Hesss Law 5 3 ENERGETICS HESSS LAW Hesss







- Slides: 22

Hess’s Law 5. 3 ENERGETICS

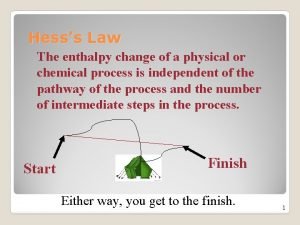
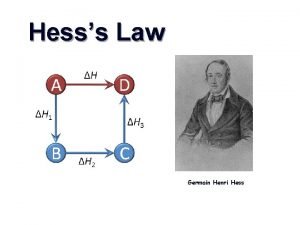
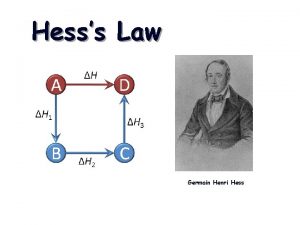
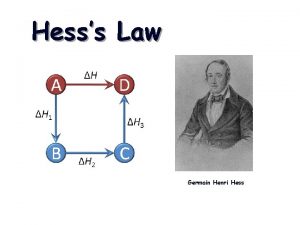
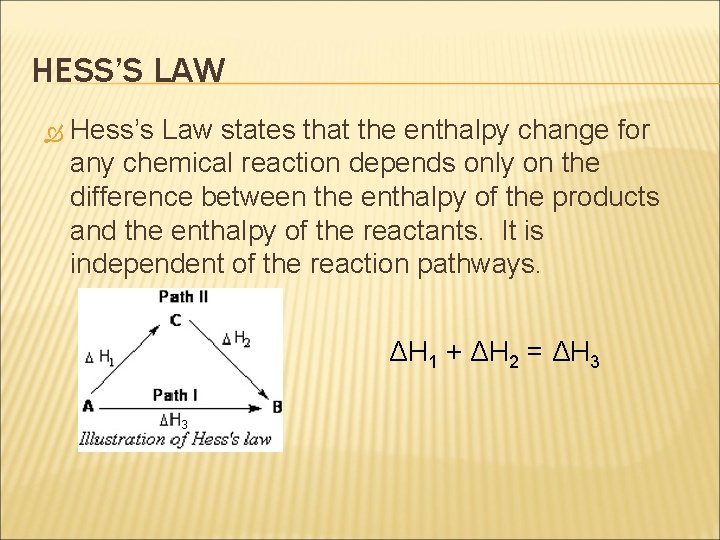
HESS’S LAW Hess’s Law states that the enthalpy change for any chemical reaction depends only on the difference between the enthalpy of the products and the enthalpy of the reactants. It is independent of the reaction pathways. ΔH 1 + ΔH 2 = ΔH 3 3

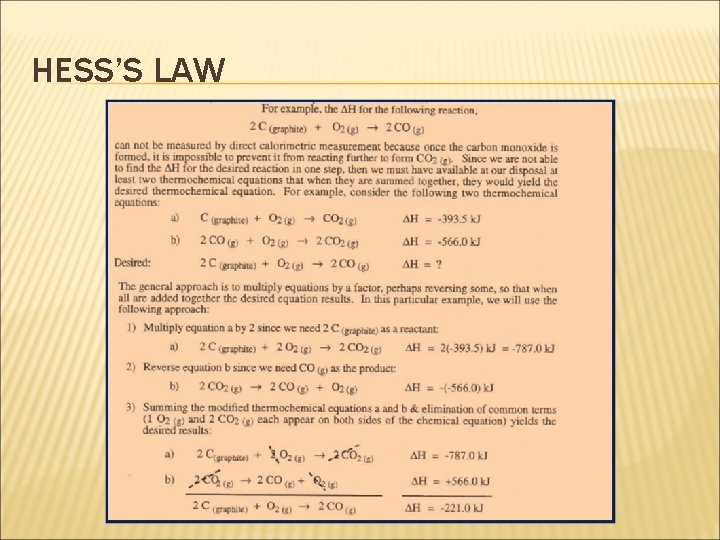
HESS’S LAW Why is this important? Hess’s Law allows us to calculate the enthalpy changes of reactions that cannot be measured directly in the laboratory. For example, although the elements carbon and hydrogen do not combine directly to form propane, the enthalpy change for the reaction: 3 C(graphite) + 4 H 2(g) C 3 H 8(g) can be calculated from the enthalpy of combustion data of the elements and the compound.

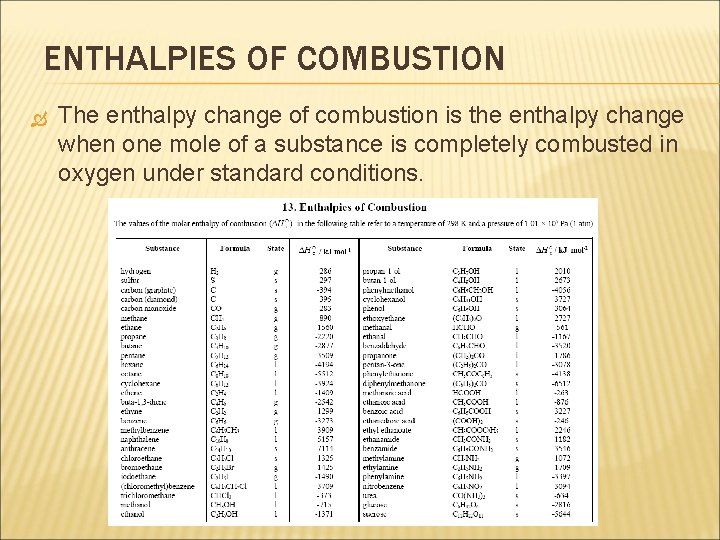
ENTHALPIES OF COMBUSTION The enthalpy change of combustion is the enthalpy change when one mole of a substance is completely combusted in oxygen under standard conditions.

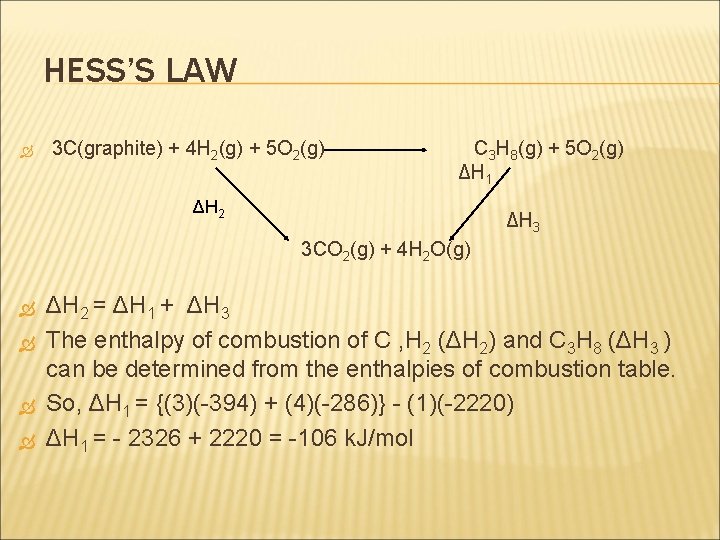
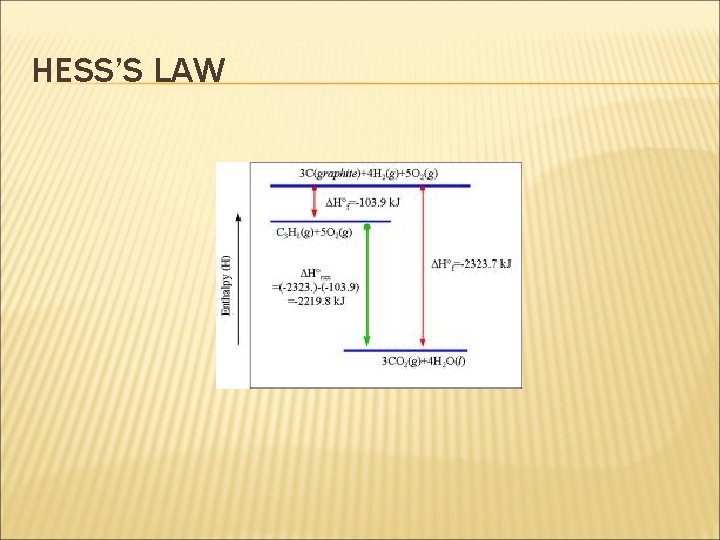
HESS’S LAW 3 C(graphite) + 4 H 2(g) + 5 O 2(g) C 3 H 8(g) + 5 O 2(g) ΔH 1 ΔH 2 ΔH 3 3 CO 2(g) + 4 H 2 O(g) ΔH 2 = ΔH 1 + ΔH 3 The enthalpy of combustion of C , H 2 (ΔH 2) and C 3 H 8 (ΔH 3 ) can be determined from the enthalpies of combustion table. So, ΔH 1 = {(3)(-394) + (4)(-286)} - (1)(-2220) ΔH 1 = - 2326 + 2220 = -106 k. J/mol

HESS’S LAW

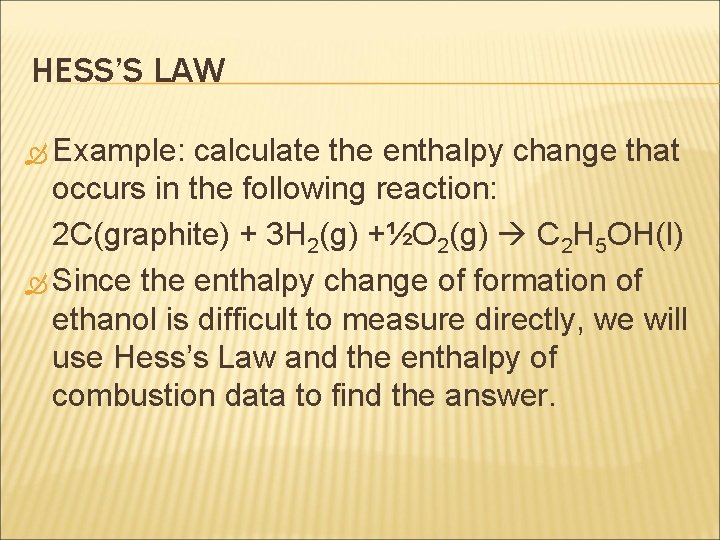
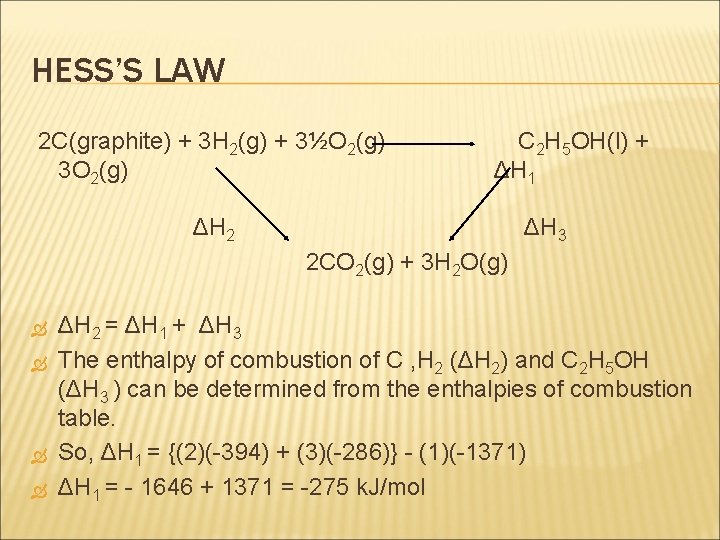
HESS’S LAW Example: calculate the enthalpy change that occurs in the following reaction: 2 C(graphite) + 3 H 2(g) +½O 2(g) C 2 H 5 OH(l) Since the enthalpy change of formation of ethanol is difficult to measure directly, we will use Hess’s Law and the enthalpy of combustion data to find the answer.

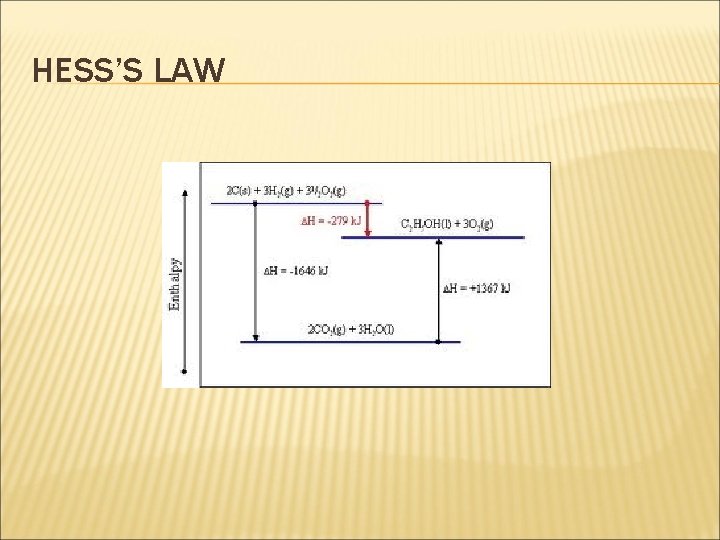
HESS’S LAW 2 C(graphite) + 3 H 2(g) + 3½O 2(g) 3 O 2(g) C 2 H 5 OH(l) + ΔH 1 ΔH 2 ΔH 3 2 CO 2(g) + 3 H 2 O(g) ΔH 2 = ΔH 1 + ΔH 3 The enthalpy of combustion of C , H 2 (ΔH 2) and C 2 H 5 OH (ΔH 3 ) can be determined from the enthalpies of combustion table. So, ΔH 1 = {(2)(-394) + (3)(-286)} - (1)(-1371) ΔH 1 = - 1646 + 1371 = -275 k. J/mol

HESS’S LAW

HESS’S LAW Exercise: Calculate the standard enthalpy change when one mole of methane is formed from its elements in their standard states. The standard enthalpies of combustion of carbon, hydrogen, and methane are -393, -286, and -890 k. J/mol, respectively.

HESS’S LAW Expressed in a different way, Hess’s Law states that if a reaction is carried out in a series of steps, Δ H for the reaction will be equal to the sum of the enthalpy changes for the individual steps.

HESS’S LAW

HESS’S LAW

HESS’S LAW Exercise: Calculate the enthalpy change, ΔHo, for the reaction: S(s) + O 2(g) SO 2(g) From the information below: S(s) +3/2 O 2(g) SO 3(g) ΔHo = -395 k. J SO 2(g) + ½ O 2(g) SO 3(g) Δho = -98 k. J

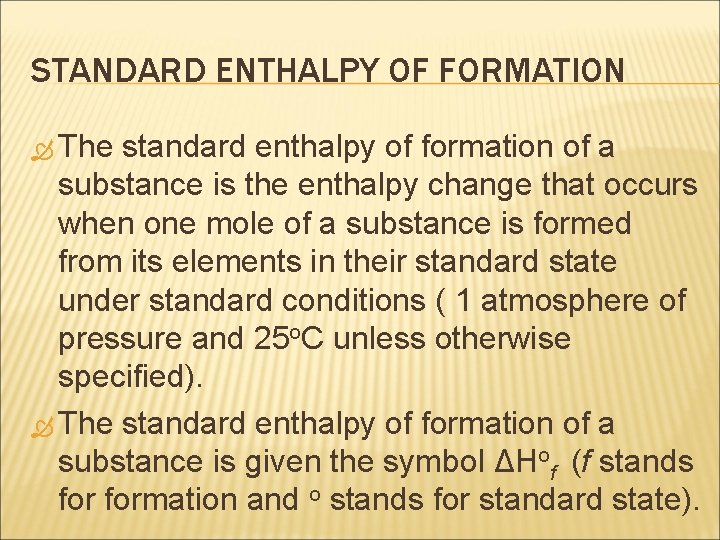
STANDARD ENTHALPY OF FORMATION The standard enthalpy of formation of a substance is the enthalpy change that occurs when one mole of a substance is formed from its elements in their standard state under standard conditions ( 1 atmosphere of pressure and 25 o. C unless otherwise specified). The standard enthalpy of formation of a substance is given the symbol ΔHof (f stands formation and o stands for standard state).

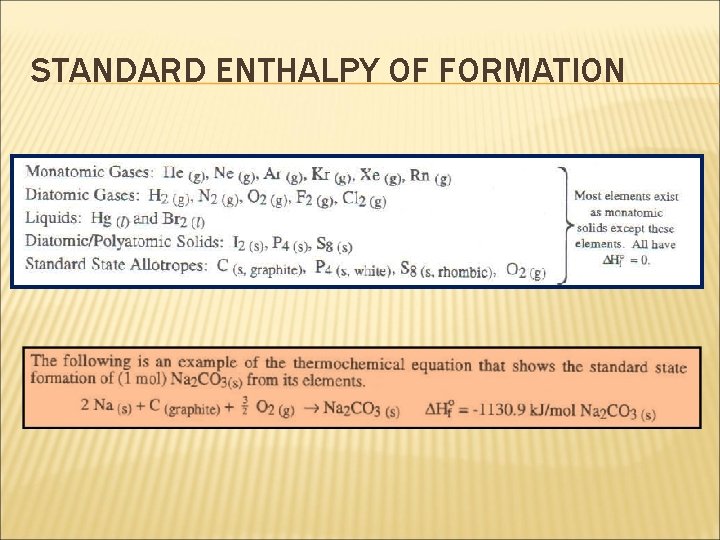
STANDARD ENTHALPY OF FORMATION The standard enthalpy change of formation of an element in its standard state = 0. Therefore, it is important that you know the physical state of the elements under standard conditions. Most elements at 1 atm and 25 o. C commonly exist as monatomic solids and in one allotropic form with the notable exceptions of:

STANDARD ENTHALPY OF FORMATION

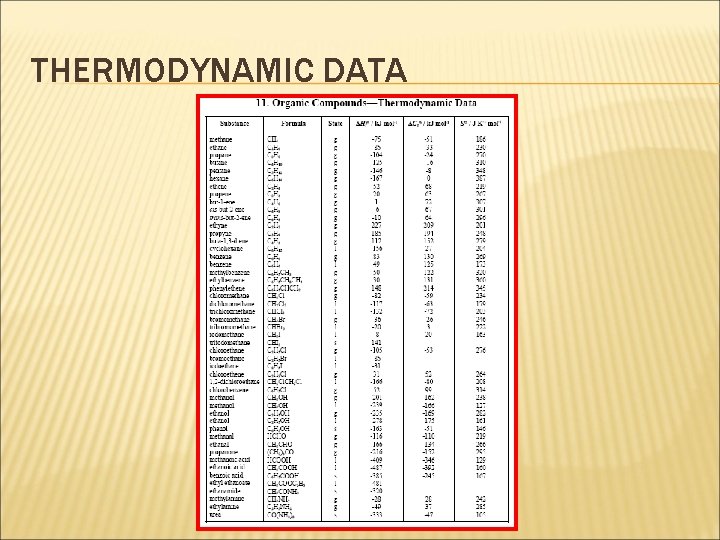
THERMODYNAMIC DATA

STANDARD ENTHALPY OF FORMATION Exercise: Which of the following does not have a standard heat of formation value of zero at 25 o. C and 1. 00 atm? A. Cl 2(g) B. I 2(s) C. Br 2(g) D. Na(s)

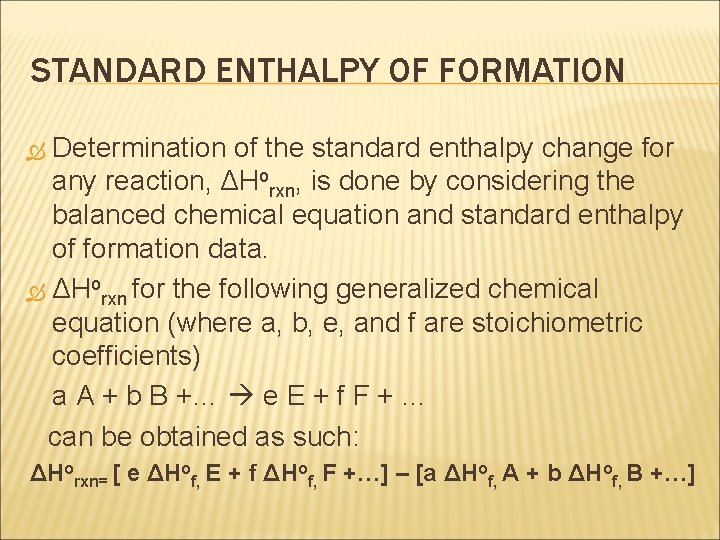
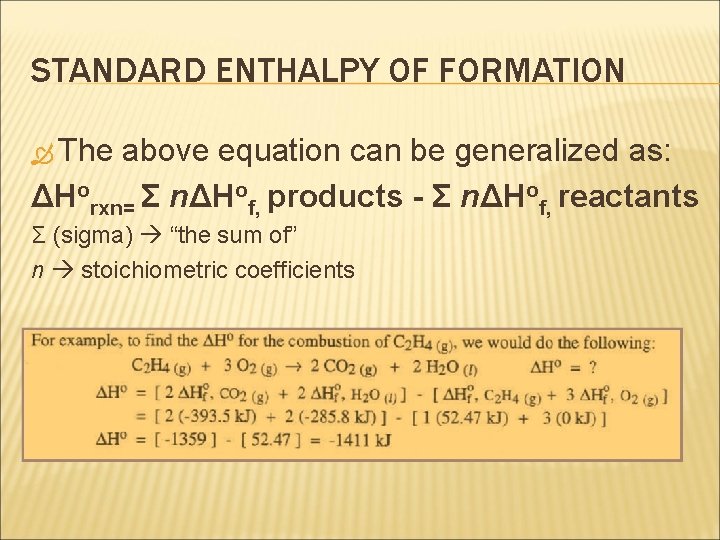
STANDARD ENTHALPY OF FORMATION Determination of the standard enthalpy change for any reaction, ΔHorxn, is done by considering the balanced chemical equation and standard enthalpy of formation data. ΔHorxn for the following generalized chemical equation (where a, b, e, and f are stoichiometric coefficients) a A + b B +… e E + f F + … can be obtained as such: ΔHorxn= [ e ΔHof, E + f ΔHof, F +…] – [a ΔHof, A + b ΔHof, B +…]

STANDARD ENTHALPY OF FORMATION The above equation can be generalized as: ΔHorxn= Σ nΔHof, products - Σ nΔHof, reactants Σ (sigma) “the sum of” n stoichiometric coefficients

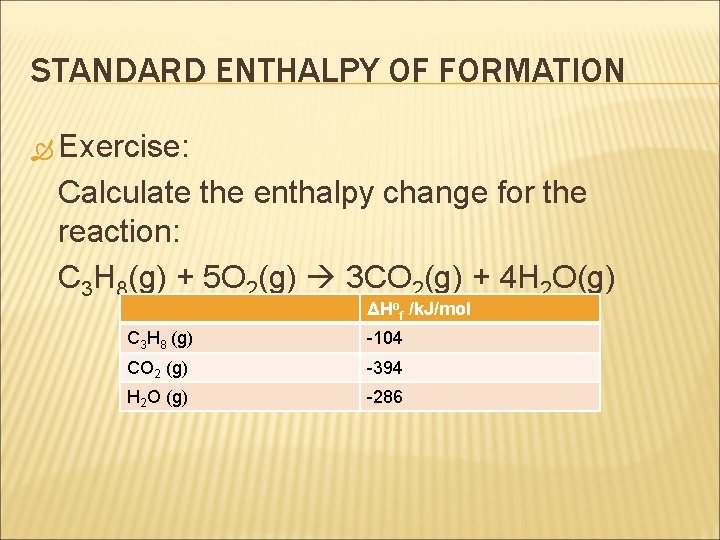
STANDARD ENTHALPY OF FORMATION Exercise: Calculate the enthalpy change for the reaction: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) ΔHof /k. J/mol C 3 H 8 (g) -104 CO 2 (g) -394 H 2 O (g) -286
 Hess's law rules
Hess's law rules Hesss law
Hesss law جرمان هنری هس
جرمان هنری هس Hesss
Hesss Significance of hmp shunt in biochemistry
Significance of hmp shunt in biochemistry Hcoh
Hcoh Energetic of glycolysis
Energetic of glycolysis Energetics of oxidative phosphorylation
Energetics of oxidative phosphorylation Energetics power tower 180
Energetics power tower 180 Energetics of gluconeogenesis
Energetics of gluconeogenesis Building energetics
Building energetics Ib energetics
Ib energetics Energetics janesville
Energetics janesville Chemsheets gcse 047 answers
Chemsheets gcse 047 answers Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion Boyles law
Boyles law Avogadro's law constants
Avogadro's law constants Raoult's law and dalton's law
Raoult's law and dalton's law Difference between civil law and criminal law
Difference between civil law and criminal law Positive law vs natural law
Positive law vs natural law Positive law
Positive law Natural law vs positive law
Natural law vs positive law