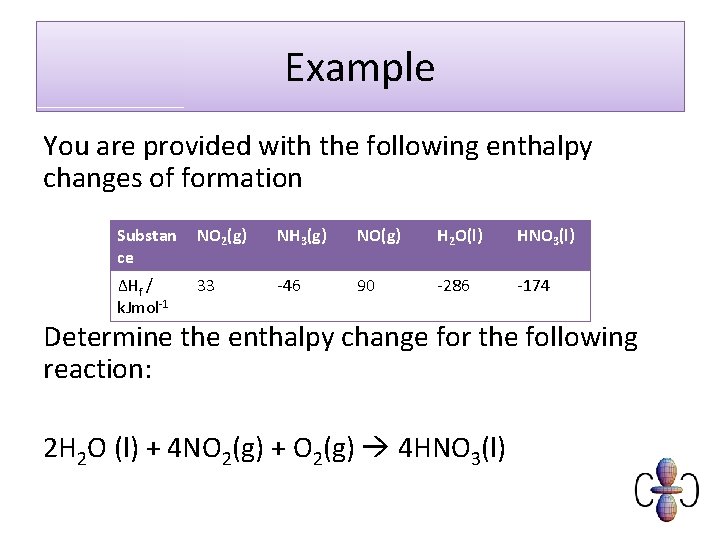
Example You are provided with the following enthalpy changes of formation Substan ce NO 2(g) NH 3(g) NO(g) H 2 O(l) HNO 3(l) ΔHf / k. Jmol-1 33 -46 90 -286 -174 Determine the enthalpy change for the following reaction: 2 H 2 O (l) + 4 NO 2(g) + O 2(g) 4 HNO 3(l)

Substan ce NO 2(g) NH 3(g) NO(g) H 2 O(l) HNO 3(l) ΔHf / k. Jmol-1 33 -46 90 -286 -174 2 H 2 O(l) + 4 NO 2 (g) + O 2(g) 4 HNO 3(l) Elements ΔHr = -(2 x -286) - (4 x 33) + (4 x -174) = -256 k. Jmol-1

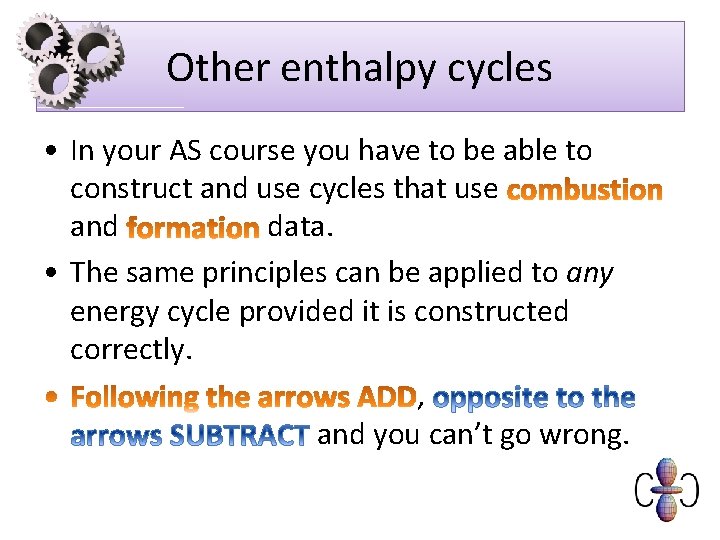
Other enthalpy cycles • In your AS course you have to be able to construct and use cycles that use and data. • The same principles can be applied to any energy cycle provided it is constructed correctly. , and you can’t go wrong.

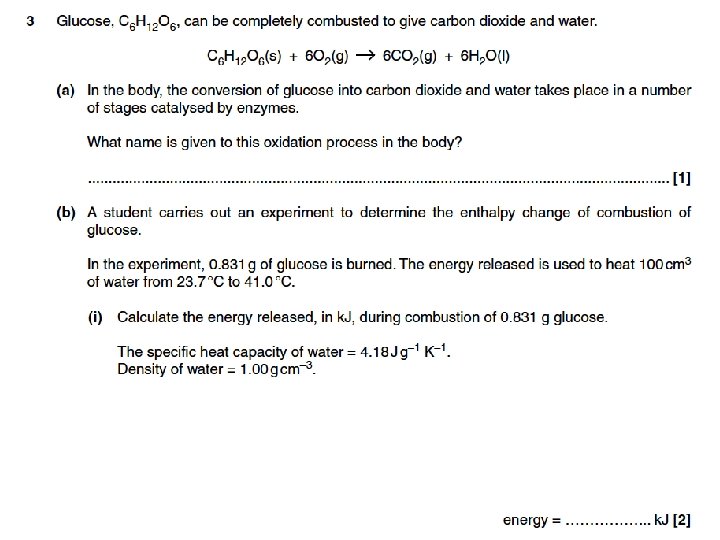
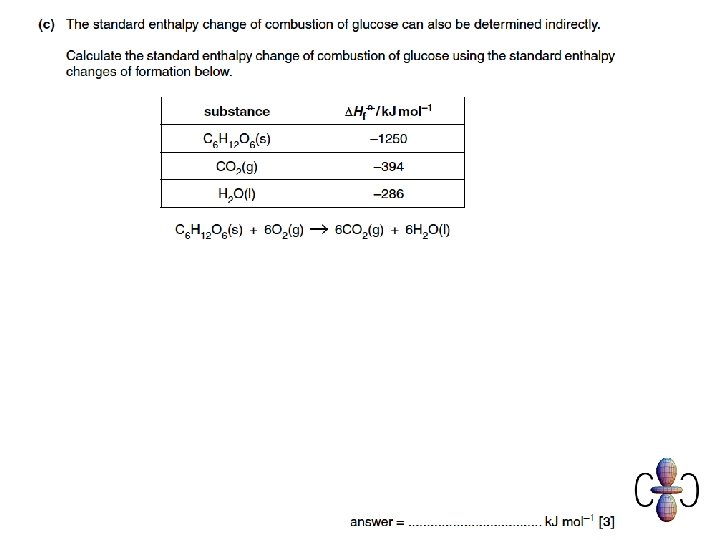
Examination question • Attempt these complete examination questions from January and June 2010 under test conditions to prepare for a mid-unit test next lesson.

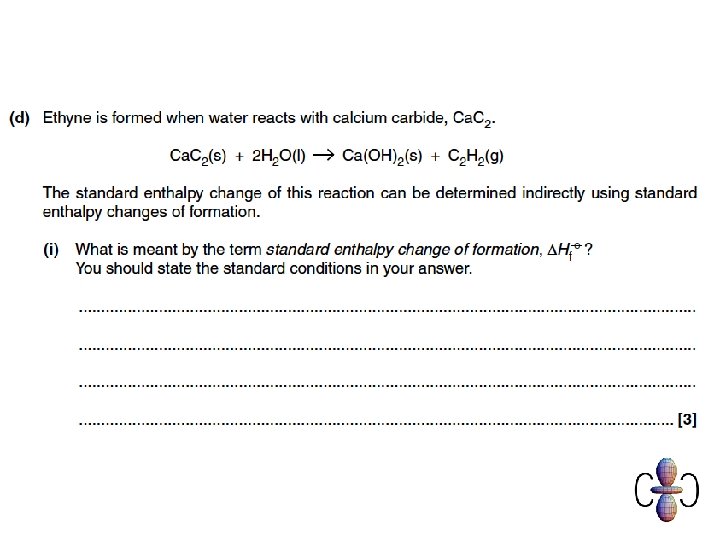
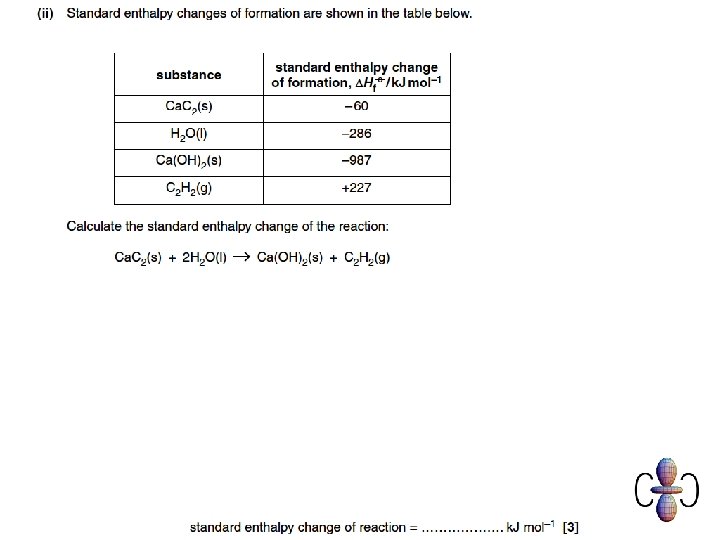
Question 1





Question 2




Mark schemes Cover until you have answered

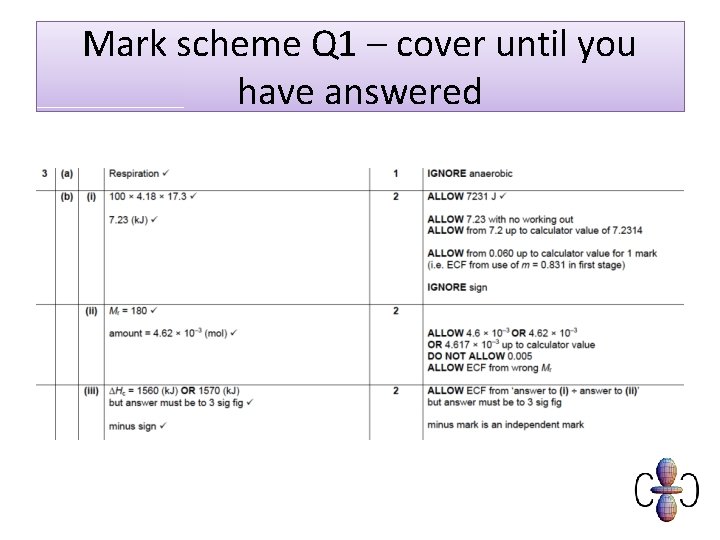
Mark scheme Q 1 – cover until you have answered

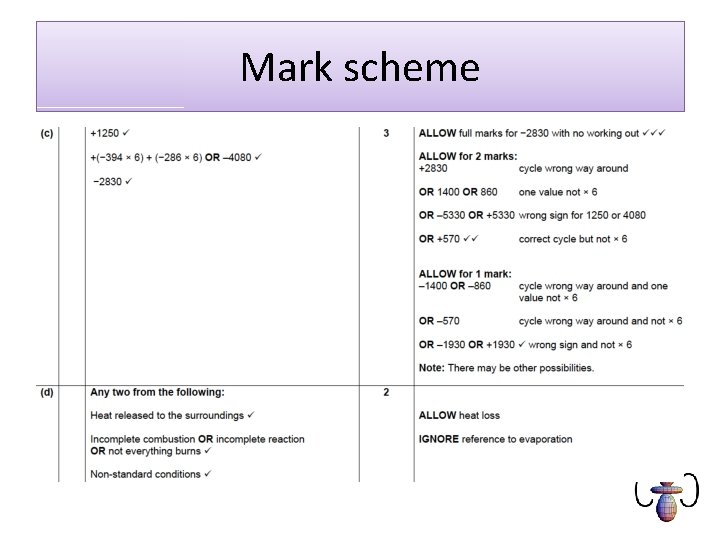
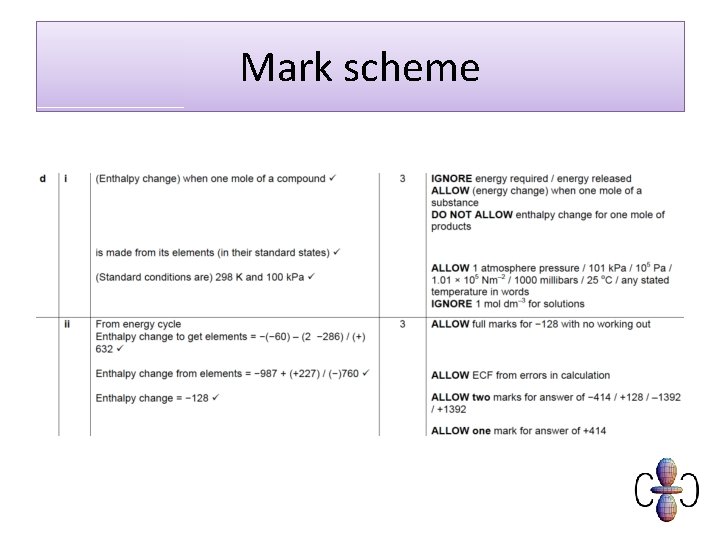
Mark scheme

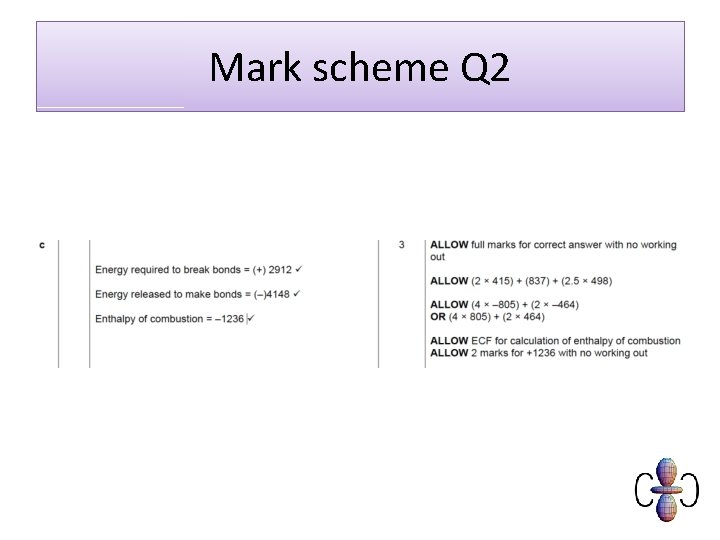
Mark scheme Q 2

Mark scheme