Methods of Calculating Enthalpy Hesss Law Standard Enthalpies




- Slides: 15

Methods of Calculating Enthalpy Hess’s Law & Standard Enthalpies of. Formation Unit 7 Lesson 3

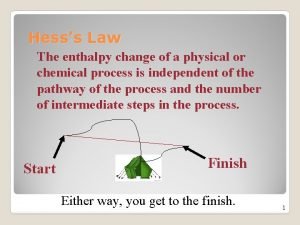
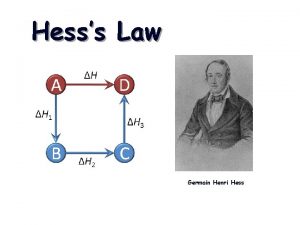
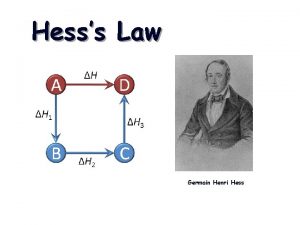
Hess’s Law H is well known for many reactions, and it is inconvenient to measure H for every reaction in which we are interested. However, we can estimate H using published H values and the properties of enthalpy.

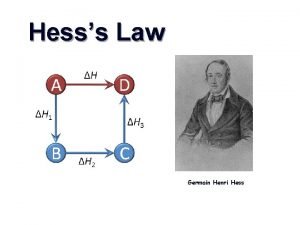
Hess’s Law • The heat released or absorbed in a chemical process is the same whether the process takes place in one or several steps • If two or more chemical equations can be added together to produce an overall equation, the sum of the enthalpy equals the enthalpy change of the overall equation. This is called the Heat of Summation, ∆H

Hess’s Law Steps Read through the whole question Plan a Strategy Evaluate the given equations. Rearrange and manipulate the equations so that they will produce the overall equation. (don’t forget to reverse the sign of ∆H if you reverse the reaction) Add the enthalpy terms.

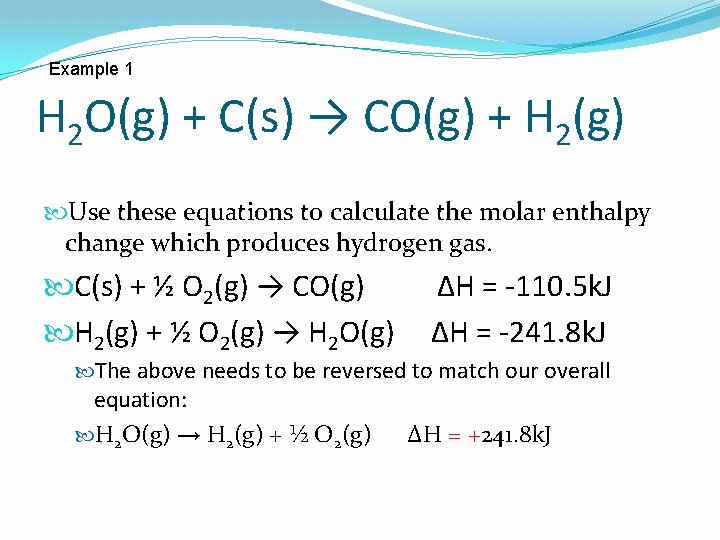
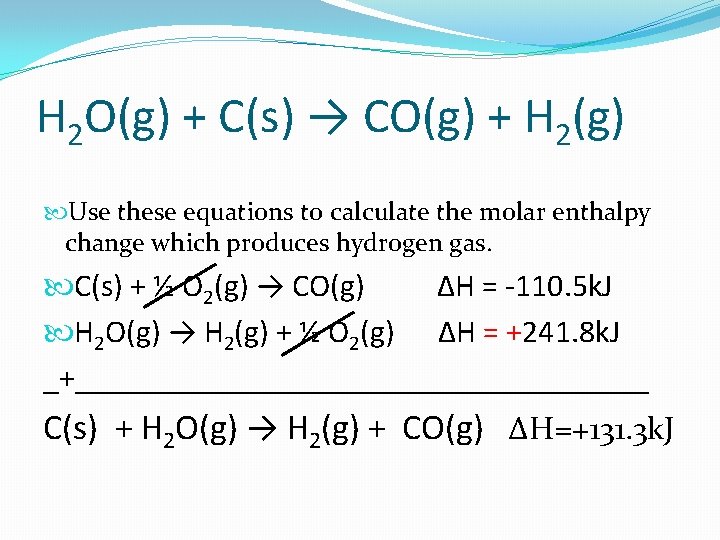
Example 1 H 2 O(g) + C(s) → CO(g) + H 2(g) Use these equations to calculate the molar enthalpy change which produces hydrogen gas. C(s) + ½ O 2(g) → CO(g) H 2(g) + ½ O 2(g) → H 2 O(g) ∆H = -110. 5 k. J ∆H = -241. 8 k. J The above needs to be reversed to match our overall equation: H 2 O(g) → H 2(g) + ½ O 2(g) ∆H = +241. 8 k. J

H 2 O(g) + C(s) → CO(g) + H 2(g) Use these equations to calculate the molar enthalpy change which produces hydrogen gas. C(s) + ½ O 2(g) → CO(g) ∆H = -110. 5 k. J H 2 O(g) → H 2(g) + ½ O 2(g) ∆H = +241. 8 k. J _+__________________ C(s) + H 2 O(g) → H 2(g) + CO(g) ∆H=+131. 3 k. J

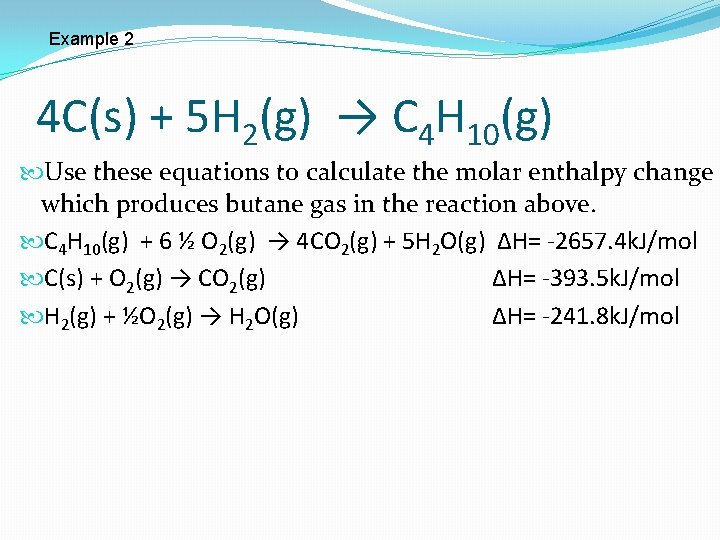
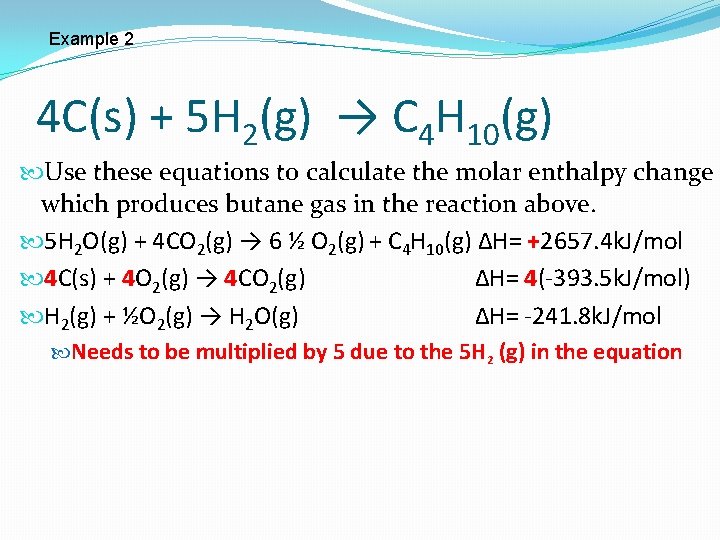
Example 2 4 C(s) + 5 H 2(g) → C 4 H 10(g) Use these equations to calculate the molar enthalpy change which produces butane gas in the reaction above. C 4 H 10(g) + 6 ½ O 2(g) → 4 CO 2(g) + 5 H 2 O(g) ∆H= -2657. 4 k. J/mol C(s) + O 2(g) → CO 2(g) ∆H= -393. 5 k. J/mol H 2(g) + ½O 2(g) → H 2 O(g) ∆H= -241. 8 k. J/mol

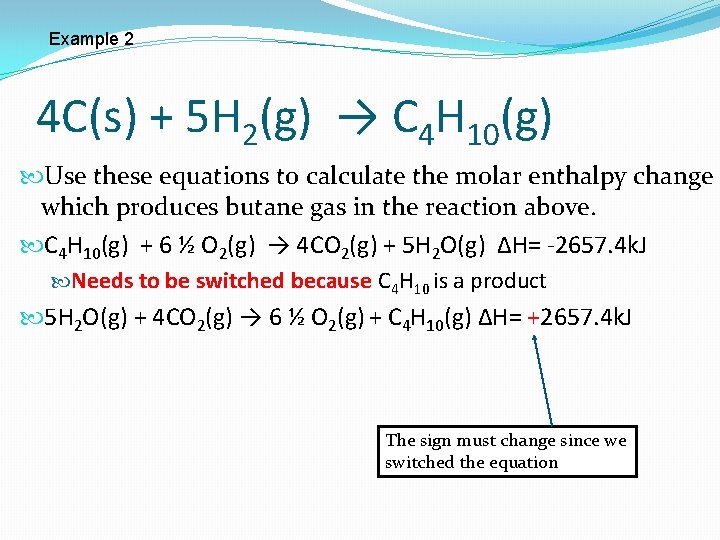
Example 2 4 C(s) + 5 H 2(g) → C 4 H 10(g) Use these equations to calculate the molar enthalpy change which produces butane gas in the reaction above. C 4 H 10(g) + 6 ½ O 2(g) → 4 CO 2(g) + 5 H 2 O(g) ∆H= -2657. 4 k. J Needs to be switched because C 4 H 10 is a product 5 H 2 O(g) + 4 CO 2(g) → 6 ½ O 2(g) + C 4 H 10(g) ∆H= +2657. 4 k. J The sign must change since we switched the equation

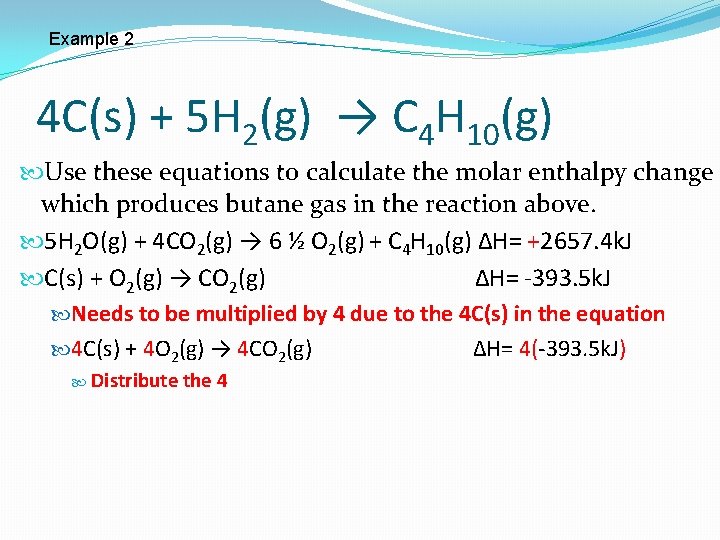
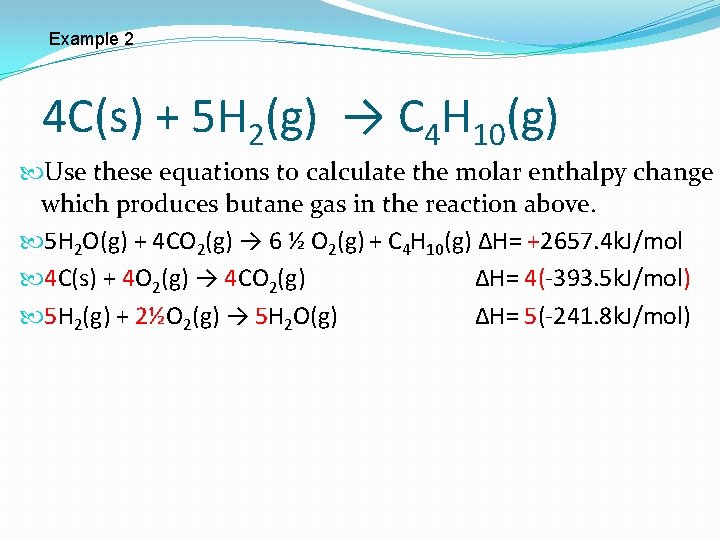
Example 2 4 C(s) + 5 H 2(g) → C 4 H 10(g) Use these equations to calculate the molar enthalpy change which produces butane gas in the reaction above. 5 H 2 O(g) + 4 CO 2(g) → 6 ½ O 2(g) + C 4 H 10(g) ∆H= +2657. 4 k. J C(s) + O 2(g) → CO 2(g) ∆H= -393. 5 k. J Needs to be multiplied by 4 due to the 4 C(s) in the equation 4 C(s) + 4 O 2(g) → 4 CO 2(g) ∆H= 4(-393. 5 k. J) Distribute the 4

Example 2 4 C(s) + 5 H 2(g) → C 4 H 10(g) Use these equations to calculate the molar enthalpy change which produces butane gas in the reaction above. 5 H 2 O(g) + 4 CO 2(g) → 6 ½ O 2(g) + C 4 H 10(g) ∆H= +2657. 4 k. J/mol 4 C(s) + 4 O 2(g) → 4 CO 2(g) ∆H= 4(-393. 5 k. J/mol) H 2(g) + ½O 2(g) → H 2 O(g) ∆H= -241. 8 k. J/mol Needs to be multiplied by 5 due to the 5 H 2 (g) in the equation

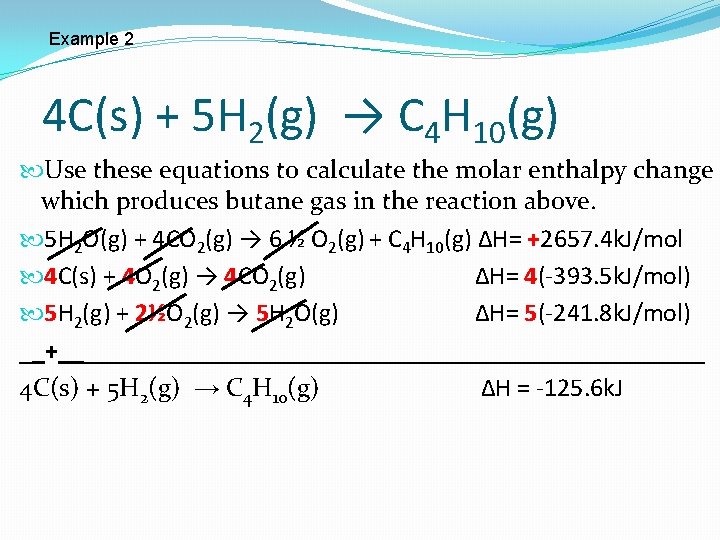
Example 2 4 C(s) + 5 H 2(g) → C 4 H 10(g) Use these equations to calculate the molar enthalpy change which produces butane gas in the reaction above. 5 H 2 O(g) + 4 CO 2(g) → 6 ½ O 2(g) + C 4 H 10(g) ∆H= +2657. 4 k. J/mol 4 C(s) + 4 O 2(g) → 4 CO 2(g) ∆H= 4(-393. 5 k. J/mol) 5 H 2(g) + 2½O 2(g) → 5 H 2 O(g) ∆H= 5(-241. 8 k. J/mol)

Example 2 4 C(s) + 5 H 2(g) → C 4 H 10(g) Use these equations to calculate the molar enthalpy change which produces butane gas in the reaction above. 5 H 2 O(g) + 4 CO 2(g) → 6 ½ O 2(g) + C 4 H 10(g) ∆H= +2657. 4 k. J/mol 4 C(s) + 4 O 2(g) → 4 CO 2(g) ∆H= 4(-393. 5 k. J/mol) 5 H 2(g) + 2½O 2(g) → 5 H 2 O(g) ∆H= 5(-241. 8 k. J/mol) __+_________________________ 4 C(s) + 5 H 2(g) → C 4 H 10(g) ∆H = -125. 6 k. J

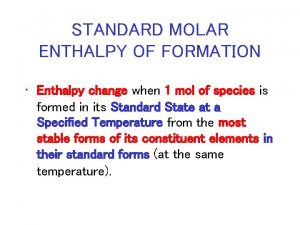
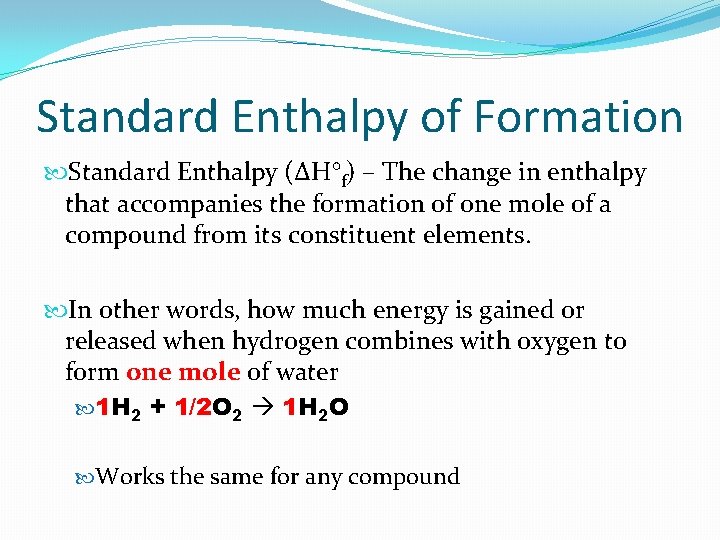
Standard Enthalpy of Formation Standard Enthalpy (ΔH°f) – The change in enthalpy that accompanies the formation of one mole of a compound from its constituent elements. In other words, how much energy is gained or released when hydrogen combines with oxygen to form one mole of water 1 H 2 + 1/2 O 2 1 H 2 O Works the same for any compound

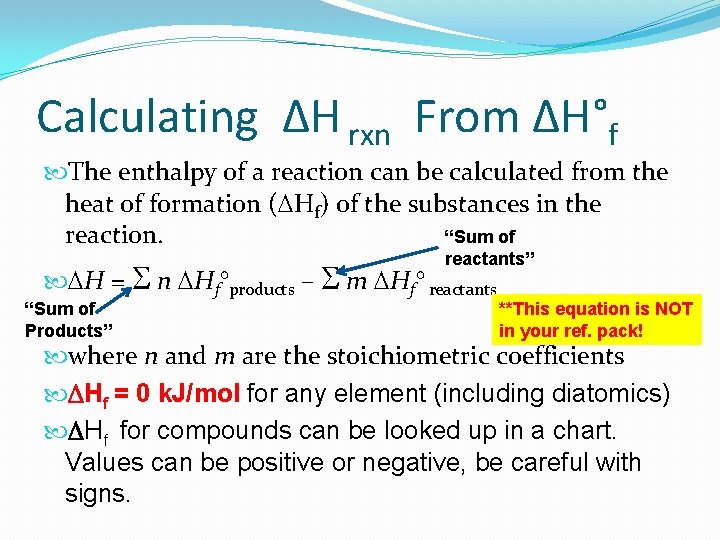
Calculating ΔH rxn From ΔH°f The enthalpy of a reaction can be calculated from the heat of formation ( Hf) of the substances in the reaction. “Sum of reactants” H = n Hf°products – m Hf° reactants “Sum of Products” **This equation is NOT in your ref. pack! where n and m are the stoichiometric coefficients DHf = 0 k. J/mol for any element (including diatomics) DHf for compounds can be looked up in a chart. Values can be positive or negative, be careful with signs.

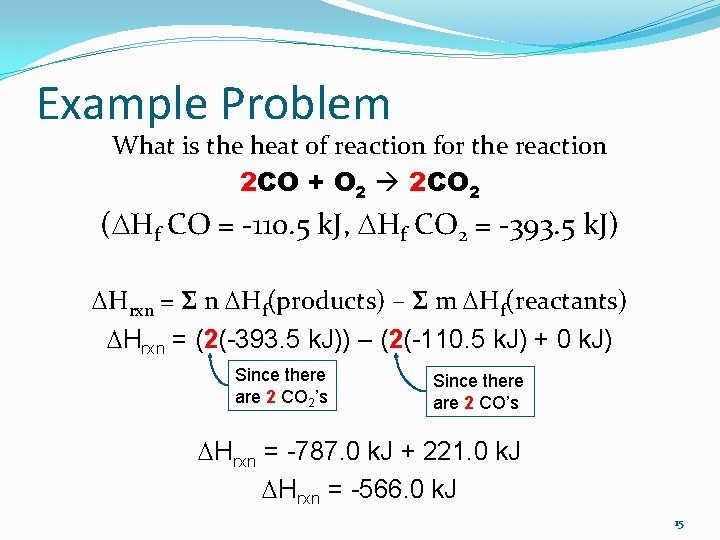
Example Problem What is the heat of reaction for the reaction 2 CO + O 2 2 CO 2 ( Hf CO = -110. 5 k. J, Hf CO 2 = -393. 5 k. J) Hrxn = Σ n Hf(products) – Σ m Hf(reactants) Hrxn = (2(-393. 5 k. J)) – (2(-110. 5 k. J) + 0 k. J) Since there are 2 CO 2’s Since there are 2 CO’s Hrxn = -787. 0 k. J + 221. 0 k. J Hrxn = -566. 0 k. J 15
 Application of hess law
Application of hess law Hesss law
Hesss law Hess's law
Hess's law Reactants minus products
Reactants minus products Hesss
Hesss Limitations of average bond enthalpies
Limitations of average bond enthalpies How to calculate enthalpy change
How to calculate enthalpy change Determine enthalpy of reaction
Determine enthalpy of reaction How to find the enthalpy change of a reaction
How to find the enthalpy change of a reaction Enthalpy change unit
Enthalpy change unit M.h.r method is used in
M.h.r method is used in Application of hess law
Application of hess law Standard enthalpy of reaction
Standard enthalpy of reaction Enthalpy of formation hess law
Enthalpy of formation hess law Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry