Hesss Law The enthalpy change of a physical




- Slides: 15

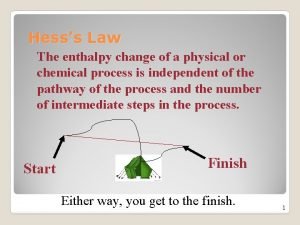
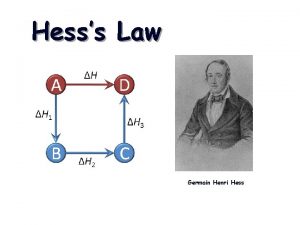
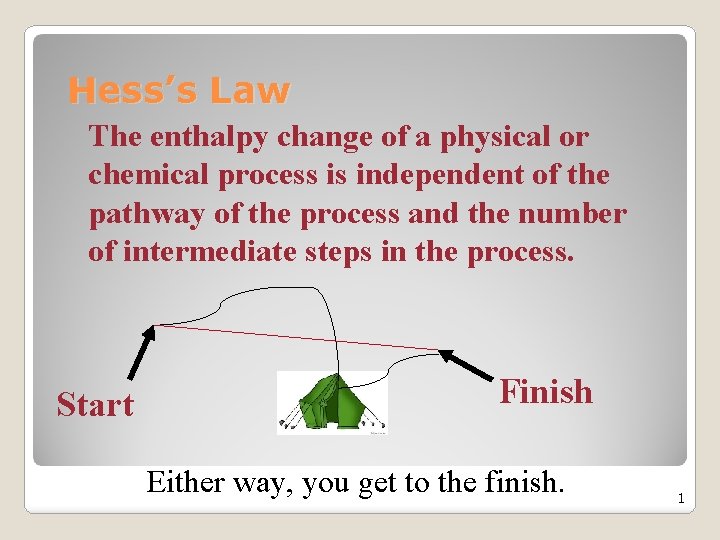
Hess’s Law The enthalpy change of a physical or chemical process is independent of the pathway of the process and the number of intermediate steps in the process. Start Finish Either way, you get to the finish. 1

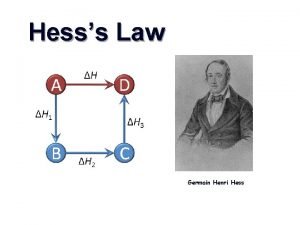
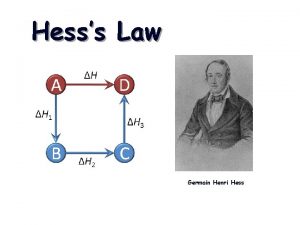
Hess’s law allows you to determine the energy of chemical reaction without directly measuring it. The enthalpy change of a chemical process is equal to the sum of the enthalpy changes of all the individual steps that make up the process. 2

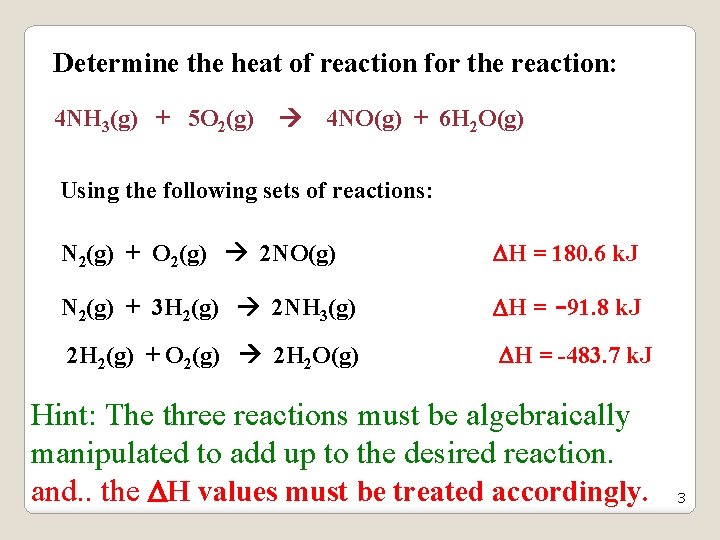
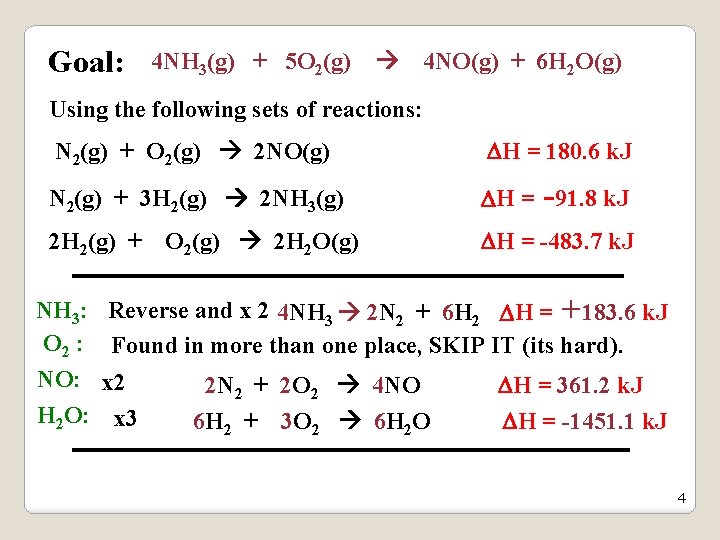
Determine the heat of reaction for the reaction: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Using the following sets of reactions: N 2(g) + O 2(g) 2 NO(g) H = 180. 6 k. J N 2(g) + 3 H 2(g) 2 NH 3(g) H = -91. 8 k. J 2 H 2(g) + O 2(g) 2 H 2 O(g) H = -483. 7 k. J Hint: The three reactions must be algebraically manipulated to add up to the desired reaction. and. . the H values must be treated accordingly. 3

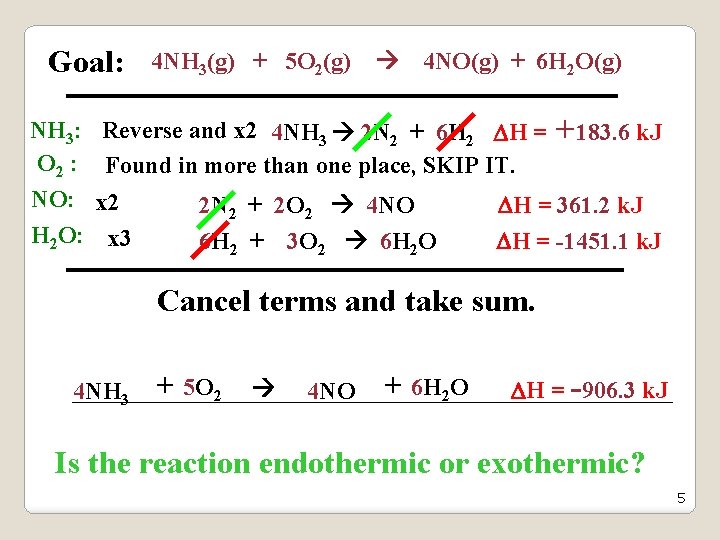
Goal: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Using the following sets of reactions: N 2(g) + O 2(g) 2 NO(g) H = 180. 6 k. J N 2(g) + 3 H 2(g) 2 NH 3(g) H = -91. 8 k. J 2 H 2(g) + O 2(g) 2 H 2 O(g) H = -483. 7 k. J NH 3: Reverse and x 2 4 NH 3 2 N 2 + 6 H 2 H = +183. 6 k. J O 2 : Found in more than one place, SKIP IT (its hard). NO: x 2 2 N 2 + 2 O 2 4 NO H = 361. 2 k. J H 2 O: x 3 6 H 2 + 3 O 2 6 H 2 O H = -1451. 1 k. J 4

Goal: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) NH 3: Reverse and x 2 4 NH 3 2 N 2 + 6 H 2 H = +183. 6 k. J O 2 : Found in more than one place, SKIP IT. NO: x 2 2 N 2 + 2 O 2 4 NO H = 361. 2 k. J H 2 O: x 3 6 H 2 + 3 O 2 6 H 2 O H = -1451. 1 k. J Cancel terms and take sum. 4 NH 3 + 5 O 2 4 NO + 6 H 2 O H = -906. 3 k. J Is the reaction endothermic or exothermic? 5

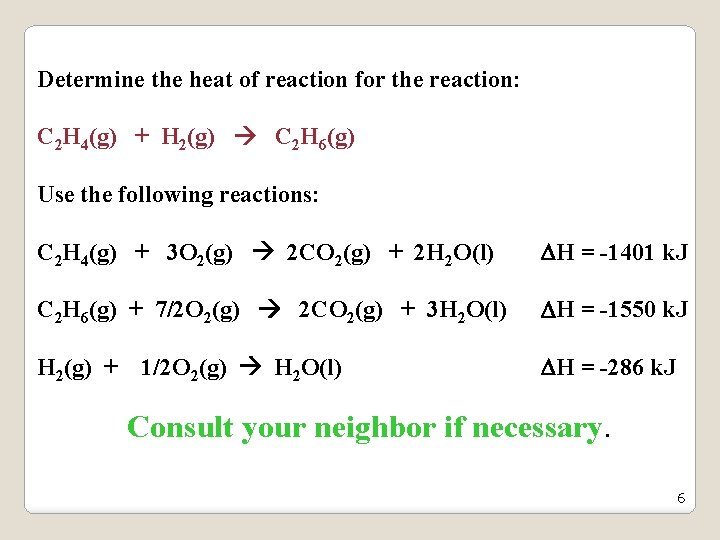
Determine the heat of reaction for the reaction: C 2 H 4(g) + H 2(g) C 2 H 6(g) Use the following reactions: C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J C 2 H 6(g) + 7/2 O 2(g) 2 CO 2(g) + 3 H 2 O(l) H = -1550 k. J H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J Consult your neighbor if necessary. 6

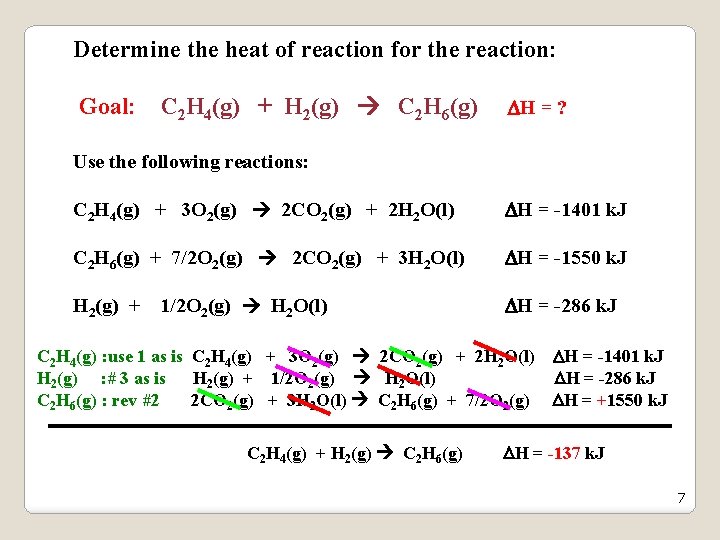
Determine the heat of reaction for the reaction: Goal: C 2 H 4(g) + H 2(g) C 2 H 6(g) H = ? Use the following reactions: C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J C 2 H 6(g) + 7/2 O 2(g) 2 CO 2(g) + 3 H 2 O(l) H = -1550 k. J H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J C 2 H 4(g) : use 1 as is C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(l) H = -1401 k. J H 2(g) : # 3 as is H 2(g) + 1/2 O 2(g) H 2 O(l) H = -286 k. J C 2 H 6(g) : rev #2 2 CO 2(g) + 3 H 2 O(l) C 2 H 6(g) + 7/2 O 2(g) H = +1550 k. J C 2 H 4(g) + H 2(g) C 2 H 6(g) H = -137 k. J 7

Homework: Page 317 #1 -3 8

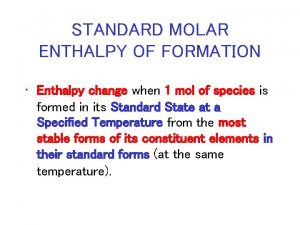
Where do the reference equations come from? Many can be determined through calorimetry. ie. combustion reactions Another type of reference equation is called standard enthalpies of formation. 9

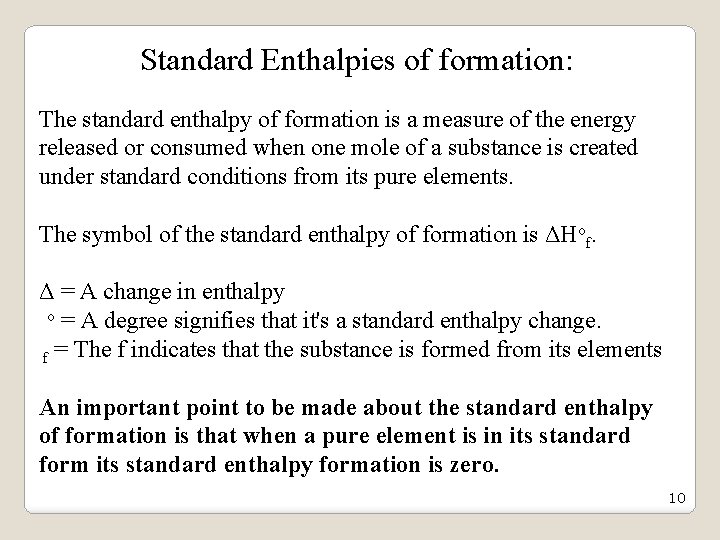
Standard Enthalpies of formation: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions from its pure elements. The symbol of the standard enthalpy of formation is ΔHof. Δ = A change in enthalpy o = A degree signifies that it's a standard enthalpy change. f = The f indicates that the substance is formed from its elements An important point to be made about the standard enthalpy of formation is that when a pure element is in its standard form its standard enthalpy formation is zero. 10

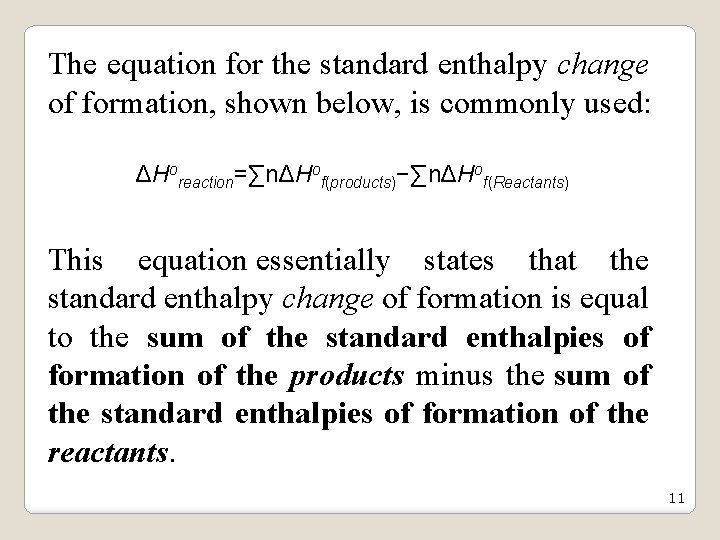
The equation for the standard enthalpy change of formation, shown below, is commonly used: ΔHoreaction=∑nΔHof(products)−∑nΔHof(Reactants) This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the products minus the sum of the standard enthalpies of formation of the reactants. 11

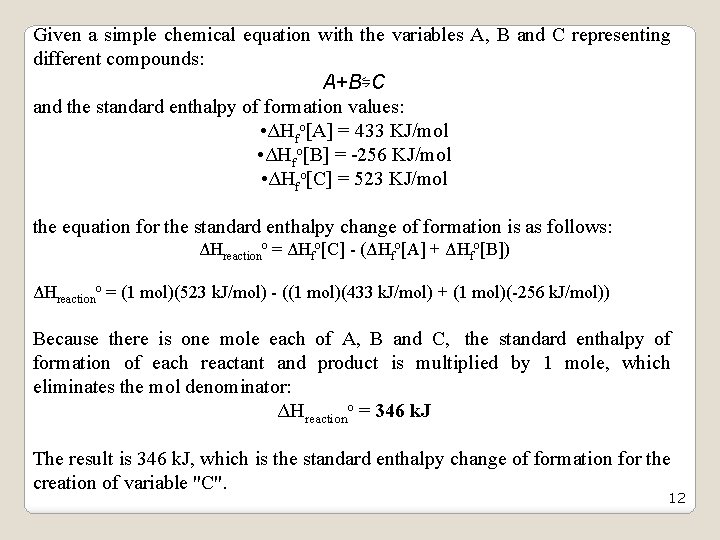
Given a simple chemical equation with the variables A, B and C representing different compounds: A+B⇋C and the standard enthalpy of formation values: • ΔHfo[A] = 433 KJ/mol • ΔHfo[B] = -256 KJ/mol • ΔHfo[C] = 523 KJ/mol the equation for the standard enthalpy change of formation is as follows: ΔHreactiono = ΔHfo[C] - (ΔHfo[A] + ΔHfo[B]) ΔHreactiono = (1 mol)(523 k. J/mol) - ((1 mol)(433 k. J/mol) + (1 mol)(-256 k. J/mol)) Because there is one mole each of A, B and C, the standard enthalpy of formation of each reactant and product is multiplied by 1 mole, which eliminates the mol denominator: ΔHreactiono = 346 k. J The result is 346 k. J, which is the standard enthalpy change of formation for the creation of variable "C". 12

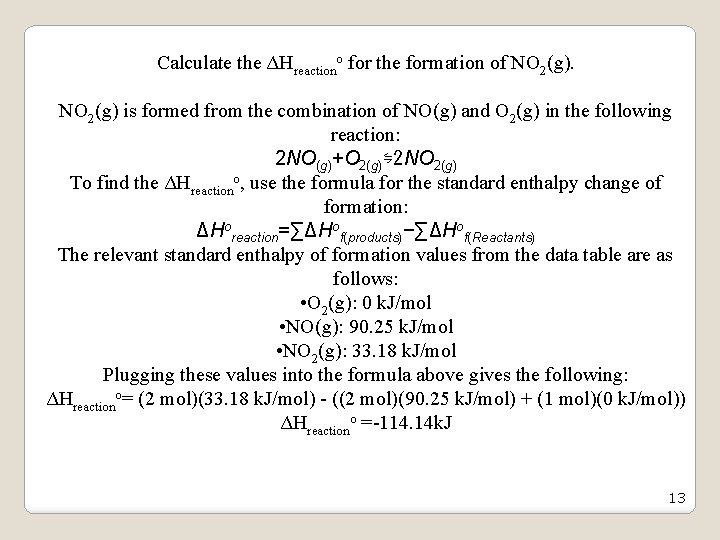
Calculate the ΔHreactiono for the formation of NO 2(g) is formed from the combination of NO(g) and O 2(g) in the following reaction: 2 NO(g)+O 2(g)⇋2 NO 2(g) To find the ΔHreactiono, use the formula for the standard enthalpy change of formation: ΔHoreaction=∑ΔHof(products)−∑ΔHof(Reactants) The relevant standard enthalpy of formation values from the data table are as follows: • O 2(g): 0 k. J/mol • NO(g): 90. 25 k. J/mol • NO 2(g): 33. 18 k. J/mol Plugging these values into the formula above gives the following: ΔHreactiono= (2 mol)(33. 18 k. J/mol) - ((2 mol)(90. 25 k. J/mol) + (1 mol)(0 k. J/mol)) ΔHreactiono =-114. 14 k. J 13

Homework: Page 324 #1 -6 14

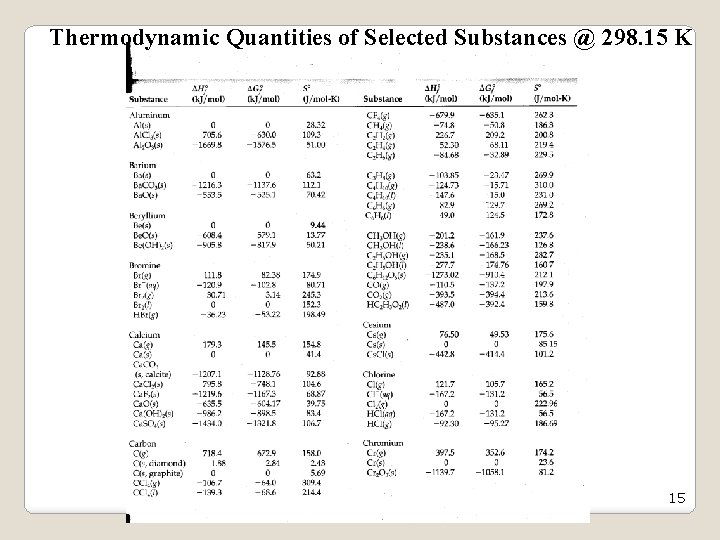
Thermodynamic Quantities of Selected Substances @ 298. 15 K 15
 Hess's law rules
Hess's law rules Hesss law
Hesss law Hess's law example
Hess's law example Hesss
Hesss Is painting a wall a physical change
Is painting a wall a physical change Physical change
Physical change Difference between physical and chemical change
Difference between physical and chemical change Examples of physical vs chemical changes
Examples of physical vs chemical changes Spare change physical versus chemical change
Spare change physical versus chemical change Whats the difference between physical and chemical change
Whats the difference between physical and chemical change Physical change
Physical change Chemical change
Chemical change Chopping of wood is which change
Chopping of wood is which change Unit of enthalpy change
Unit of enthalpy change Enthalpy change in a potential energy diagram
Enthalpy change in a potential energy diagram Molar enthalpy change formula
Molar enthalpy change formula