5 4 Hesss Law The laws of thermodynamics




- Slides: 11

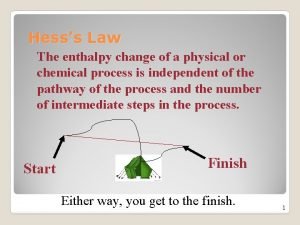
5. 4 Hess’s Law • The laws of thermodynamics only concern themselves with the initial and final enthalpy states. • Enthalpy change is independent of the reaction pathway, process or number of steps. • Whether one takes the stairs or the elevator doesn’t change the fact that they have moved from one floor to another. 1

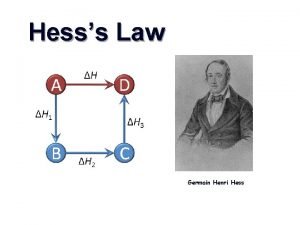
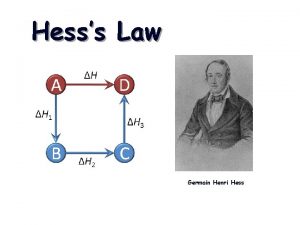
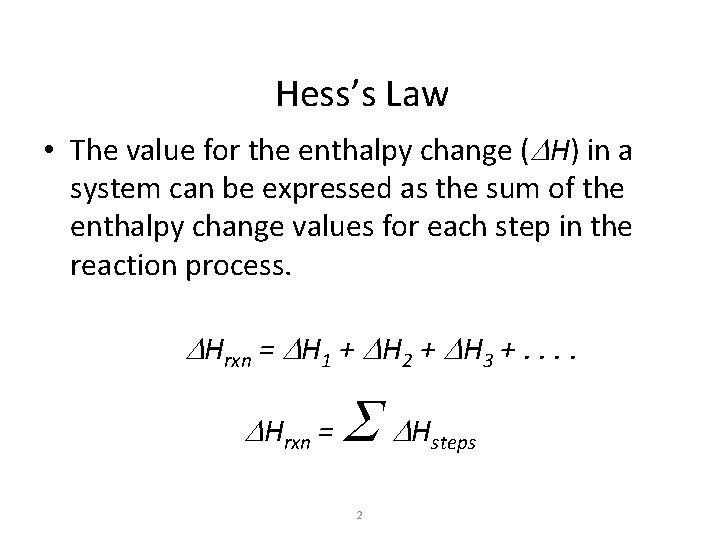
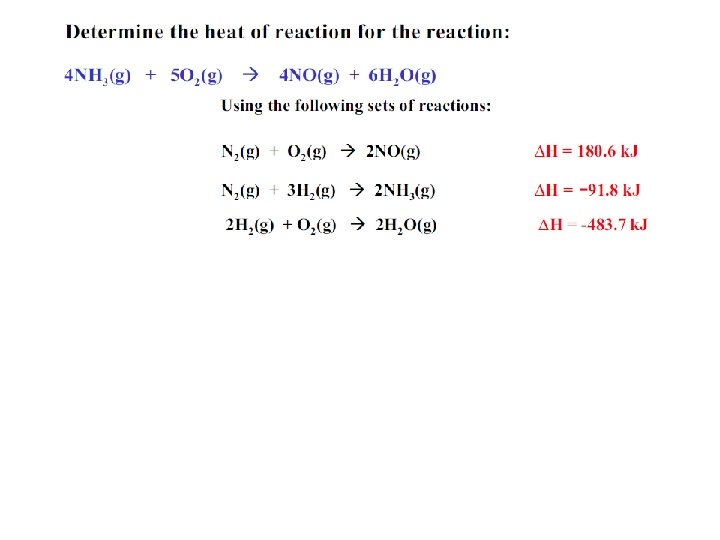
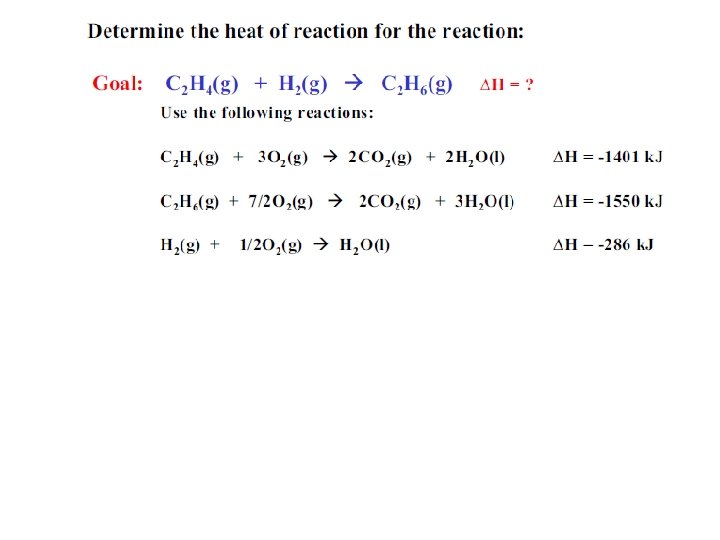
Hess’s Law • The value for the enthalpy change (DH) in a system can be expressed as the sum of the enthalpy change values for each step in the reaction process. DHrxn = DH 1 + DH 2 + DH 3 +. . DHrxn = S DH 2 steps

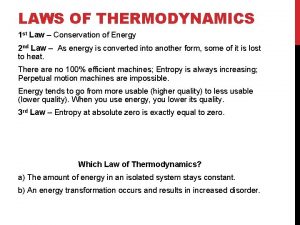
Hess’s Law • Rules to application of Hess’s Law; 1 - If a chemical reaction is reversed, then the sign of the DH changes. (- DH + DH) 2 - If the coefficients of a chemical reaction are multiplied by a factor, then the DH value is also multiplied by that same factor. 3

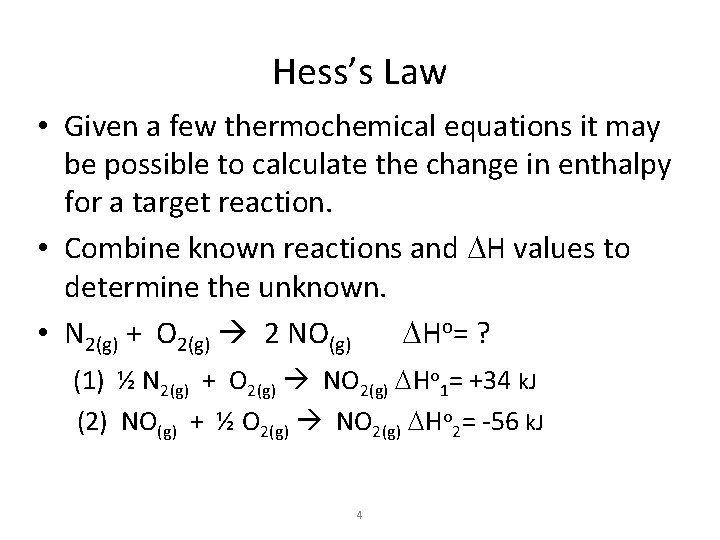
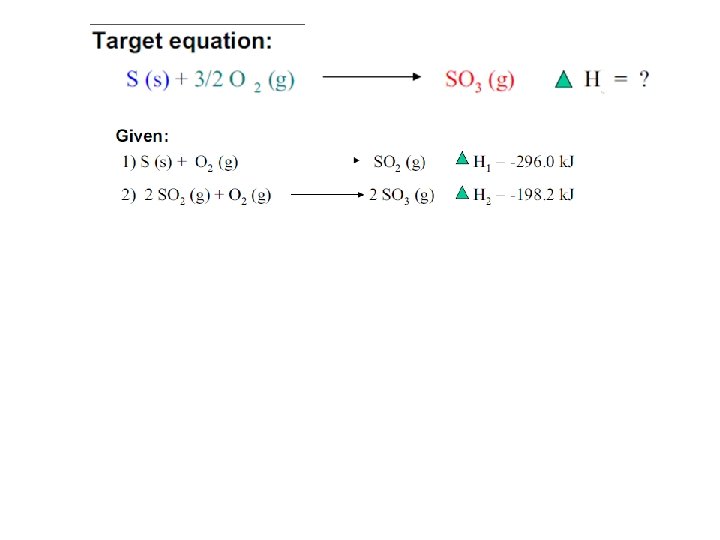
Hess’s Law • Given a few thermochemical equations it may be possible to calculate the change in enthalpy for a target reaction. • Combine known reactions and DH values to determine the unknown. • N 2(g) + O 2(g) 2 NO(g) DHo= ? (1) ½ N 2(g) + O 2(g) NO 2(g) DHo 1= +34 k. J (2) NO(g) + ½ O 2(g) NO 2(g) DHo 2= -56 k. J 4

Hess’s Law DHo= ? • N 2(g) + O 2(g) 2 NO(g) (1) ½ N 2(g) + O 2(g) NO 2(g) DHo 1= +34 k. J/mol (2) NO(g) + ½ O 2(g) NO 2(g) DHo 2= -56 k. J/mol 5

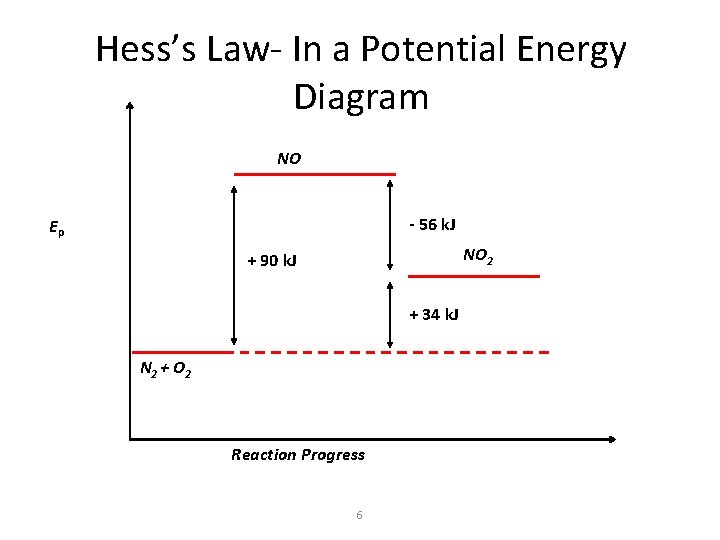
Hess’s Law- In a Potential Energy Diagram NO - 56 k. J Ep NO 2 + 90 k. J + 34 k. J N 2 + O 2 Reaction Progress 6

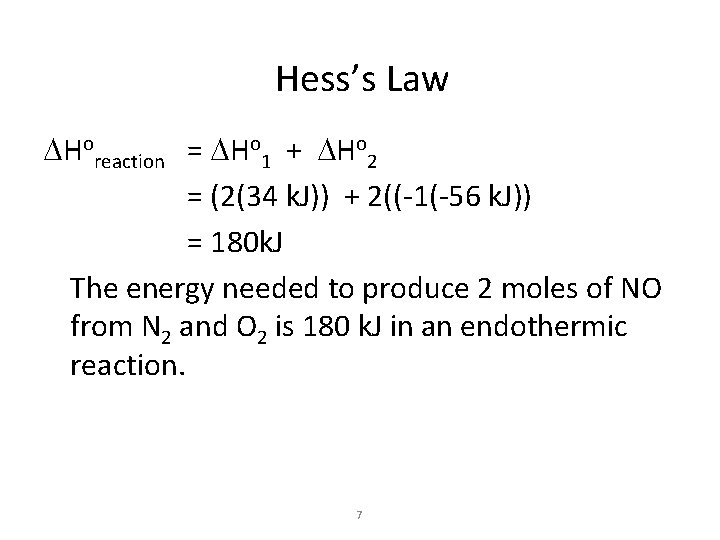
Hess’s Law DHoreaction = DHo 1 + DHo 2 = (2(34 k. J)) + 2((-1(-56 k. J)) = 180 k. J The energy needed to produce 2 moles of NO from N 2 and O 2 is 180 k. J in an endothermic reaction. 7




Work • Pg. 318 # 4 -8