CHEMSHEETS ELECTROCHEMISTRY www chemsheets co uk A 2






















- Slides: 46

CHEMSHEETS ELECTROCHEMISTRY © www. chemsheets. co. uk A 2 1077 2 -July-

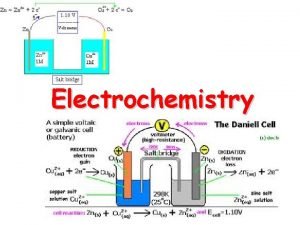
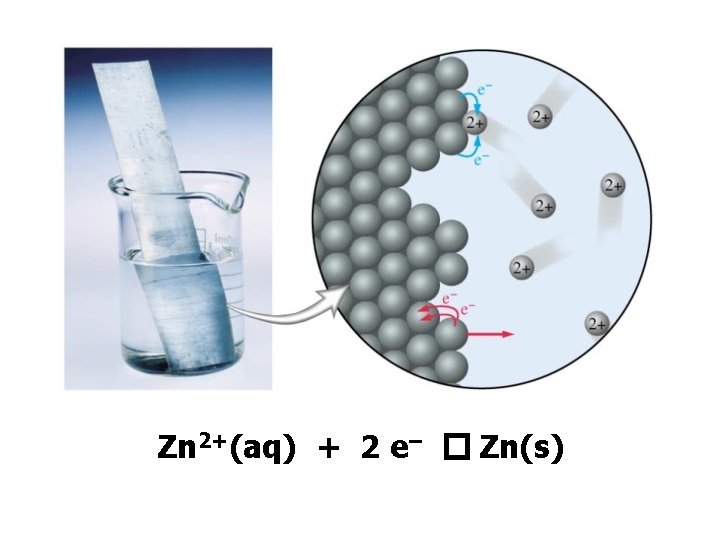
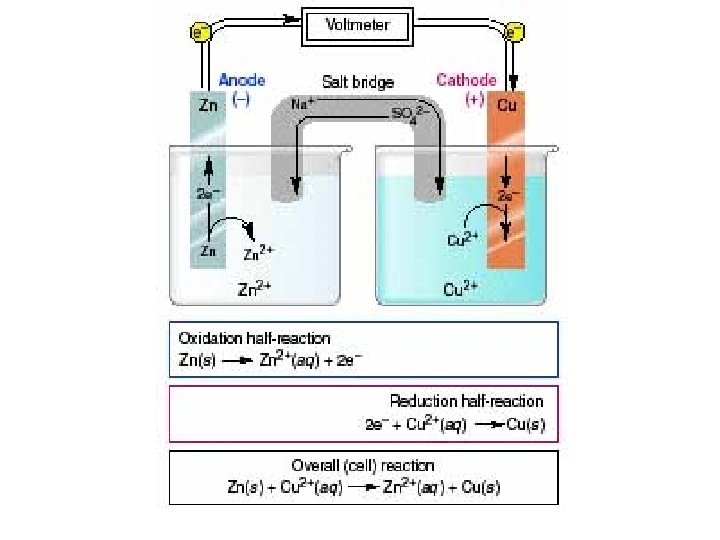
Zn 2+(aq) + 2 e– � Zn(s)

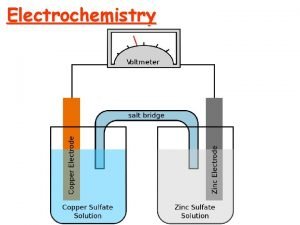
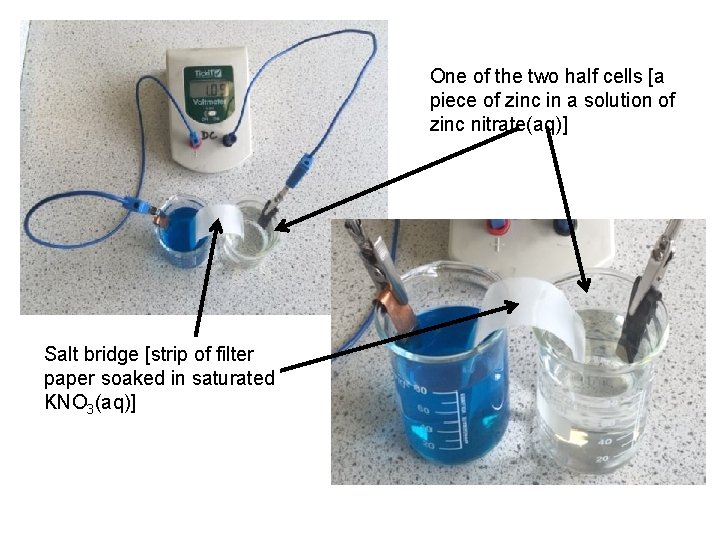
One of the two half cells [a piece of zinc in a solution of zinc nitrate(aq)] Salt bridge [strip of filter paper soaked in saturated KNO 3(aq)]




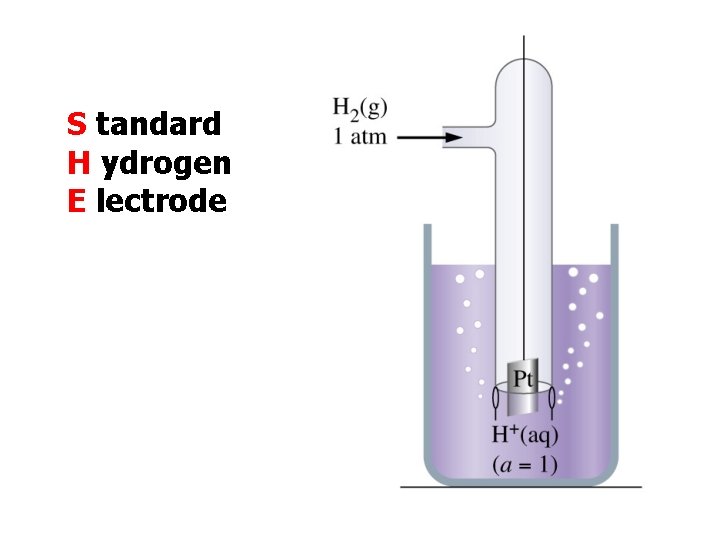
Standard Conditions Concentration 1. 0 mol dm-3 (ions involved in ½ equation) Temperature 298 K Pressure 100 k. Pa (if gases involved in ½ equation) Current Zero (use high resistance voltmeter) © www. chemsheets. co. uk A 2 1077 2 -July-

S tandard H ydrogen E lectrode

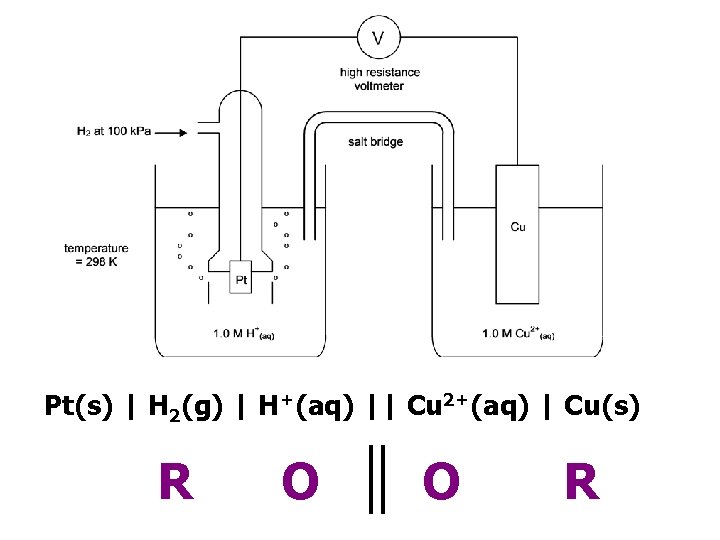
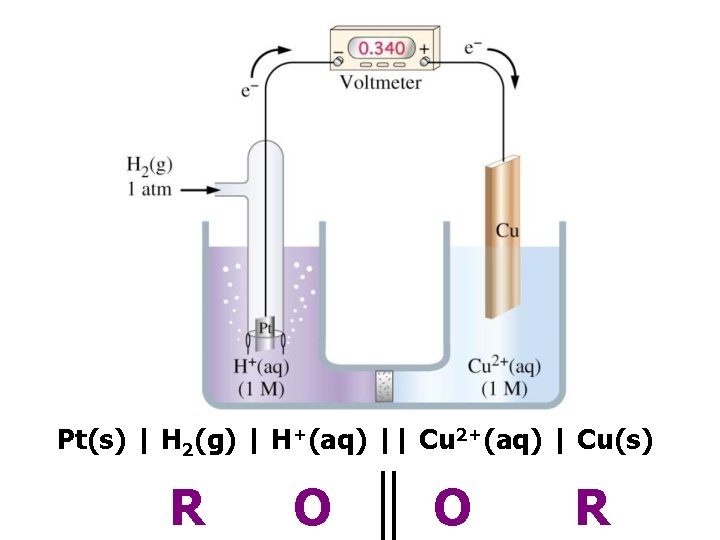
Pt(s) | H 2(g) | H+(aq) || Cu 2+(aq) | Cu(s) R O O R

R O O R Pt(s) | H 2(g) | H+(aq) || Cu 2+(aq) | Cu(s)

Pt(s) | H 2(g) | H+(aq) || Cu 2+(aq) | Cu(s) R O O R

Pt(s) | H 2(g) | H+(aq) || Zn 2+(aq) | Zn(s) R O O R

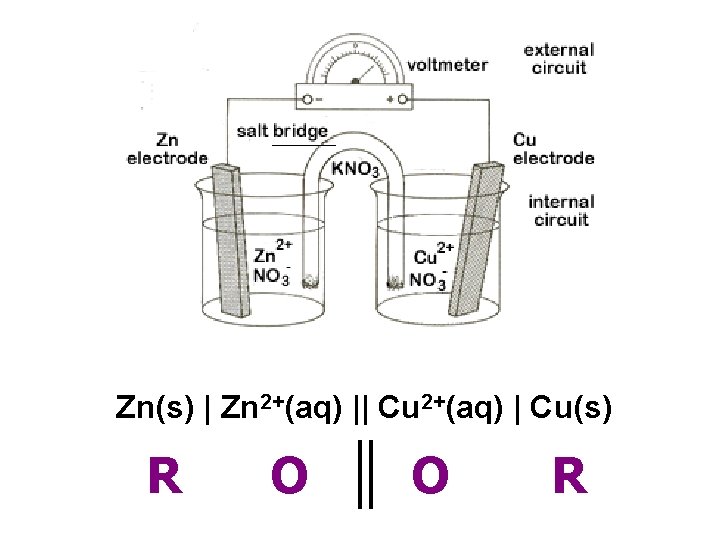
Zn(s) | Zn 2+(aq) || Cu 2+(aq) | Cu(s) R O O R

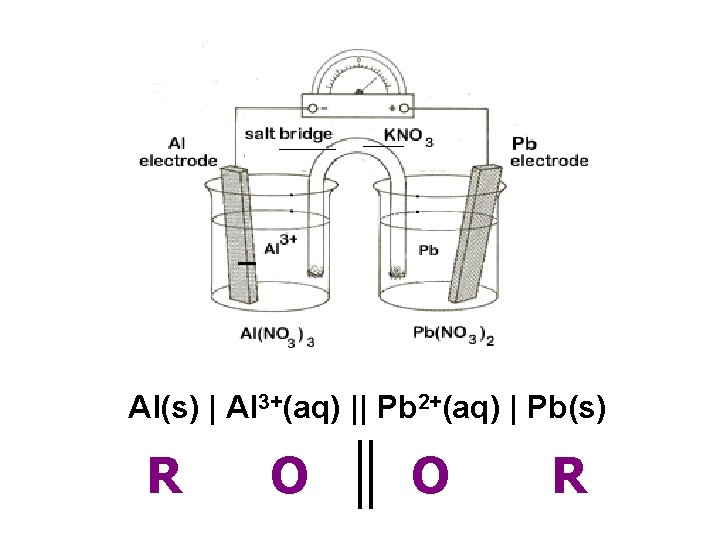
Al(s) | Al 3+(aq) || Pb 2+(aq) | Pb(s) R O O R

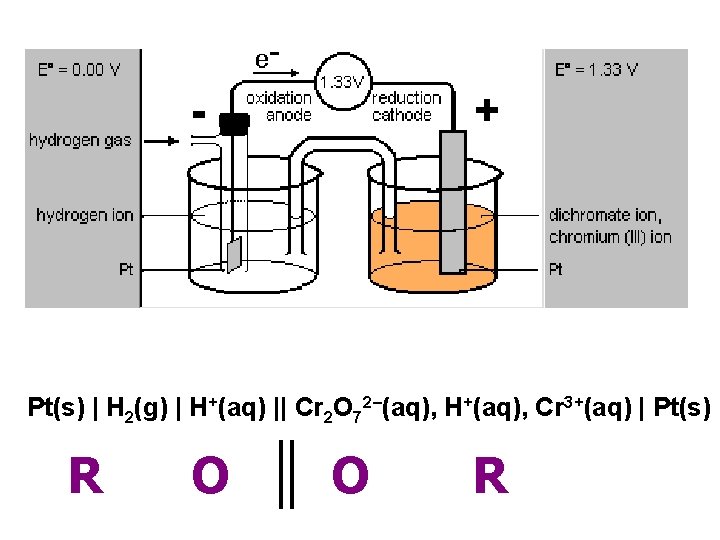
Pt(s) | H 2(g) | H+(aq) || Cr 2 O 72–(aq), H+(aq), Cr 3+(aq) | Pt(s) R O O R

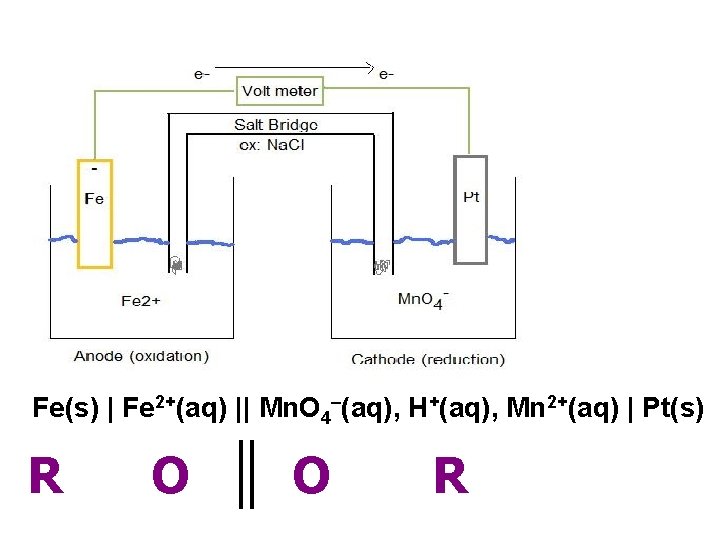
Fe(s) | Fe 2+(aq) || Mn. O 4–(aq), H+(aq), Mn 2+(aq) | Pt(s) R O O R

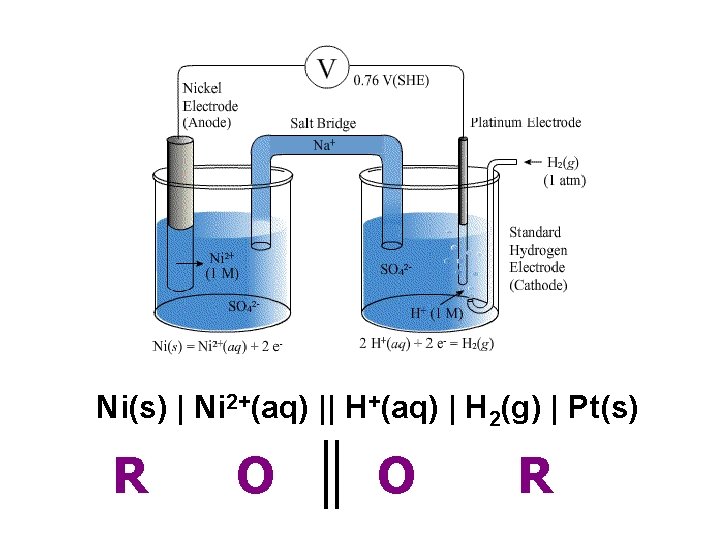
Ni(s) | Ni 2+(aq) || H+(aq) | H 2(g) | Pt(s) R O O R

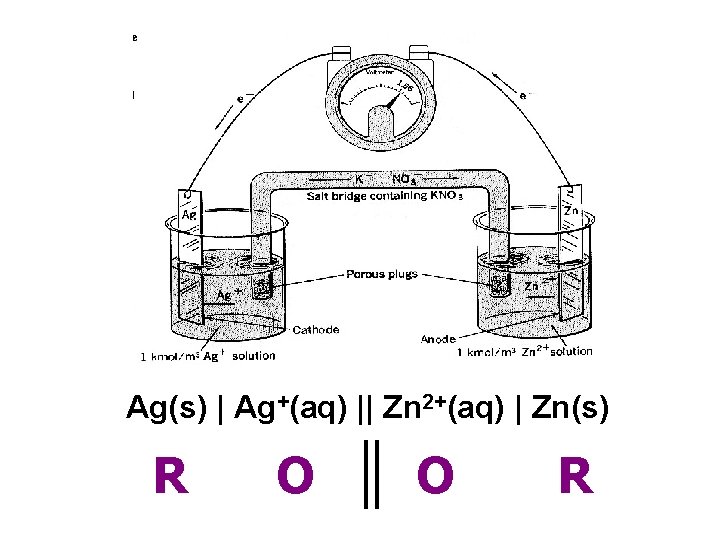
Ag(s) | Ag+(aq) || Zn 2+(aq) | Zn(s) R O O R

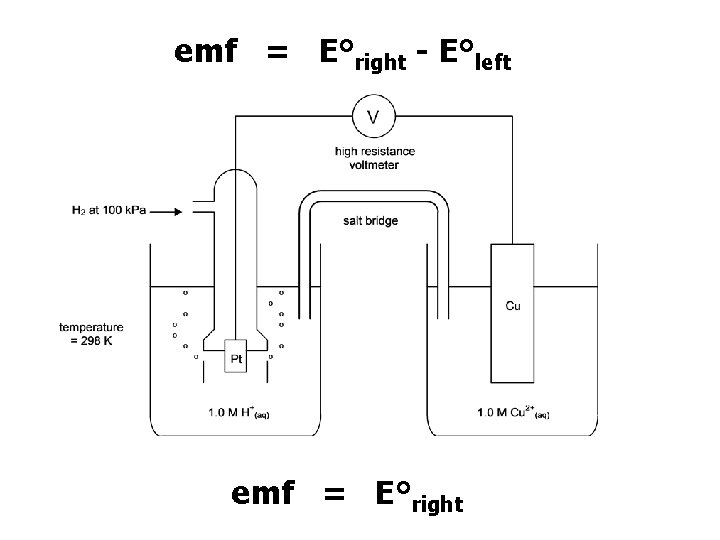
emf = E°right - E°left emf = E°right

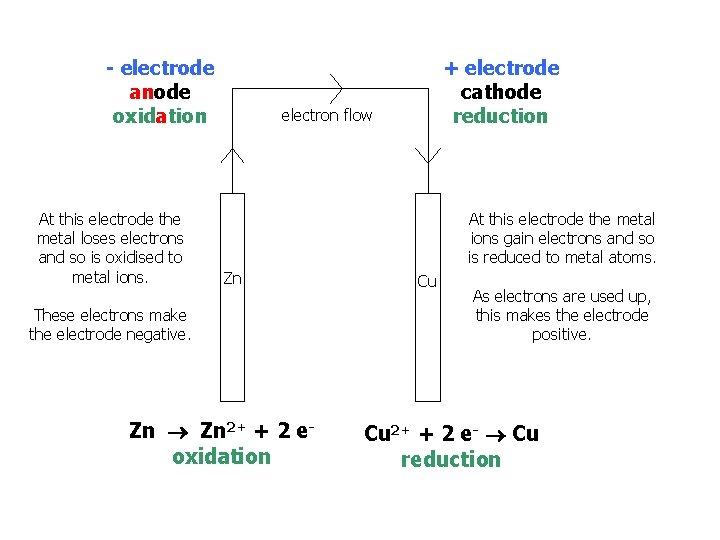
- electrode anode oxidation At this electrode the metal loses electrons and so is oxidised to metal ions. + electrode cathode reduction electron flow At this electrode the metal ions gain electrons and so is reduced to metal atoms. Zn These electrons make the electrode negative. Zn 2+ + 2 eoxidation Cu As electrons are used up, this makes the electrode positive. Cu 2+ + 2 e- Cu reduction

The electrochemical series best oxidising agents best reducing agents

The more +ve electrode gains electrons (+ charge attracts electrons)

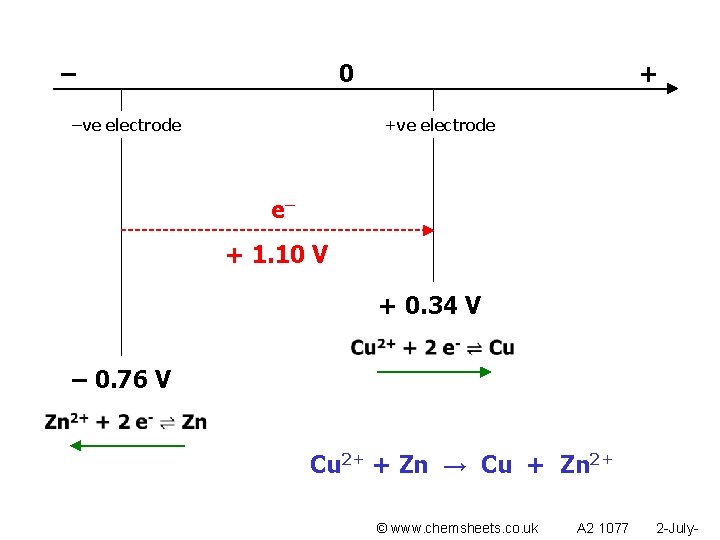
– 0 –ve electrode + +ve electrode e– + 1. 10 V + 0. 34 V – 0. 76 V Cu 2+ + Zn → Cu + Zn 2+ © www. chemsheets. co. uk A 2 1077 2 -July-

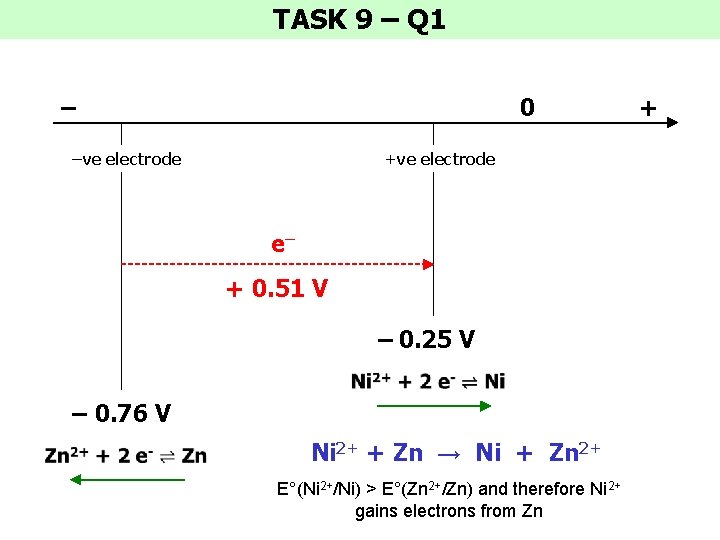
TASK 9 – Q 1 – 0 –ve electrode +ve electrode e– + 0. 51 V – 0. 25 V – 0. 76 V Ni 2+ + Zn → Ni + Zn 2+ E°(Ni 2+/Ni) > E°(Zn 2+/Zn) and therefore Ni 2+ gains electrons from Zn +

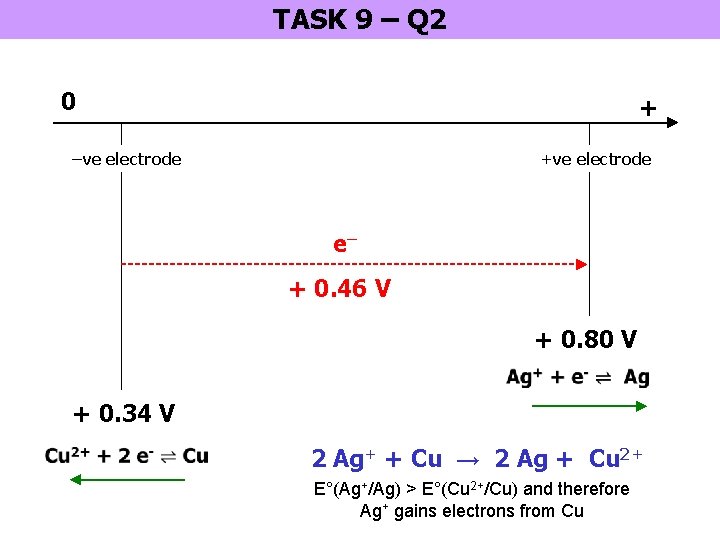
TASK 9 – Q 2 0 + –ve electrode +ve electrode e– + 0. 46 V + 0. 80 V + 0. 34 V 2 Ag+ + Cu → 2 Ag + Cu 2+ E°(Ag+/Ag) > E°(Cu 2+/Cu) and therefore Ag+ gains electrons from Cu

TASK 9 – Q 3 + 0 + 1. 36 V Cl 2 + 2 e- � 2 Cl- + 0. 77 V NO + 1. 51 V + 1. 33 V YES NO Mn. O 4– will oxidise Cl– to form Cl 2 as E°(Mn. O 4–/Mn 2+) > E°(Cl 2/ Cl–) and therefore Mn. O 4– gains electrons from Cl–

TASK 9 – Q 4 a Pt(s)|H 2(g)|H+(aq)||Br 2(aq), Br-(aq)|Pt(s) + 0 –ve electrode )anode +ve electrode e– + 1. 09 V 0. 00 V H 2 + Br 2 → 2 H+ + 2 Br. E°(Br 2/Br–) > E°(H+/H 2) and therefore Br 2 gains electrons from H 2

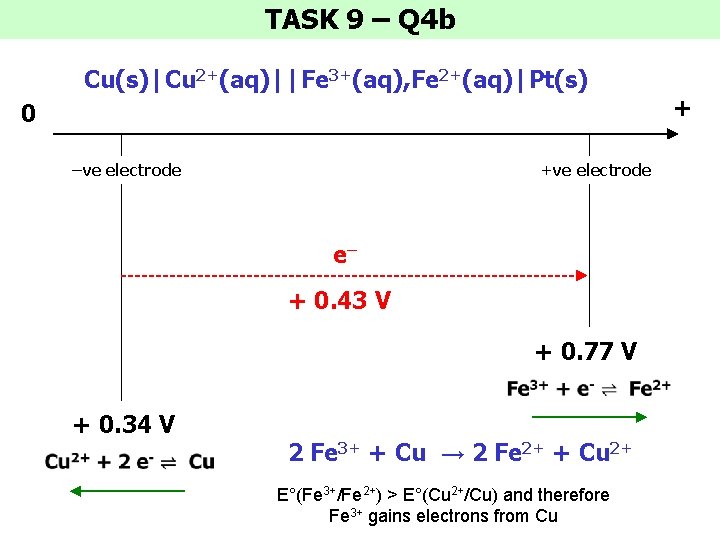
TASK 9 – Q 4 b Cu(s)|Cu 2+(aq)||Fe 3+(aq), Fe 2+(aq)|Pt(s) 0 –ve electrode +ve electrode e– + 0. 43 V + 0. 77 V + 0. 34 V 2 Fe 3+ + Cu → 2 Fe 2+ + Cu 2+ E°(Fe 3+/Fe 2+) > E°(Cu 2+/Cu) and therefore Fe 3+ gains electrons from Cu +

TASK 9 – Q 6 a Fe(s)|Fe 2+(aq)||H+(aq)|H 2(g)|Pt(s) – 0 –ve electrode +ve electrode e– + 0. 44 V 0. 00 V – 0. 44 V YES: H+ oxidises Fe to Fe 2+ E°(H+/H 2) > E°(Fe 2+/Fe) and therefore H+ gains electrons from Fe

TASK 9 – Q 6 b 0 + –ve electrode +ve electrode e– + 0. 34 V 0. 00 V NO: H+ won’t oxidise Cu to Cu 2+ E°(H+/H 2) < E°(Cu 2+/Cu) and therefore H+ cannot gain electrons from Cu

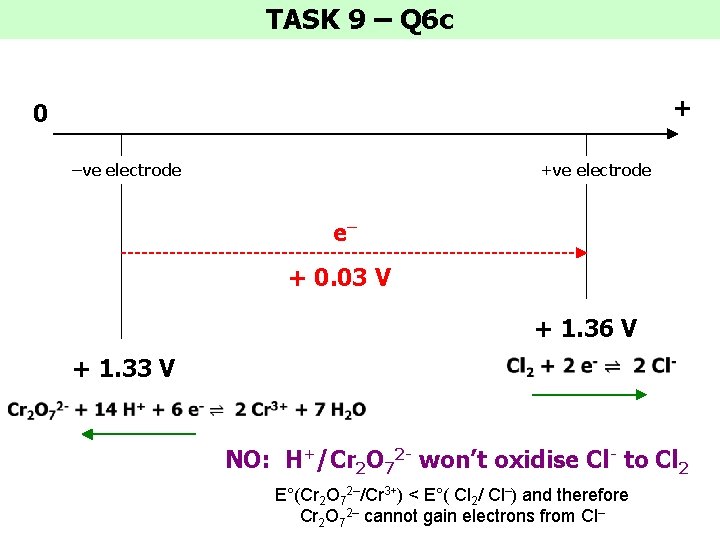
TASK 9 – Q 6 c + 0 –ve electrode +ve electrode e– + 0. 03 V + 1. 36 V + 1. 33 V NO: H+/Cr 2 O 72 - won’t oxidise Cl- to Cl 2 E°(Cr 2 O 72–/Cr 3+) < E°( Cl 2/ Cl–) and therefore Cr 2 O 72– cannot gain electrons from Cl–

TASK 9 – Q 6 d Pt(s)|Cl-(aq)|Cl 2(g)||Mn. O 4 - (aq), H+(aq), Mn 2+(aq)|Pt(s) + 0 –ve electrode +ve electrode e– + 0. 03 V + 1. 51 V + 1. 36 V YES: H+/Mn. O 4 - oxidises Cl- to Cl 2 E°(Mn. O 4–/Mn 2+) > E°(Cl 2/ Cl–) and therefore Mn. O 4– gains electrons from Cl–

TASK 9 – Q 6 e Mg(s)|Mg 2+(aq)||V 3+(aq), V 2+(aq)|Pt(s) – 0 –ve electrode +ve electrode e– + 2. 10 V – 0. 26 V – 2. 36 V YES: Mg reduces V 3+ to V 2+ E°(V 3+/V 2+) > E°(Mg 2+/Mg) and therefore V 3+ gains electrons from Mg

Commercial Cells rechargeable Non

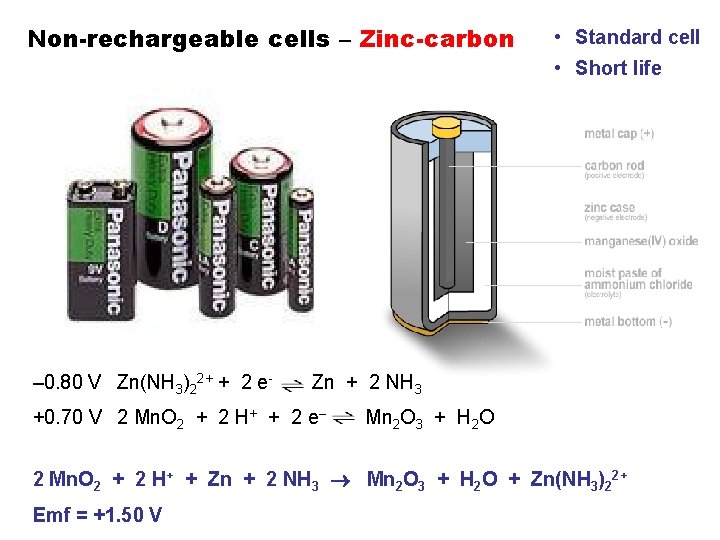
Non-rechargeable cells – Zinc-carbon • Standard cell • Short life – 0. 80 V Zn(NH 3)22+ + 2 e- Zn + 2 NH 3 +0. 70 V 2 Mn. O 2 + 2 H+ + 2 e– Mn 2 O 3 + H 2 O 2 Mn. O 2 + 2 H+ + Zn + 2 NH 3 Mn 2 O 3 + H 2 O + Zn(NH 3)22+ Emf = +1. 50 V

Non-rechargeable cells – alkaline – 0. 76 V Zn 2+ + 2 e– Zn +0. 84 V Mn. O 2 + H 2 O + e– Mn. O(OH) + OH– 2 Mn. O 2 + 2 H 2 O + Zn 2 Mn. O(OH) + 2 OH– + Zn 2+ Emf = +1. 60 V • Longer life

Commercial Cells Rechargeable

Rechargeable cells – Li ion • Rechargeable • Most common rechargeable cell +0. 60 V Li+ + Co. O 2 + e– – 3. 00 V Li+ + e– Li. Co. O 2 Li In use: Co. O 2 + Li Li. Co. O 2 Charging: Li. Co. O 2 + Li Emf = +3. 60 V

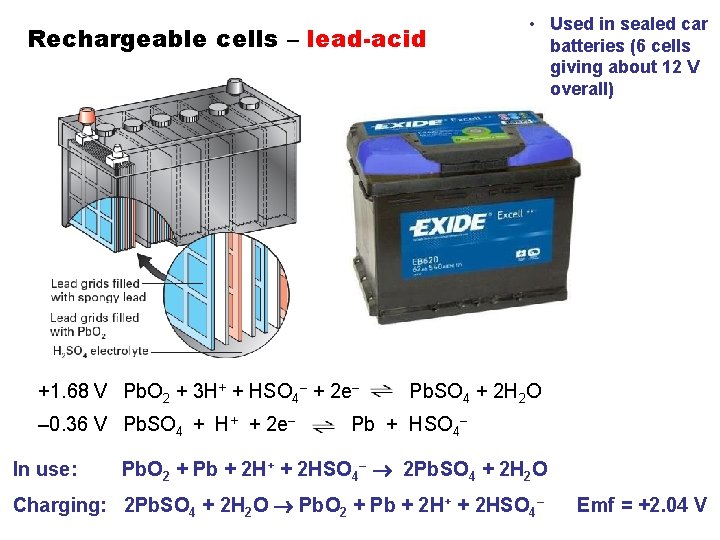
Rechargeable cells – lead-acid +1. 68 V Pb. O 2 + 3 H+ + HSO 4– + 2 e– – 0. 36 V Pb. SO 4 + H+ + 2 e– In use: • Used in sealed car batteries (6 cells giving about 12 V overall) Pb. SO 4 + 2 H 2 O Pb + HSO 4– Pb. O 2 + Pb + 2 H+ + 2 HSO 4– 2 Pb. SO 4 + 2 H 2 O Charging: 2 Pb. SO 4 + 2 H 2 O Pb. O 2 + Pb + 2 H+ + 2 HSO 4– Emf = +2. 04 V

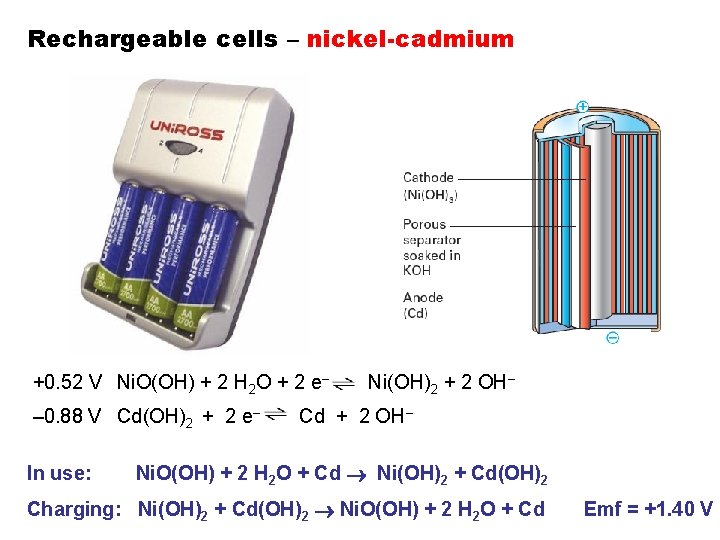
Rechargeable cells – nickel-cadmium +0. 52 V Ni. O(OH) + 2 H 2 O + 2 e– – 0. 88 V Cd(OH)2 + 2 e– In use: Ni(OH)2 + 2 OH– Cd + 2 OH– Ni. O(OH) + 2 H 2 O + Cd Ni(OH)2 + Cd(OH)2 Charging: Ni(OH)2 + Cd(OH)2 Ni. O(OH) + 2 H 2 O + Cd Emf = +1. 40 V

Commercial Cells Fuel


Hydrogen-Oxygen FUEL CELLS

Hydrogen-Oxygen FUEL CELLS • High efficiency (more efficient than burning hydrogen) • How is H 2 made? Determine: a) cell emf b) overall reaction • Input of H 2/O 2 to replenish so no need to recharge

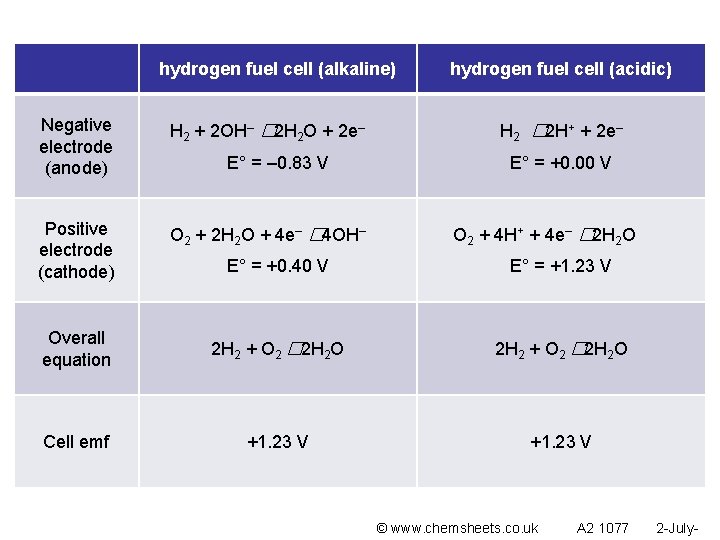
hydrogen fuel cell (alkaline) Negative electrode (anode) H 2 + 2 OH– � 2 H 2 O + 2 e– Positive electrode (cathode) O 2 + 2 H 2 O + 4 e– � 4 OH– E° = – 0. 83 V hydrogen fuel cell (acidic) H 2 � 2 H+ + 2 e– E° = +0. 00 V O 2 + 4 H+ + 4 e– � 2 H 2 O E° = +0. 40 V E° = +1. 23 V Overall equation 2 H 2 + O 2 � 2 H 2 O Cell emf +1. 23 V © www. chemsheets. co. uk A 2 1077 2 -July-

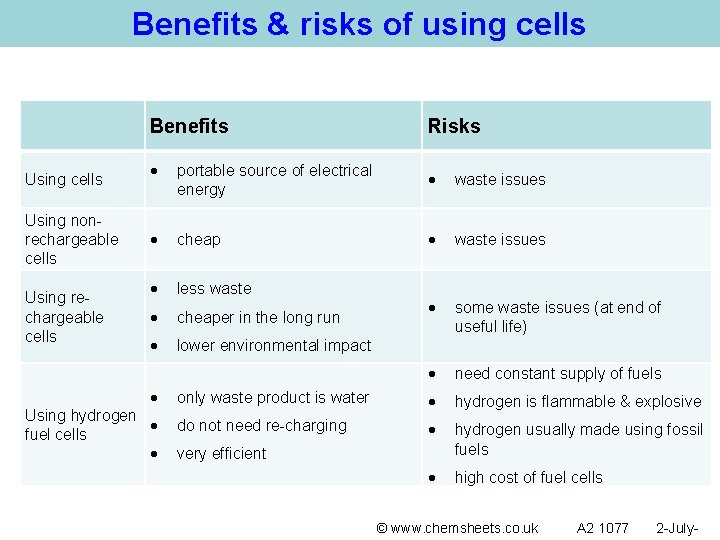
Benefits & risks of using cells Benefits Using cells Using nonrechargeable cells Using rechargeable cells Risks portable source of electrical energy waste issues cheap waste issues less waste cheaper in the long run some waste issues (at end of useful life) lower environmental impact need constant supply of fuels only waste product is water hydrogen is flammable & explosive do not need re-charging hydrogen usually made using fossil fuels high cost of fuel cells Using hydrogen fuel cells very efficient © www. chemsheets. co. uk A 2 1077 2 -July-
 Fundamentals of electrochemistry
Fundamentals of electrochemistry Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Chapter 21 electrochemistry
Chapter 21 electrochemistry What is electrochemistry
What is electrochemistry Ir drop in electrochemistry
Ir drop in electrochemistry Diagonal rule electrochemistry
Diagonal rule electrochemistry E cell formula
E cell formula Spontaneity of redox reaction
Spontaneity of redox reaction Electrochemistry khan academy
Electrochemistry khan academy Fundamentals of electrochemistry
Fundamentals of electrochemistry Chemistry
Chemistry Junction potential
Junction potential What is electrochemistry
What is electrochemistry Electrochemistry ap chem
Electrochemistry ap chem Chapter 20 electrochemistry
Chapter 20 electrochemistry Electrochemistry balancing equations
Electrochemistry balancing equations Electrochemistry balancing equations
Electrochemistry balancing equations What is polarization in electrochemistry
What is polarization in electrochemistry Electroanalytical
Electroanalytical Koh alkaline
Koh alkaline Basic electrochemistry
Basic electrochemistry What is electrochemistry
What is electrochemistry Mass transport electrochemistry
Mass transport electrochemistry Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test What is electrochemistry in chemistry
What is electrochemistry in chemistry What are redox reactions examples
What are redox reactions examples Electrochemistry stoichiometry
Electrochemistry stoichiometry Whats electrochemistry
Whats electrochemistry Electrochemistry lesson plan
Electrochemistry lesson plan Electrochemistry tutorial
Electrochemistry tutorial Electrochemistry eds
Electrochemistry eds Balance redox reactions
Balance redox reactions Electrochemistry
Electrochemistry Concentration cell
Concentration cell Diagonal rule electrochemistry
Diagonal rule electrochemistry Introduction of electrochemistry
Introduction of electrochemistry Kno3 salt bridge
Kno3 salt bridge Chapter 21 electrochemistry
Chapter 21 electrochemistry Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Voltaic aim
Voltaic aim Branches of electrochemistry
Branches of electrochemistry State hittorf s rule
State hittorf s rule Chemsheets as 1079
Chemsheets as 1079 Chemsheets as 1017 shapes of molecules
Chemsheets as 1017 shapes of molecules Group 2 questions chemsheets answers
Group 2 questions chemsheets answers Chemsheets energetics introduction answers
Chemsheets energetics introduction answers Chemsheets shapes of molecules
Chemsheets shapes of molecules