HESSS LAW 17 1 cont HESSS LAW The

- Slides: 11

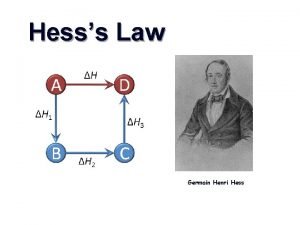
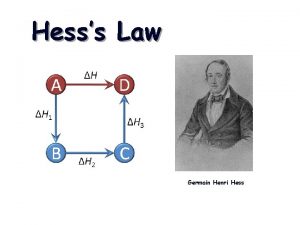
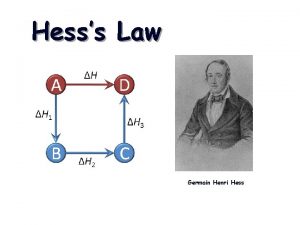
HESS’S LAW (17 -1 cont. )

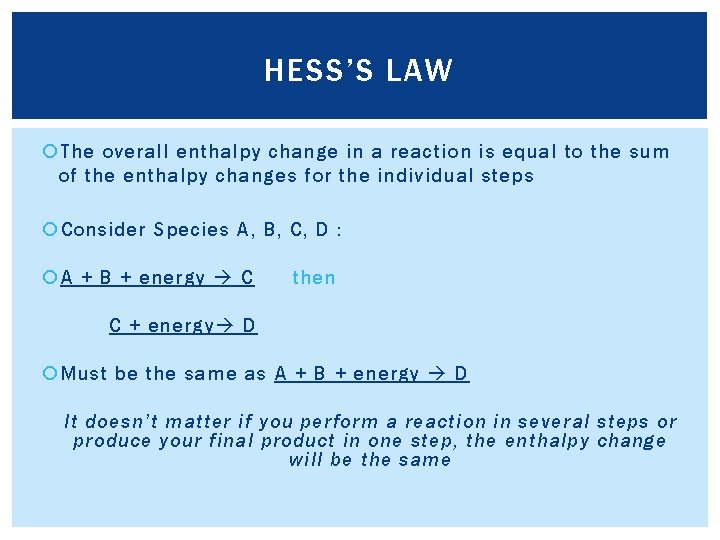
HESS’S LAW The overall enthalpy change in a reaction is equal to the sum of the enthalpy changes for the individual steps Consider Species A, B, C, D : A + B + energy C then C + energy D Must be the same as A + B + energy D It doesn’t matter if you perform a reaction in several steps or produce your final product in one step, the enthalpy change will be the same

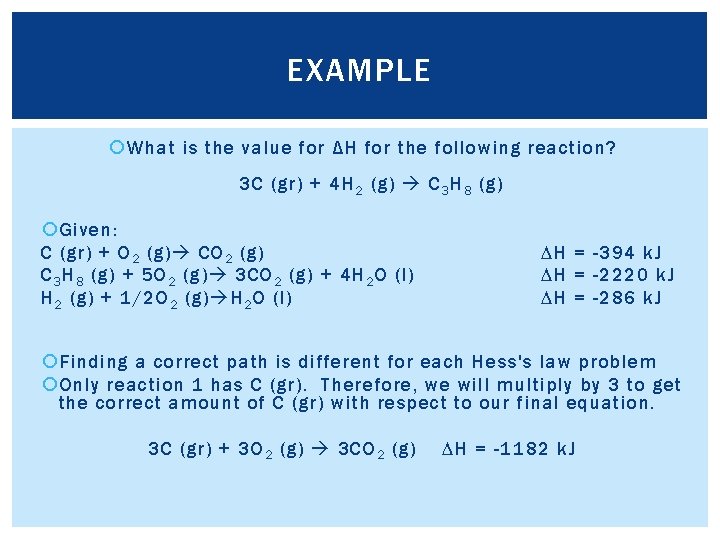
EXAMPLE What is the value for ΔH for the following reaction? 3 C (gr) + 4 H 2 (g) C 3 H 8 (g) Given: C (gr) + O 2 (g) C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) H 2 (g) + 1/2 O 2 (g) H 2 O (l) DH = -394 k. J DH = -2220 k. J DH = -286 k. J Finding a correct path is different for each Hess's law problem Only reaction 1 has C (gr). Therefore, we will multiply by 3 to get the correct amount of C (gr) with respect to our final equation. 3 C (gr) + 3 O 2 (g) 3 CO 2 (g) DH = -1182 k. J

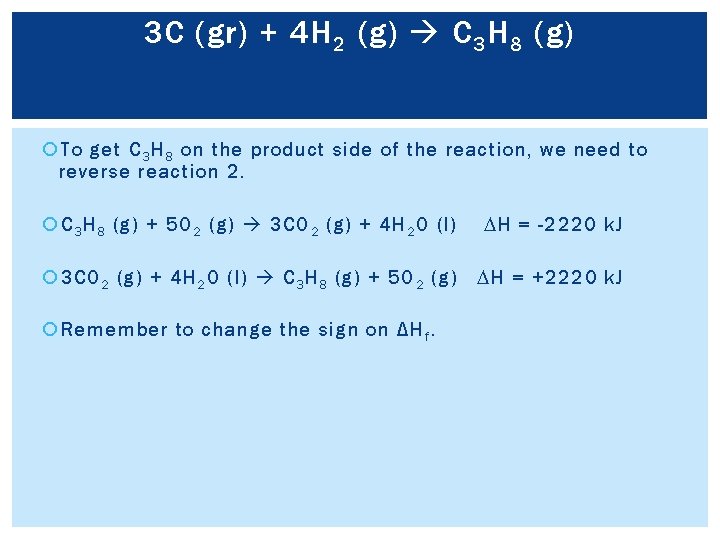
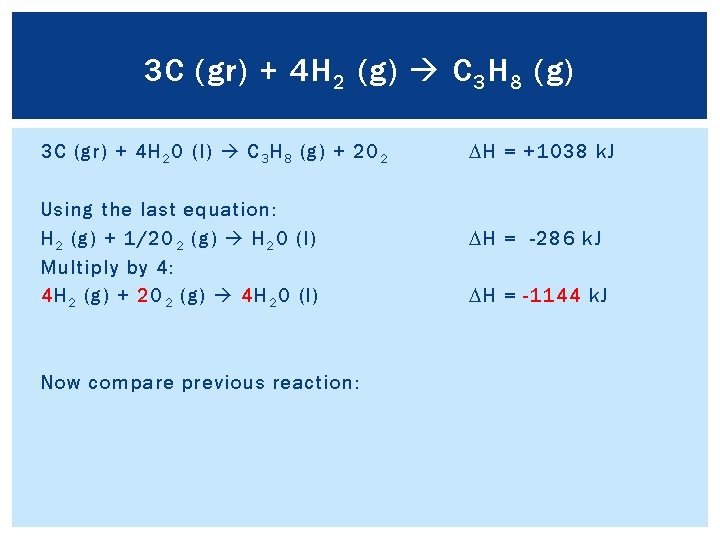
3 C (gr) + 4 H 2 (g) C 3 H 8 (g) To get C 3 H 8 on the product side of the reaction, we need to reverse reaction 2. C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) DH = -2220 k. J 3 CO 2 (g) + 4 H 2 O (l) C 3 H 8 (g) + 5 O 2 (g) DH = +2220 k. J Remember to change the sign on ΔH f.

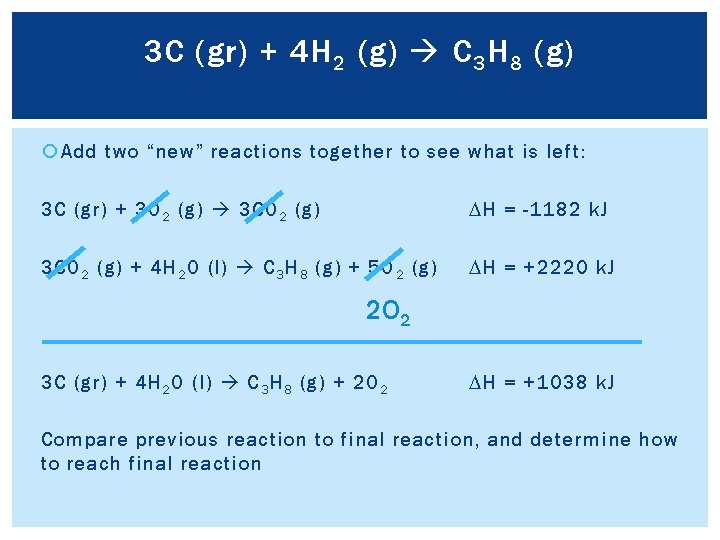
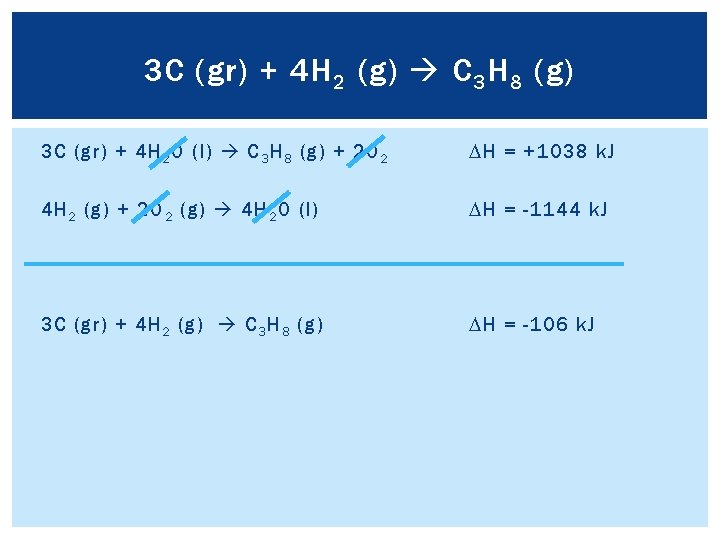
3 C (gr) + 4 H 2 (g) C 3 H 8 (g) Add two “new” reactions together to see what is left: 3 C (gr) + 3 O 2 (g) 3 CO 2 (g) DH = -1182 k. J 3 CO 2 (g) + 4 H 2 O (l) C 3 H 8 (g) + 5 O 2 (g) DH = +2220 k. J 2 O 2 3 C (gr) + 4 H 2 O (l) C 3 H 8 (g) + 2 O 2 DH = +1038 k. J Compare previous reaction to final reaction, and determine how to reach final reaction

3 C (gr) + 4 H 2 (g) C 3 H 8 (g) 3 C (gr) + 4 H 2 O (l) C 3 H 8 (g) + 2 O 2 Using the last equation: H 2 (g) + 1/2 O 2 (g) H 2 O (l) Multiply by 4: 4 H 2 (g) + 2 O 2 (g) 4 H 2 O (l) Now compare previous reaction: DH = +1038 k. J DH = -286 k. J DH = -1144 k. J

3 C (gr) + 4 H 2 (g) C 3 H 8 (g) 3 C (gr) + 4 H 2 O (l) C 3 H 8 (g) + 2 O 2 DH = +1038 k. J 4 H 2 (g) + 2 O 2 (g) 4 H 2 O (l) DH = -1144 k. J 3 C (gr) + 4 H 2 (g) C 3 H 8 (g) DH = -106 k. J

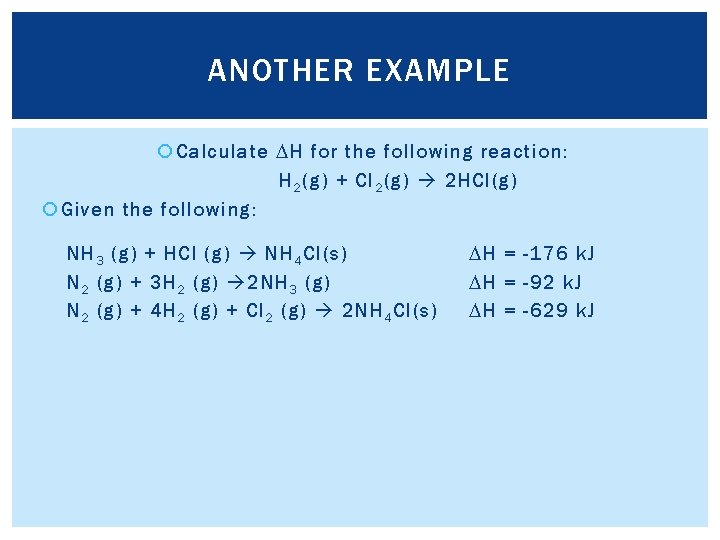
ANOTHER EXAMPLE Calculate DH for the following reaction: H 2 (g) + Cl 2 (g) 2 HCl(g) Given the following: NH 3 (g) + HCl (g) NH 4 Cl(s) N 2 (g) + 3 H 2 (g) 2 NH 3 (g) N 2 (g) + 4 H 2 (g) + Cl 2 (g) 2 NH 4 Cl(s) DH = -176 k. J DH = -92 k. J DH = -629 k. J

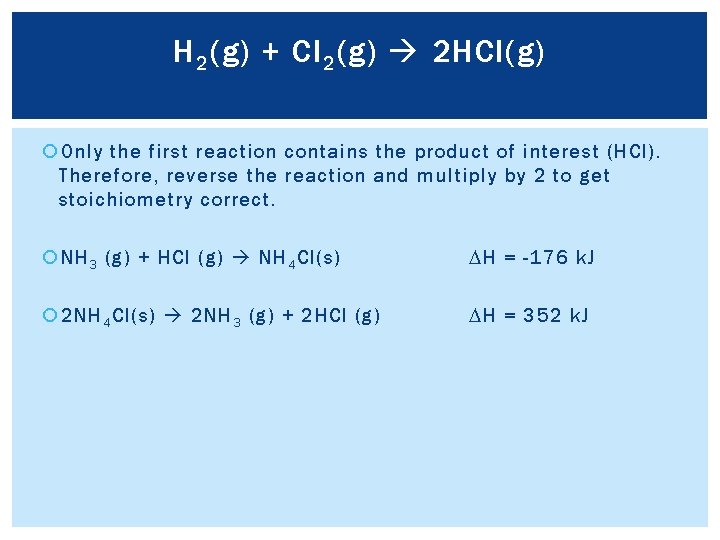
H 2 (g) + Cl 2 (g) 2 HCl(g) Only the first reaction contains the product of interest (HCl). Therefore, reverse the reaction and multiply by 2 to get stoichiometry correct. NH 3 (g) + HCl (g) NH 4 Cl(s) DH = -176 k. J 2 NH 4 Cl(s) 2 NH 3 (g) + 2 HCl (g) DH = 352 k. J

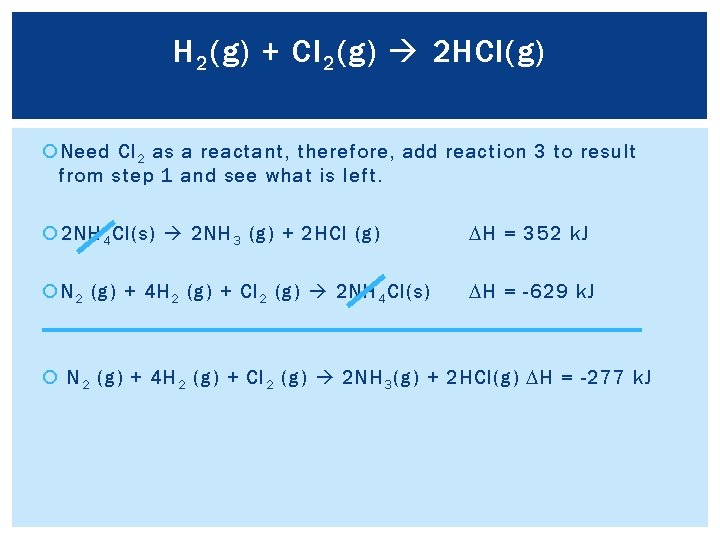
H 2 (g) + Cl 2 (g) 2 HCl(g) Need Cl 2 as a reactant, therefore, add reaction 3 to result from step 1 and see what is left. 2 NH 4 Cl(s) 2 NH 3 (g) + 2 HCl (g) DH = 352 k. J N 2 (g) + 4 H 2 (g) + Cl 2 (g) 2 NH 4 Cl(s) DH = -629 k. J N 2 (g) + 4 H 2 (g) + Cl 2 (g) 2 NH 3 (g) + 2 HCl(g) DH = -277 k. J

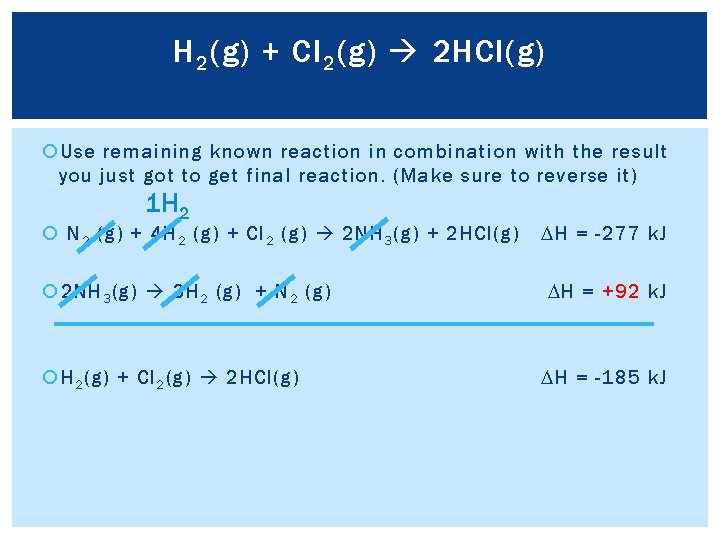
H 2 (g) + Cl 2 (g) 2 HCl(g) Use remaining known reaction in combination with the result you just got to get final reaction. (Make sure to reverse it) 1 H 2 N 2 (g) + 4 H 2 (g) + Cl 2 (g) 2 NH 3 (g) + 2 HCl(g) 2 NH 3 (g) 3 H 2 (g) + N 2 (g) H 2 (g) + Cl 2 (g) 2 HCl(g) DH = -277 k. J DH = +92 k. J DH = -185 k. J