CALORIMETRY www chemsheets co uk AS 1048 30






- Slides: 42

CALORIMETRY © www. chemsheets. co. uk AS 1048 30 -Jun-2015

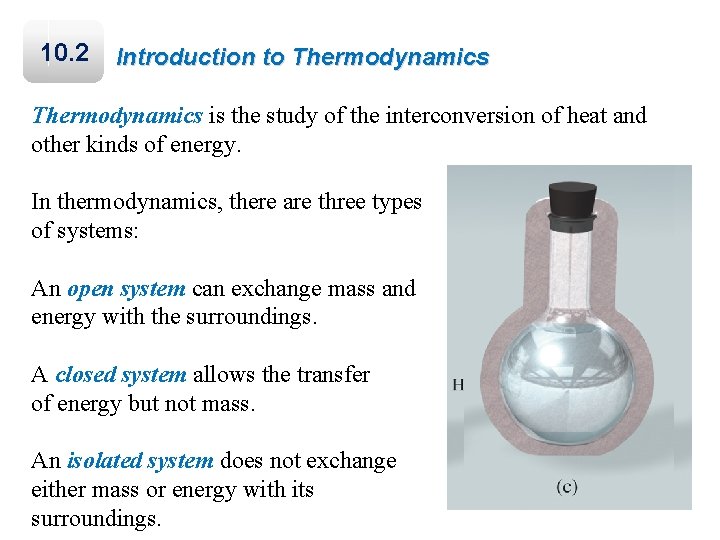
10. 2 Introduction to Thermodynamics is the study of the interconversion of heat and other kinds of energy. In thermodynamics, there are three types of systems: An open system can exchange mass and energy with the surroundings. A closed system allows the transfer of energy but not mass. An isolated system does not exchange either mass or energy with its surroundings.

Calorimetry Fuse school ( calorimeters and heats of combustion ) https: //www. youtube. com/watch? v=if. Ttdv. F 98 T 8

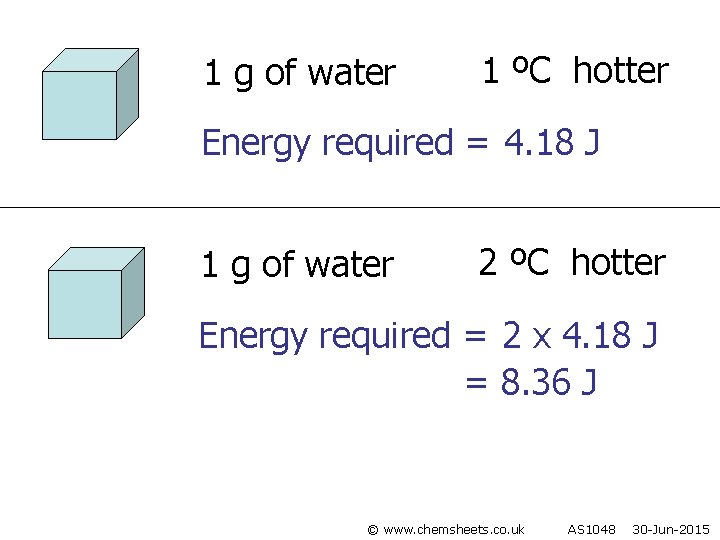
1 g of water 1 ºC hotter Energy required = 4. 18 J 1 g of water 2 ºC hotter Energy required = 2 x 4. 18 J = 8. 36 J © www. chemsheets. co. uk AS 1048 30 -Jun-2015

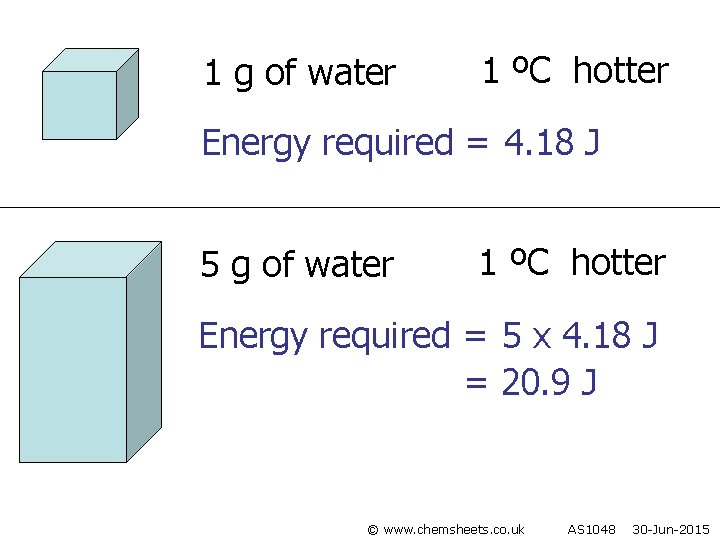
1 g of water 1 ºC hotter Energy required = 4. 18 J 5 g of water 1 ºC hotter Energy required = 5 x 4. 18 J = 20. 9 J © www. chemsheets. co. uk AS 1048 30 -Jun-2015

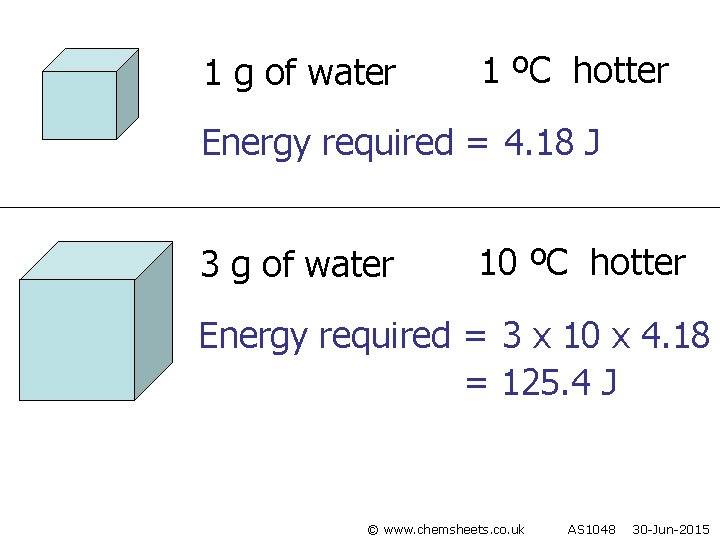
1 g of water 1 ºC hotter Energy required = 4. 18 J 3 g of water 10 ºC hotter Energy required = 3 x 10 x 4. 18 = 125. 4 J © www. chemsheets. co. uk AS 1048 30 -Jun-2015

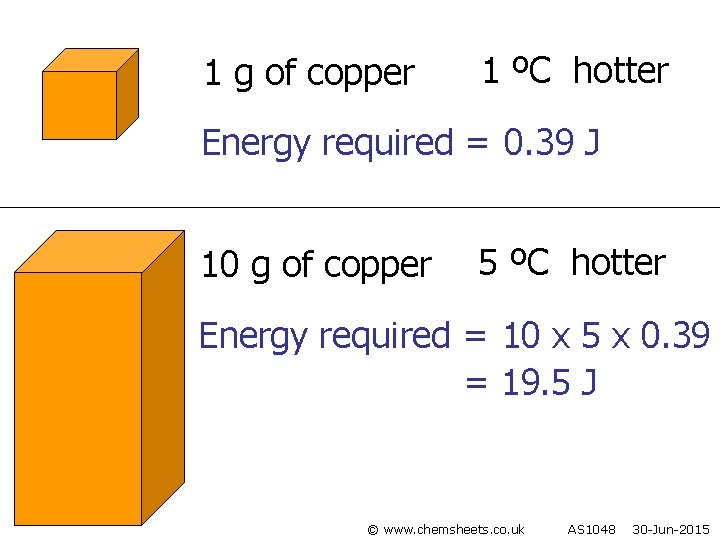
1 g of copper 1 ºC hotter Energy required = 0. 39 J 10 g of copper 5 ºC hotter Energy required = 10 x 5 x 0. 39 = 19. 5 J © www. chemsheets. co. uk AS 1048 30 -Jun-2015

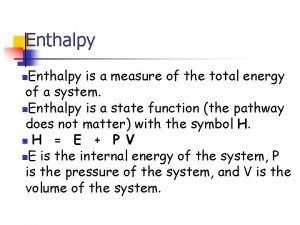
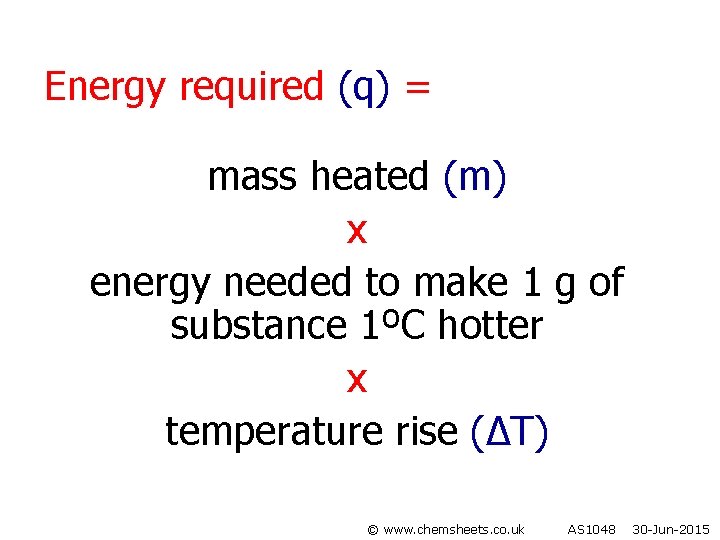
Energy required (q) = mass heated (m) x energy needed to make 1 g of substance 1ºC hotter x temperature rise (∆T) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

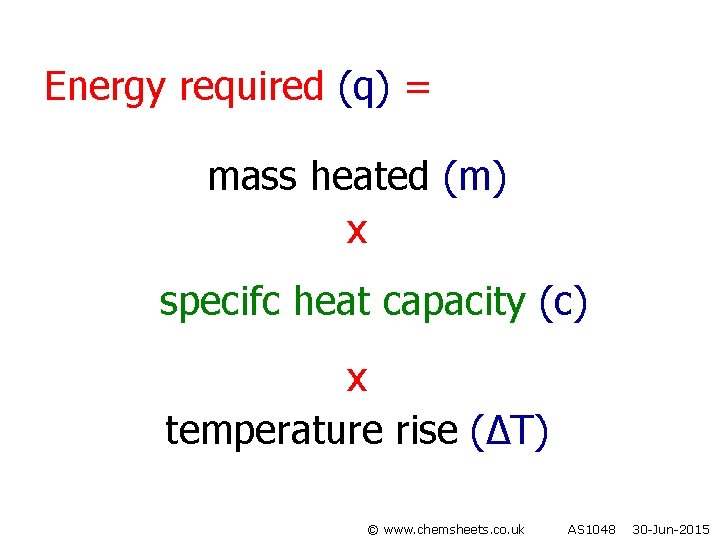
Energy required (q) = mass heated (m) x specifc heat capacity (c) x temperature rise (∆T) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

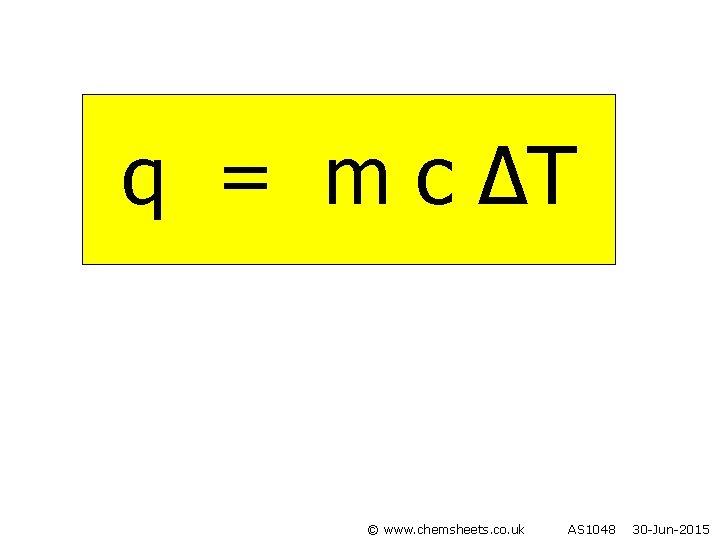
q = m c ∆T © www. chemsheets. co. uk AS 1048 30 -Jun-2015

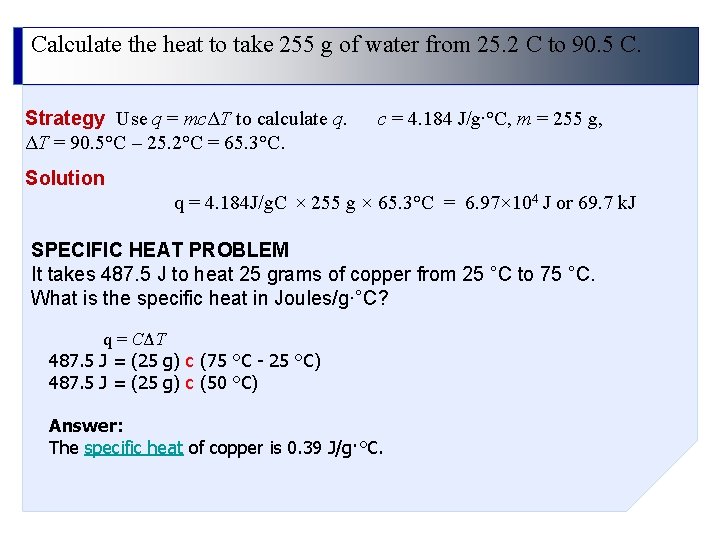
Calculate the heat to take 255 g of water from 25. 2 C to 90. 5 C. Strategy Use q = mcΔT to calculate q. ΔT = 90. 5°C – 25. 2°C = 65. 3°C. c = 4. 184 J/g∙°C, m = 255 g, Solution q = 4. 184 J/g. C × 255 g × 65. 3°C = 6. 97× 104 J or 69. 7 k. J SPECIFIC HEAT PROBLEM It takes 487. 5 J to heat 25 grams of copper from 25 °C to 75 °C. What is the specific heat in Joules/g·°C? q = CΔT 487. 5 J = (25 g) c (75 °C - 25 °C) 487. 5 J = (25 g) c (50 °C) Answer: The specific heat of copper is 0. 39 J/g·°C.

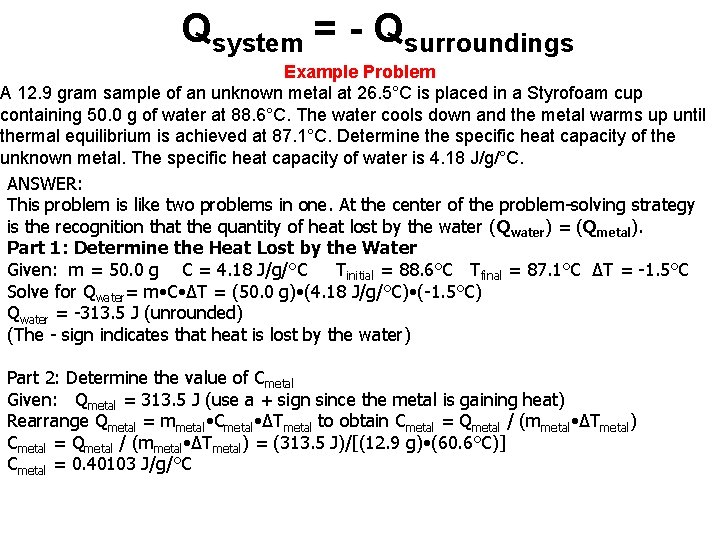
Qsystem = - Qsurroundings Example Problem A 12. 9 gram sample of an unknown metal at 26. 5°C is placed in a Styrofoam cup containing 50. 0 g of water at 88. 6°C. The water cools down and the metal warms up until thermal equilibrium is achieved at 87. 1°C. Determine the specific heat capacity of the unknown metal. The specific heat capacity of water is 4. 18 J/g/°C. ANSWER: This problem is like two problems in one. At the center of the problem-solving strategy is the recognition that the quantity of heat lost by the water (Qwater) = (Qmetal). Part 1: Determine the Heat Lost by the Water Given: m = 50. 0 g C = 4. 18 J/g/°C Tinitial = 88. 6°C Tfinal = 87. 1°C ΔT = -1. 5°C Solve for Qwater= m • C • ΔT = (50. 0 g) • (4. 18 J/g/°C) • (-1. 5°C) Qwater = -313. 5 J (unrounded) (The - sign indicates that heat is lost by the water) Part 2: Determine the value of Cmetal Given: Qmetal = 313. 5 J (use a + sign since the metal is gaining heat) Rearrange Qmetal = mmetal • Cmetal • ΔTmetal to obtain Cmetal = Qmetal / (mmetal • ΔTmetal) = (313. 5 J)/[(12. 9 g) • (60. 6°C)] Cmetal = 0. 40103 J/g/°C

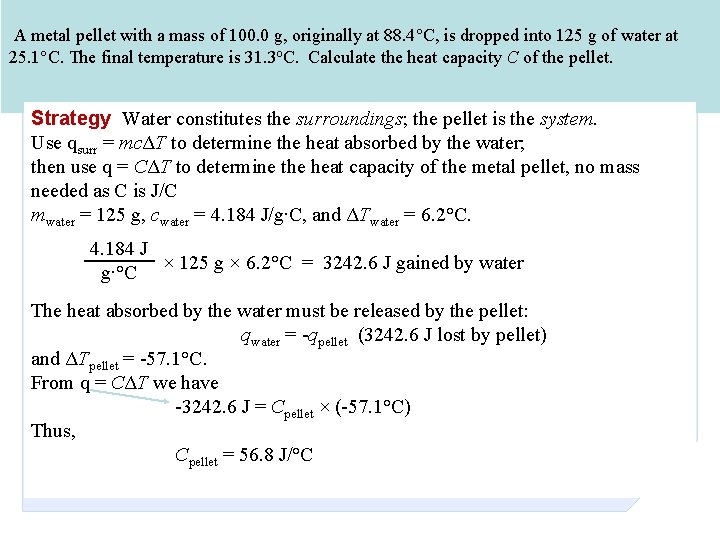
A metal pellet with a mass of 100. 0 g, originally at 88. 4°C, is dropped into 125 g of water at 25. 1°C. The final temperature is 31. 3 o. C. Calculate the heat capacity C of the pellet. Strategy Water constitutes the surroundings; the pellet is the system. Use qsurr = mcΔT to determine the heat absorbed by the water; then use q = CΔT to determine the heat capacity of the metal pellet, no mass needed as C is J/C mwater = 125 g, cwater = 4. 184 J/g∙C, and ΔTwater = 6. 2°C. 4. 184 J × 125 g × 6. 2°C = 3242. 6 J gained by water g∙°C The heat absorbed by the water must be released by the pellet: qwater = -qpellet (3242. 6 J lost by pellet) and ΔTpellet = -57. 1°C. From q = CΔT we have -3242. 6 J = Cpellet × (-57. 1°C) Thus, Cpellet = 56. 8 J/°C

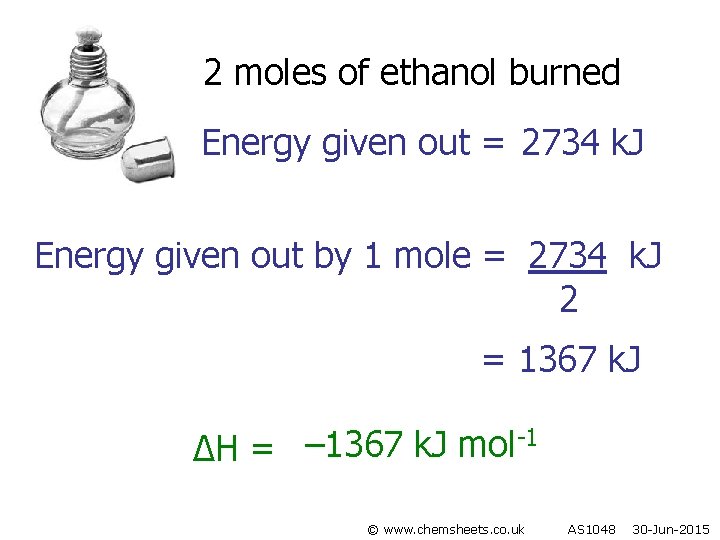
2 moles of ethanol burned Energy given out = 2734 k. J Energy given out by 1 mole = 2734 k. J 2 = 1367 k. J -1 – 1367 k. J mol ∆H = © www. chemsheets. co. uk AS 1048 30 -Jun-2015

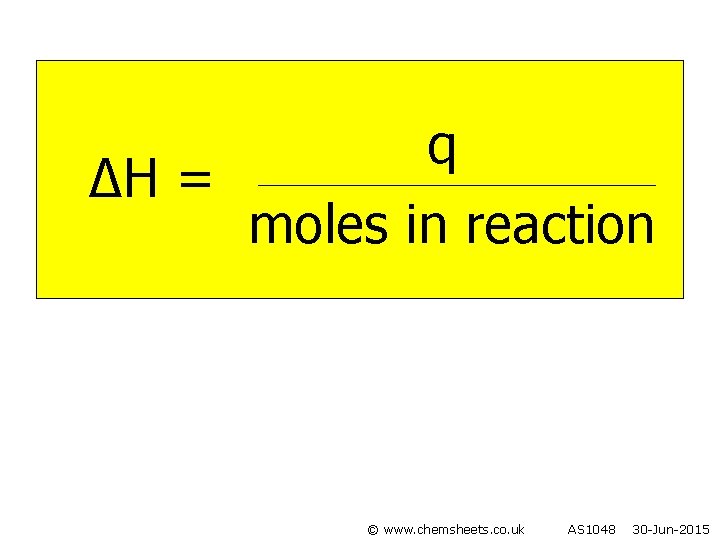
q ∆H = moles in reaction © www. chemsheets. co. uk AS 1048 30 -Jun-2015

© www. chemsheets. co. uk AS 1048 30 -Jun-2015

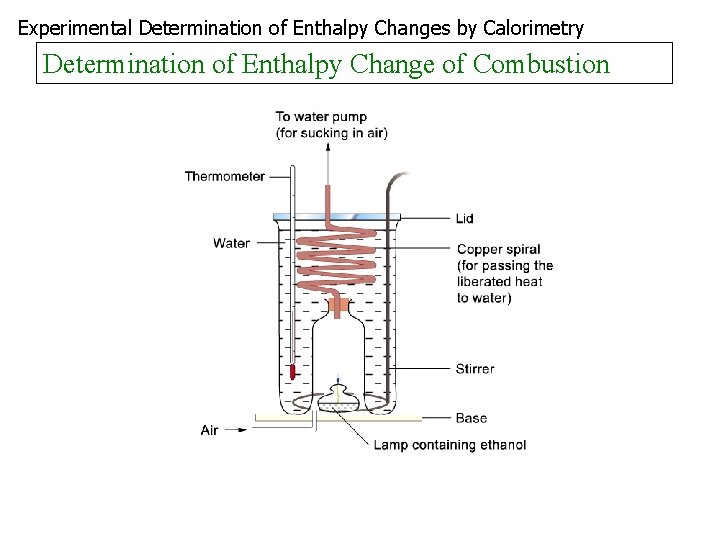
Experimental Determination of Enthalpy Changes by Calorimetry Determination of Enthalpy Change of Combustion

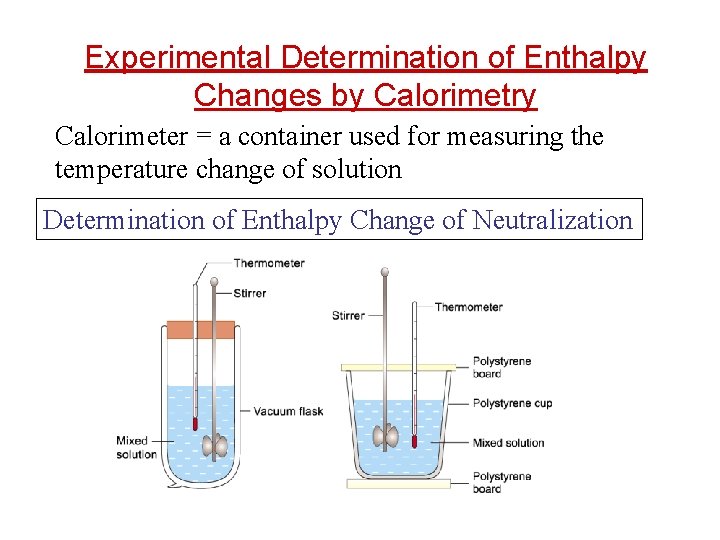
Experimental Determination of Enthalpy Changes by Calorimetry Calorimeter = a container used for measuring the temperature change of solution Determination of Enthalpy Change of Neutralization

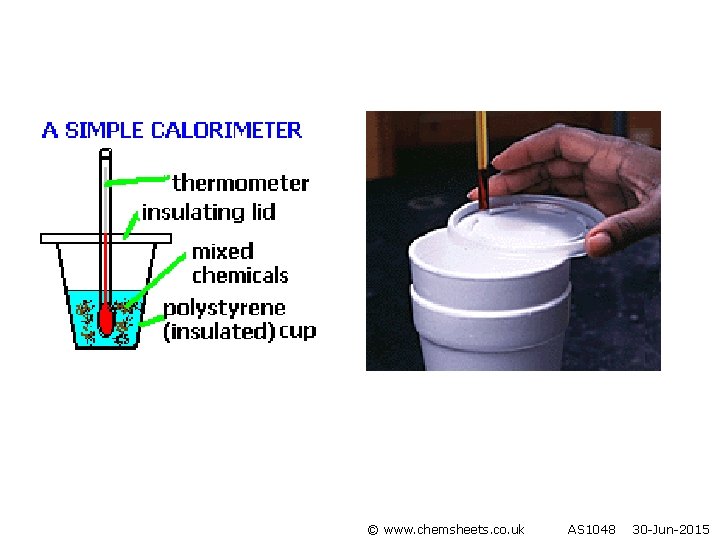
Specific heat capacity is the amount of heat needed to raise the temperature of 1 g of substance by 1 K Specific heat capacity of water = 4. 18 KJ kg-1 K-1 or 4. 18 J g -1 K 1 Be careful with the units it could also be quoted as KJ g-1 K-1 Ensure you use the correct units in your calculation! To measure the heat released in a process we arrange for the heat to be transferred to a substance (usually water) then measure the temperature rise.

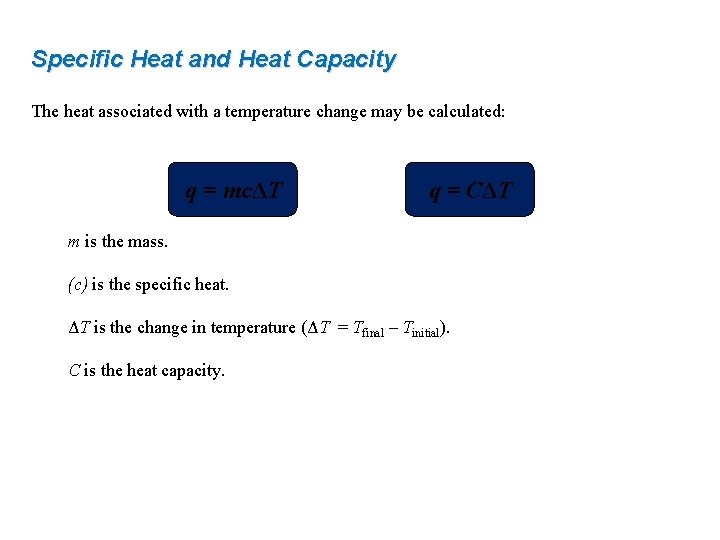
Specific Heat and Heat Capacity The heat associated with a temperature change may be calculated: q = mcΔT q = CΔT m is the mass. (c) is the specific heat. ΔT is the change in temperature (ΔT = Tfinal – Tinitial). C is the heat capacity.

Cal Problems worked 4 min https: //www. youtube. com/watc h? v=Hs 5 x 0 -IU 2 F 4

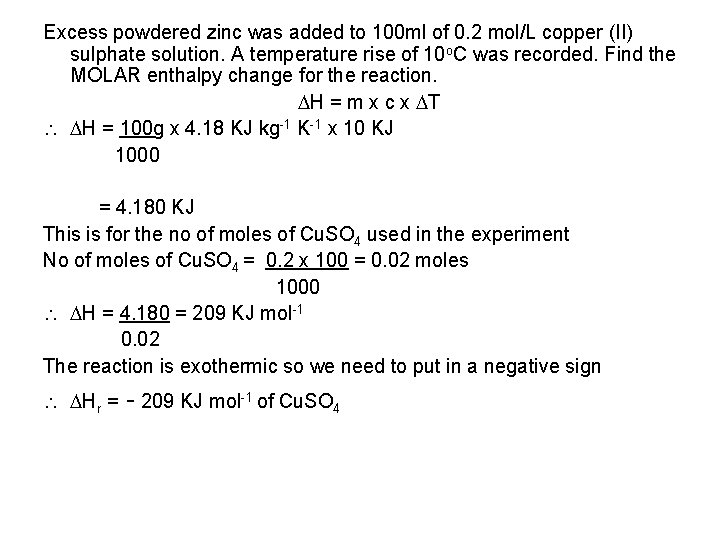
Excess powdered zinc was added to 100 ml of 0. 2 mol/L copper (II) sulphate solution. A temperature rise of 10 o. C was recorded. Find the MOLAR enthalpy change for the reaction. H = m x c x T H = 100 g x 4. 18 KJ kg-1 K-1 x 10 KJ 1000 = 4. 180 KJ This is for the no of moles of Cu. SO 4 used in the experiment No of moles of Cu. SO 4 = 0. 2 x 100 = 0. 02 moles 1000 H = 4. 180 = 209 KJ mol-1 0. 02 The reaction is exothermic so we need to put in a negative sign Hr = - 209 KJ mol-1 of Cu. SO 4

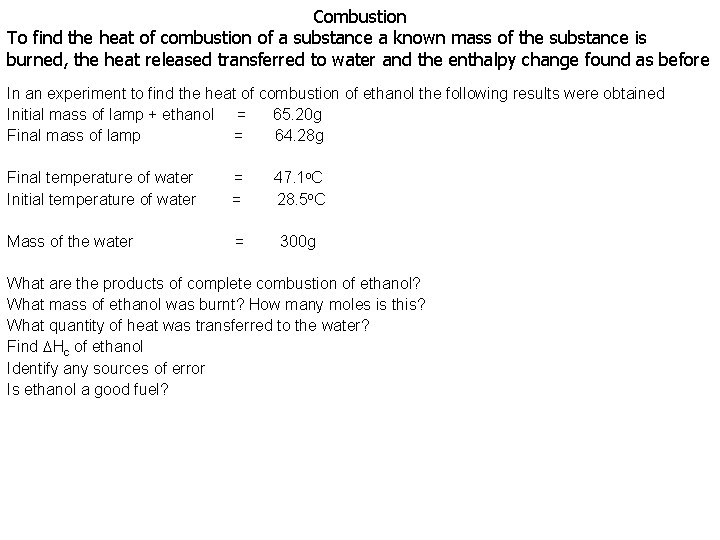
Combustion To find the heat of combustion of a substance a known mass of the substance is burned, the heat released transferred to water and the enthalpy change found as before In an experiment to find the heat of combustion of ethanol the following results were obtained Initial mass of lamp + ethanol = 65. 20 g Final mass of lamp = 64. 28 g Final temperature of water Initial temperature of water = = 47. 1 o. C 28. 5 o. C Mass of the water = 300 g What are the products of complete combustion of ethanol? What mass of ethanol was burnt? How many moles is this? What quantity of heat was transferred to the water? Find Hc of ethanol Identify any sources of error Is ethanol a good fuel?

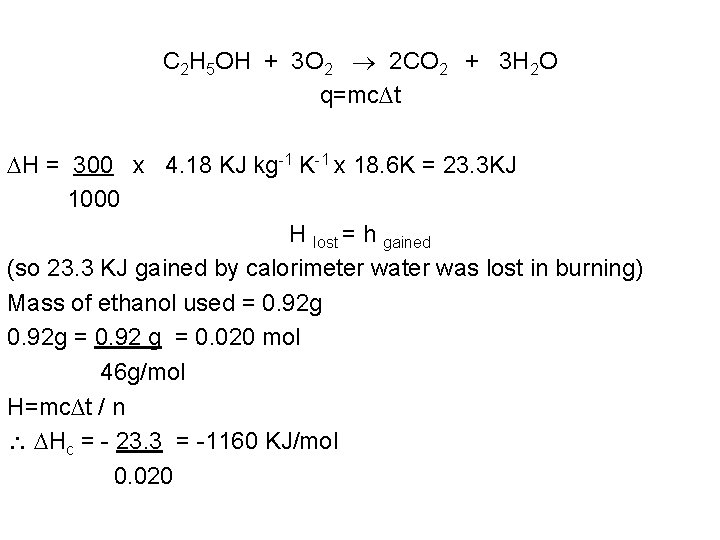
C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O q=mc∆t H = 300 x 4. 18 KJ kg-1 K-1 x 18. 6 K = 23. 3 KJ 1000 H lost = h gained (so 23. 3 KJ gained by calorimeter was lost in burning) Mass of ethanol used = 0. 92 g = 0. 92 g = 0. 020 mol 46 g/mol H=mc∆t / n Hc = - 23. 3 = -1160 KJ/mol 0. 020

Errors Heat lost to surroundings (air, can thermometer) Errors in measuring temperature change (unavoidable error in reading thermometer) Errors in measuring masses (unavoidable error in reading balance) The enthalpy change of combustion is high ethanol is a good fuel

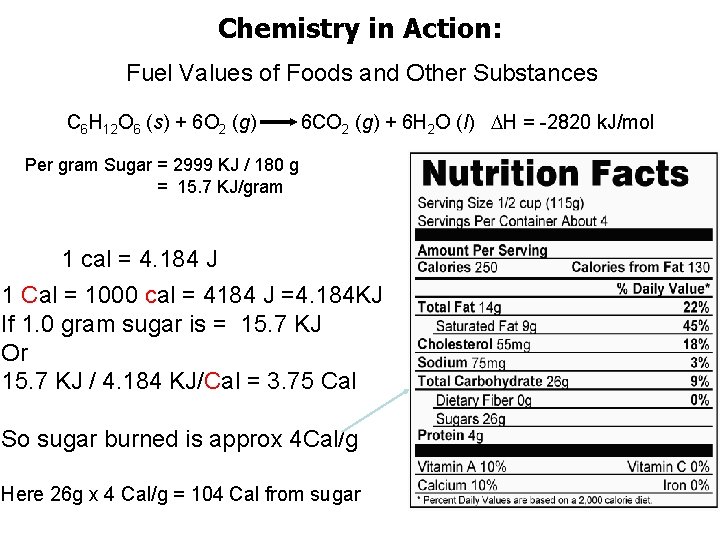
Chemistry in Action: Fuel Values of Foods and Other Substances C 6 H 12 O 6 (s) + 6 O 2 (g) 6 CO 2 (g) + 6 H 2 O (l) H = -2820 k. J/mol Per gram Sugar = 2999 KJ / 180 g = 15. 7 KJ/gram 1 cal = 4. 184 J 1 Cal = 1000 cal = 4184 J =4. 184 KJ If 1. 0 gram sugar is = 15. 7 KJ Or 15. 7 KJ / 4. 184 KJ/Cal = 3. 75 Cal So sugar burned is approx 4 Cal/g Here 26 g x 4 Cal/g = 104 Cal from sugar

Foods and Fuels Cheetos burned v Cereal https: //www. youtube. com/watch? v=UDgea. AM d. YIY Foods • 1 nutritional Calorie, 1 Cal = 1000 cal = 1 kcal. • Energy in us comes from carbohydrates and fats TED X (3 min, Calories) https: //www. youtube. com/watch? v=VEQa. H 4 Lru. Uo • Intestines: carbohydrates converted into glucose: C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O, H = -2816 k. J • Fats break down as follows: 2 C 57 H 110 O 6 + 163 O 2 114 CO 2 + 110 H 2 O, H = -75, 520 k. J Fats contain more energy; are not water soluble, so are good for energy storage.

The END

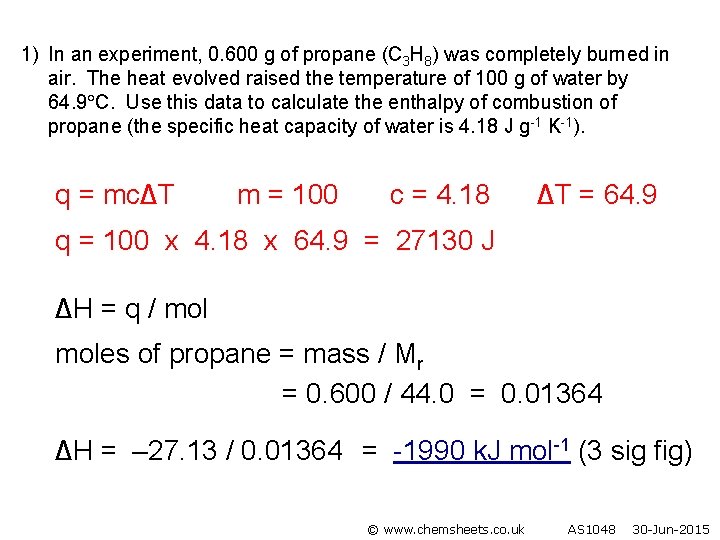
1) In an experiment, 0. 600 g of propane (C 3 H 8) was completely burned in air. The heat evolved raised the temperature of 100 g of water by 64. 9 C. Use this data to calculate the enthalpy of combustion of propane (the specific heat capacity of water is 4. 18 J g-1 K-1). q = mc∆T m = 100 c = 4. 18 ∆T = 64. 9 q = 100 x 4. 18 x 64. 9 = 27130 J ∆H = q / moles of propane = mass / Mr = 0. 600 / 44. 0 = 0. 01364 ∆H = – 27. 13 / 0. 01364 = -1990 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

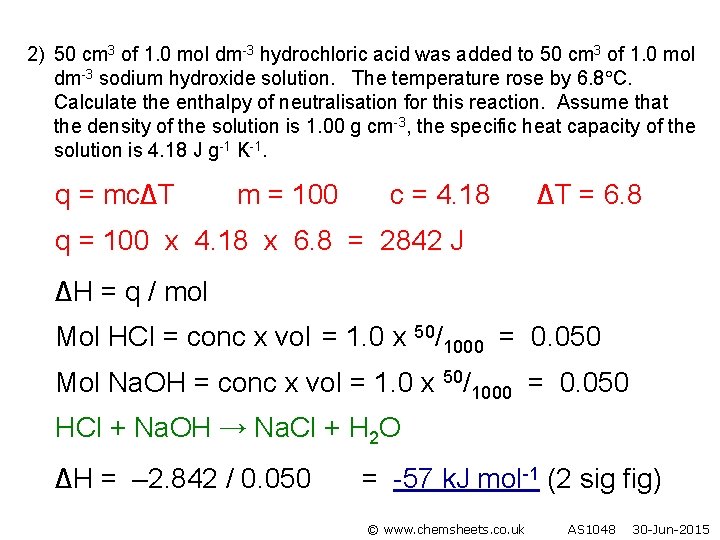
2) 50 cm 3 of 1. 0 mol dm-3 hydrochloric acid was added to 50 cm 3 of 1. 0 mol dm-3 sodium hydroxide solution. The temperature rose by 6. 8 C. Calculate the enthalpy of neutralisation for this reaction. Assume that the density of the solution is 1. 00 g cm-3, the specific heat capacity of the solution is 4. 18 J g-1 K-1. q = mc∆T m = 100 c = 4. 18 ∆T = 6. 8 q = 100 x 4. 18 x 6. 8 = 2842 J ∆H = q / mol Mol HCl = conc x vol = 1. 0 x 50/1000 = 0. 050 Mol Na. OH = conc x vol = 1. 0 x 50/1000 = 0. 050 HCl + Na. OH → Na. Cl + H 2 O ∆H = – 2. 842 / 0. 050 = -57 k. J mol-1 (2 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

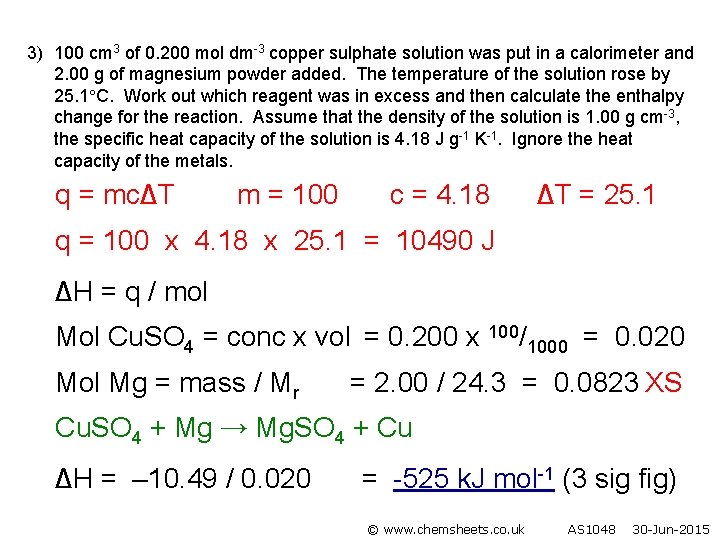
3) 100 cm 3 of 0. 200 mol dm-3 copper sulphate solution was put in a calorimeter and 2. 00 g of magnesium powder added. The temperature of the solution rose by 25. 1 C. Work out which reagent was in excess and then calculate the enthalpy change for the reaction. Assume that the density of the solution is 1. 00 g cm-3, the specific heat capacity of the solution is 4. 18 J g-1 K-1. Ignore the heat capacity of the metals. q = mc∆T m = 100 c = 4. 18 ∆T = 25. 1 q = 100 x 4. 18 x 25. 1 = 10490 J ∆H = q / mol Mol Cu. SO 4 = conc x vol = 0. 200 x 100/1000 = 0. 020 Mol Mg = mass / Mr = 2. 00 / 24. 3 = 0. 0823 XS Cu. SO 4 + Mg → Mg. SO 4 + Cu ∆H = – 10. 49 / 0. 020 = -525 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

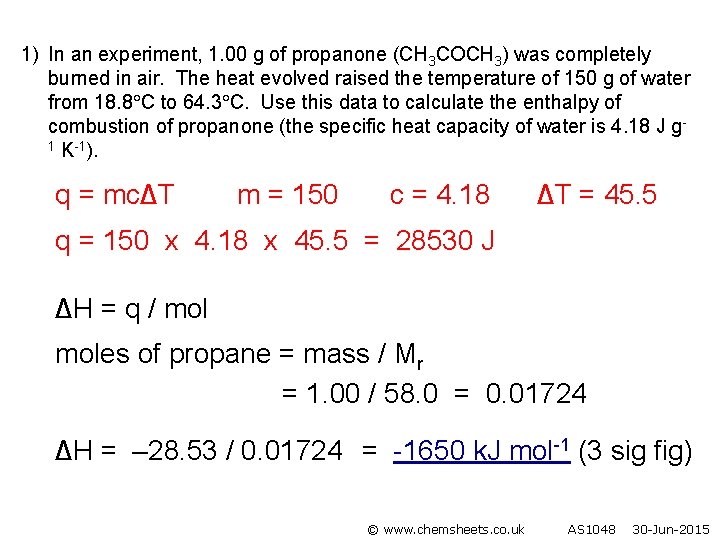
1) In an experiment, 1. 00 g of propanone (CH 3 COCH 3) was completely burned in air. The heat evolved raised the temperature of 150 g of water from 18. 8 C to 64. 3 C. Use this data to calculate the enthalpy of combustion of propanone (the specific heat capacity of water is 4. 18 J g 1 K-1). q = mc∆T m = 150 c = 4. 18 ∆T = 45. 5 q = 150 x 4. 18 x 45. 5 = 28530 J ∆H = q / moles of propane = mass / Mr = 1. 00 / 58. 0 = 0. 01724 ∆H = – 28. 53 / 0. 01724 = -1650 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

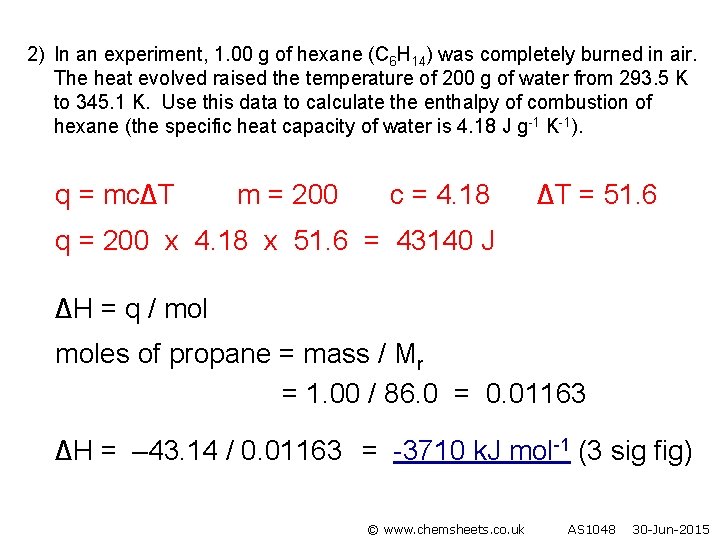
2) In an experiment, 1. 00 g of hexane (C 6 H 14) was completely burned in air. The heat evolved raised the temperature of 200 g of water from 293. 5 K to 345. 1 K. Use this data to calculate the enthalpy of combustion of hexane (the specific heat capacity of water is 4. 18 J g-1 K-1). q = mc∆T m = 200 c = 4. 18 ∆T = 51. 6 q = 200 x 4. 18 x 51. 6 = 43140 J ∆H = q / moles of propane = mass / Mr = 1. 00 / 86. 0 = 0. 01163 ∆H = – 43. 14 / 0. 01163 = -3710 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

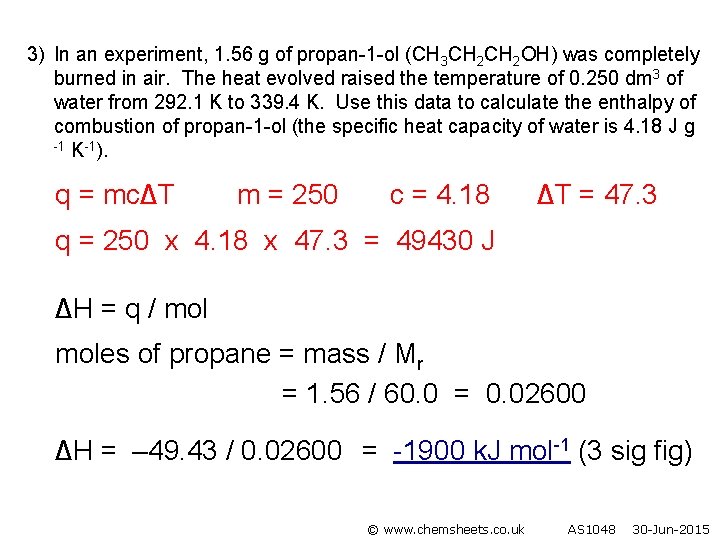
3) In an experiment, 1. 56 g of propan-1 -ol (CH 3 CH 2 OH) was completely burned in air. The heat evolved raised the temperature of 0. 250 dm 3 of water from 292. 1 K to 339. 4 K. Use this data to calculate the enthalpy of combustion of propan-1 -ol (the specific heat capacity of water is 4. 18 J g -1 K-1). q = mc∆T m = 250 c = 4. 18 ∆T = 47. 3 q = 250 x 4. 18 x 47. 3 = 49430 J ∆H = q / moles of propane = mass / Mr = 1. 56 / 60. 0 = 0. 02600 ∆H = – 49. 43 / 0. 02600 = -1900 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

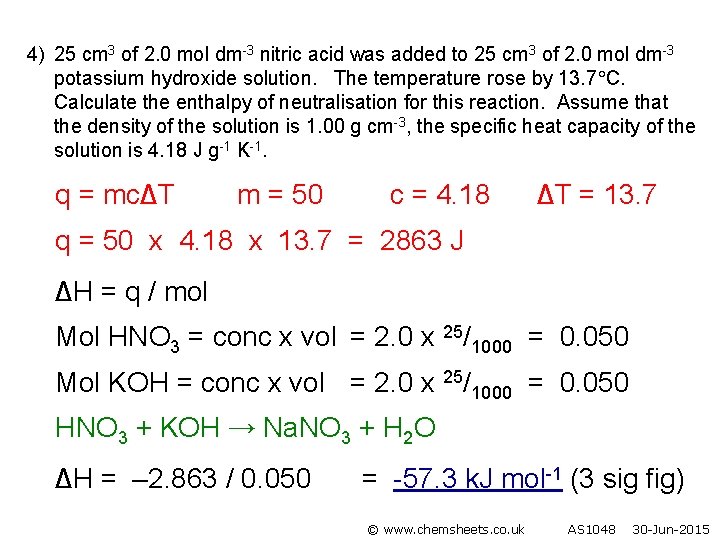
4) 25 cm 3 of 2. 0 mol dm-3 nitric acid was added to 25 cm 3 of 2. 0 mol dm-3 potassium hydroxide solution. The temperature rose by 13. 7 C. Calculate the enthalpy of neutralisation for this reaction. Assume that the density of the solution is 1. 00 g cm-3, the specific heat capacity of the solution is 4. 18 J g-1 K-1. q = mc∆T m = 50 c = 4. 18 ∆T = 13. 7 q = 50 x 4. 18 x 13. 7 = 2863 J ∆H = q / mol Mol HNO 3 = conc x vol = 2. 0 x 25/1000 = 0. 050 Mol KOH = conc x vol = 2. 0 x 25/1000 = 0. 050 HNO 3 + KOH → Na. NO 3 + H 2 O ∆H = – 2. 863 / 0. 050 = -57. 3 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

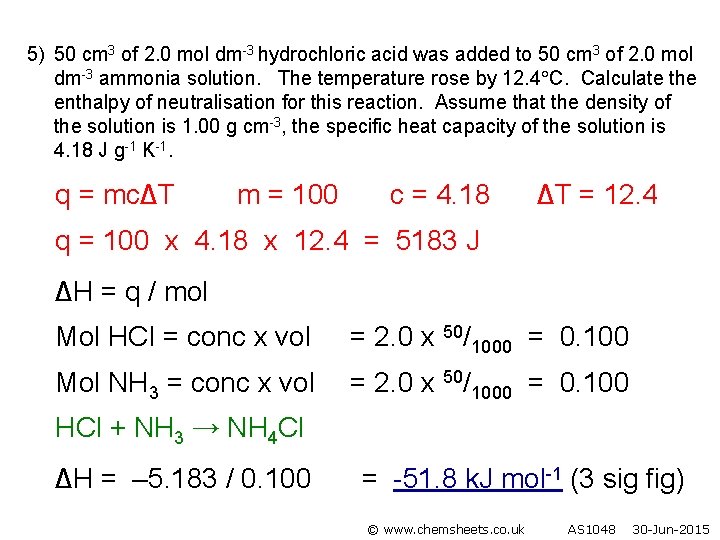
5) 50 cm 3 of 2. 0 mol dm-3 hydrochloric acid was added to 50 cm 3 of 2. 0 mol dm-3 ammonia solution. The temperature rose by 12. 4 C. Calculate the enthalpy of neutralisation for this reaction. Assume that the density of the solution is 1. 00 g cm-3, the specific heat capacity of the solution is 4. 18 J g-1 K-1. q = mc∆T m = 100 c = 4. 18 ∆T = 12. 4 q = 100 x 4. 18 x 12. 4 = 5183 J ∆H = q / mol Mol HCl = conc x vol = 2. 0 x 50/1000 = 0. 100 Mol NH 3 = conc x vol = 2. 0 x 50/1000 = 0. 100 HCl + NH 3 → NH 4 Cl ∆H = – 5. 183 / 0. 100 = -51. 8 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

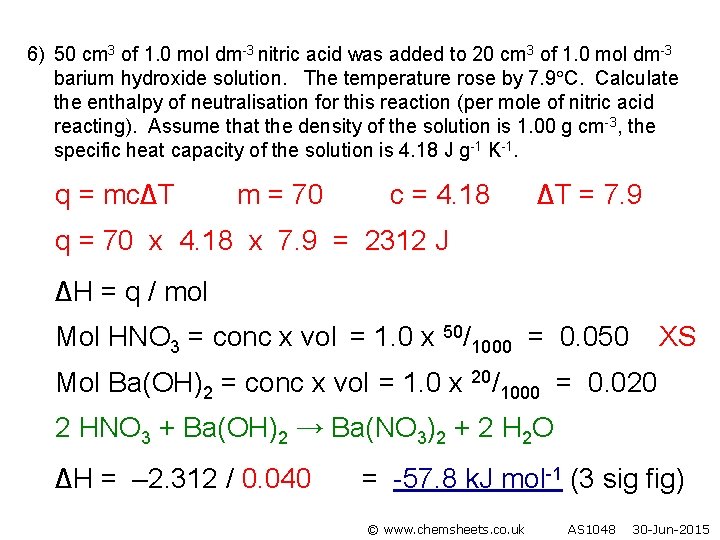
6) 50 cm 3 of 1. 0 mol dm-3 nitric acid was added to 20 cm 3 of 1. 0 mol dm-3 barium hydroxide solution. The temperature rose by 7. 9 C. Calculate the enthalpy of neutralisation for this reaction (per mole of nitric acid reacting). Assume that the density of the solution is 1. 00 g cm-3, the specific heat capacity of the solution is 4. 18 J g-1 K-1. q = mc∆T m = 70 c = 4. 18 ∆T = 7. 9 q = 70 x 4. 18 x 7. 9 = 2312 J ∆H = q / mol Mol HNO 3 = conc x vol = 1. 0 x 50/1000 = 0. 050 XS Mol Ba(OH)2 = conc x vol = 1. 0 x 20/1000 = 0. 020 2 HNO 3 + Ba(OH)2 → Ba(NO 3)2 + 2 H 2 O ∆H = – 2. 312 / 0. 040 = -57. 8 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

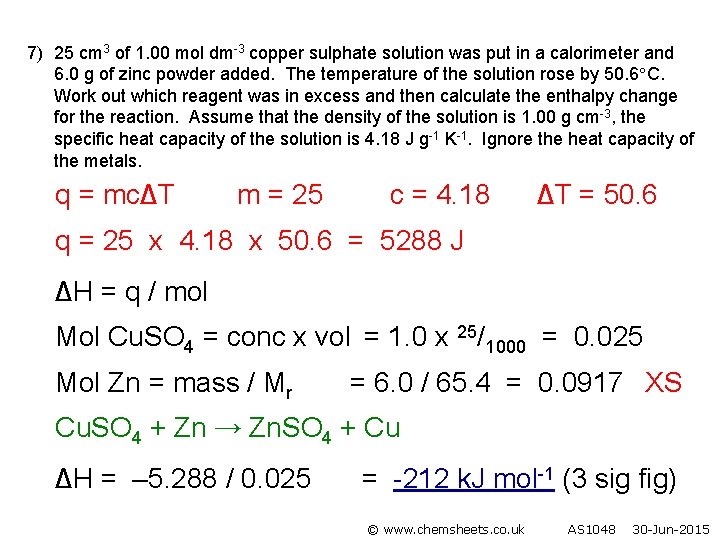
7) 25 cm 3 of 1. 00 mol dm-3 copper sulphate solution was put in a calorimeter and 6. 0 g of zinc powder added. The temperature of the solution rose by 50. 6 C. Work out which reagent was in excess and then calculate the enthalpy change for the reaction. Assume that the density of the solution is 1. 00 g cm-3, the specific heat capacity of the solution is 4. 18 J g-1 K-1. Ignore the heat capacity of the metals. q = mc∆T m = 25 c = 4. 18 ∆T = 50. 6 q = 25 x 4. 18 x 50. 6 = 5288 J ∆H = q / mol Mol Cu. SO 4 = conc x vol = 1. 0 x 25/1000 = 0. 025 Mol Zn = mass / Mr = 6. 0 / 65. 4 = 0. 0917 XS Cu. SO 4 + Zn → Zn. SO 4 + Cu ∆H = – 5. 288 / 0. 025 = -212 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

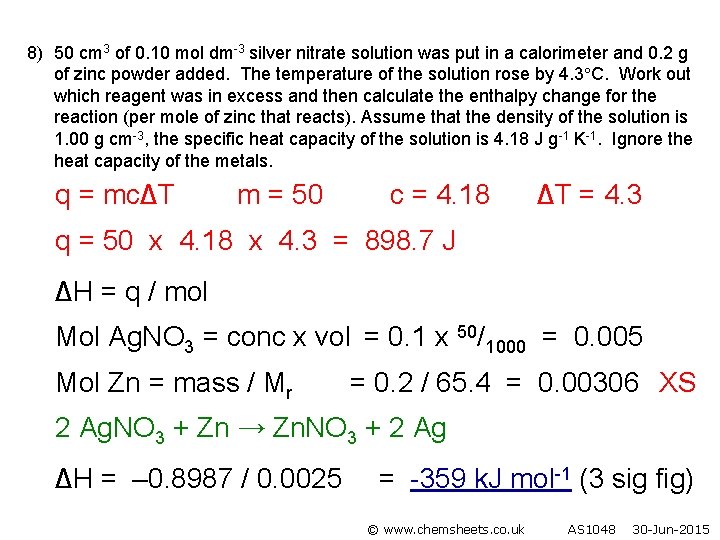
8) 50 cm 3 of 0. 10 mol dm-3 silver nitrate solution was put in a calorimeter and 0. 2 g of zinc powder added. The temperature of the solution rose by 4. 3 C. Work out which reagent was in excess and then calculate the enthalpy change for the reaction (per mole of zinc that reacts). Assume that the density of the solution is 1. 00 g cm-3, the specific heat capacity of the solution is 4. 18 J g-1 K-1. Ignore the heat capacity of the metals. q = mc∆T m = 50 c = 4. 18 ∆T = 4. 3 q = 50 x 4. 18 x 4. 3 = 898. 7 J ∆H = q / mol Mol Ag. NO 3 = conc x vol = 0. 1 x 50/1000 = 0. 005 Mol Zn = mass / Mr = 0. 2 / 65. 4 = 0. 00306 XS 2 Ag. NO 3 + Zn → Zn. NO 3 + 2 Ag ∆H = – 0. 8987 / 0. 0025 = -359 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

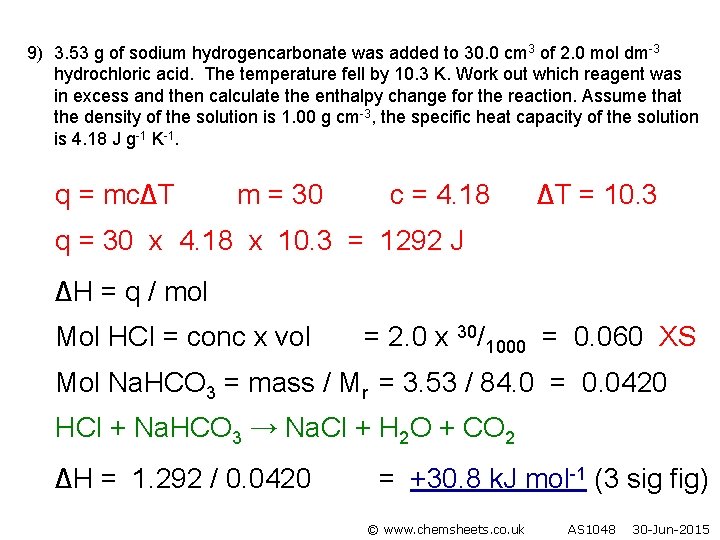
9) 3. 53 g of sodium hydrogencarbonate was added to 30. 0 cm 3 of 2. 0 mol dm-3 hydrochloric acid. The temperature fell by 10. 3 K. Work out which reagent was in excess and then calculate the enthalpy change for the reaction. Assume that the density of the solution is 1. 00 g cm-3, the specific heat capacity of the solution is 4. 18 J g-1 K-1. q = mc∆T m = 30 c = 4. 18 ∆T = 10. 3 q = 30 x 4. 18 x 10. 3 = 1292 J ∆H = q / mol Mol HCl = conc x vol = 2. 0 x 30/1000 = 0. 060 XS Mol Na. HCO 3 = mass / Mr = 3. 53 / 84. 0 = 0. 0420 HCl + Na. HCO 3 → Na. Cl + H 2 O + CO 2 ∆H = 1. 292 / 0. 0420 = +30. 8 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015

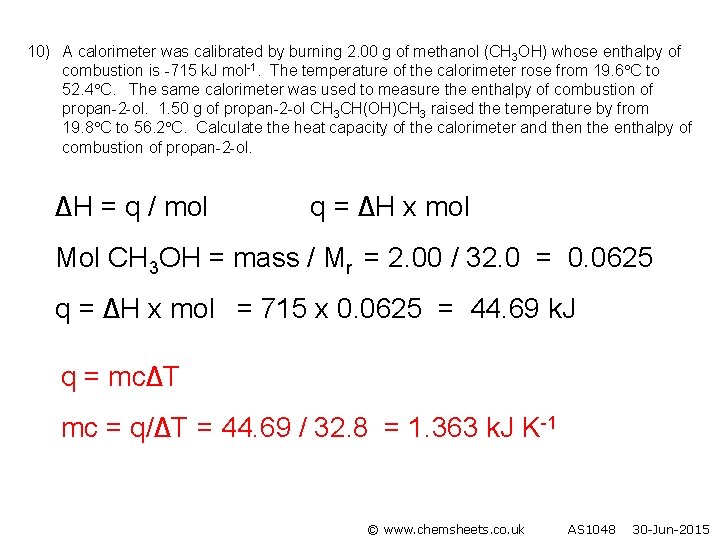
10) A calorimeter was calibrated by burning 2. 00 g of methanol (CH 3 OH) whose enthalpy of combustion is -715 k. J mol-1. The temperature of the calorimeter rose from 19. 6 C to 52. 4 C. The same calorimeter was used to measure the enthalpy of combustion of propan-2 -ol. 1. 50 g of propan-2 -ol CH 3 CH(OH)CH 3 raised the temperature by from 19. 8 C to 56. 2 C. Calculate the heat capacity of the calorimeter and then the enthalpy of combustion of propan-2 -ol. ∆H = q / mol q = ∆H x mol Mol CH 3 OH = mass / Mr = 2. 00 / 32. 0 = 0. 0625 q = ∆H x mol = 715 x 0. 0625 = 44. 69 k. J q = mc∆T mc = q/∆T = 44. 69 / 32. 8 = 1. 363 k. J K-1 © www. chemsheets. co. uk AS 1048 30 -Jun-2015

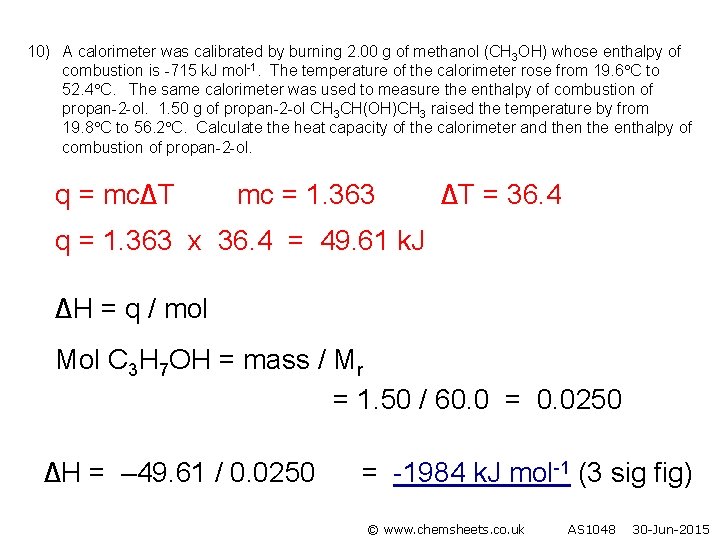
10) A calorimeter was calibrated by burning 2. 00 g of methanol (CH 3 OH) whose enthalpy of combustion is -715 k. J mol-1. The temperature of the calorimeter rose from 19. 6 C to 52. 4 C. The same calorimeter was used to measure the enthalpy of combustion of propan-2 -ol. 1. 50 g of propan-2 -ol CH 3 CH(OH)CH 3 raised the temperature by from 19. 8 C to 56. 2 C. Calculate the heat capacity of the calorimeter and then the enthalpy of combustion of propan-2 -ol. q = mc∆T mc = 1. 363 ∆T = 36. 4 q = 1. 363 x 36. 4 = 49. 61 k. J ∆H = q / mol Mol C 3 H 7 OH = mass / Mr = 1. 50 / 60. 0 = 0. 0250 ∆H = – 49. 61 / 0. 0250 = -1984 k. J mol-1 (3 sig fig) © www. chemsheets. co. uk AS 1048 30 -Jun-2015
 Chemsheets
Chemsheets Lei 1048/2000
Lei 1048/2000 Calorimetry equation
Calorimetry equation Bomb calorimeter equation
Bomb calorimeter equation Enthalpy is the measure of
Enthalpy is the measure of Isothermal calorimetry principle
Isothermal calorimetry principle Bomb calorimetry
Bomb calorimetry Calorimetry questions
Calorimetry questions Calorimetry
Calorimetry State and explain hess's law of constant heat summation
State and explain hess's law of constant heat summation Calorimetry practice problems
Calorimetry practice problems Cmmsw adalah
Cmmsw adalah Calorimetry virtual lab
Calorimetry virtual lab Calorimetry lesson
Calorimetry lesson Bomb calorimetry equation
Bomb calorimetry equation Microscale combustion calorimetry
Microscale combustion calorimetry Enthalpy change of combustion
Enthalpy change of combustion Chemsheets crystal types answers
Chemsheets crystal types answers Condensation polymers chemsheets answers
Condensation polymers chemsheets answers Butanone + acidified kcn
Butanone + acidified kcn Amines chemsheets
Amines chemsheets Chemsheets a2 1025
Chemsheets a2 1025 Chemsheets as 1079
Chemsheets as 1079 Chemsheets periodicity
Chemsheets periodicity Writing half equations worksheet
Writing half equations worksheet Io2f lewis structure
Io2f lewis structure Chemsheets as 1017
Chemsheets as 1017 Chemsheets
Chemsheets Chemsheets as 1052
Chemsheets as 1052 Chemsheets as 1063 answers
Chemsheets as 1063 answers Iof4- shape
Iof4- shape Shapes of molecules a level
Shapes of molecules a level Chemsheets
Chemsheets Chemsheets reactions of halogenoalkanes 2
Chemsheets reactions of halogenoalkanes 2 Chemsheets as 1052
Chemsheets as 1052 Amino acids 2 chemsheets
Amino acids 2 chemsheets Chemsheets shapes of molecules
Chemsheets shapes of molecules