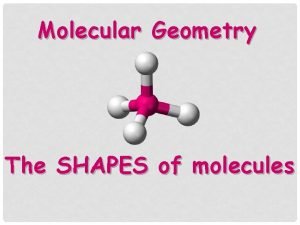
CHEMSHEETS SHAPES OF MOLECULES Chemsheets AS 1025 07

- Slides: 27

CHEMSHEETS SHAPES OF MOLECULES Chemsheets AS 1025 07 -Jun-2015

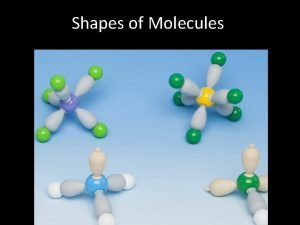
BASIC IDEAS Shapes of molecules and ions depends on total number of electron pairs around the central atom. Two types of electron pairs: • Bonding pairs (i. e. e-’s in a covalent bond) • Non-bonding pairs (i. e. lone pairs) These electron pairs repel as far as possible (but lone pairs repel more than bonding pairs).

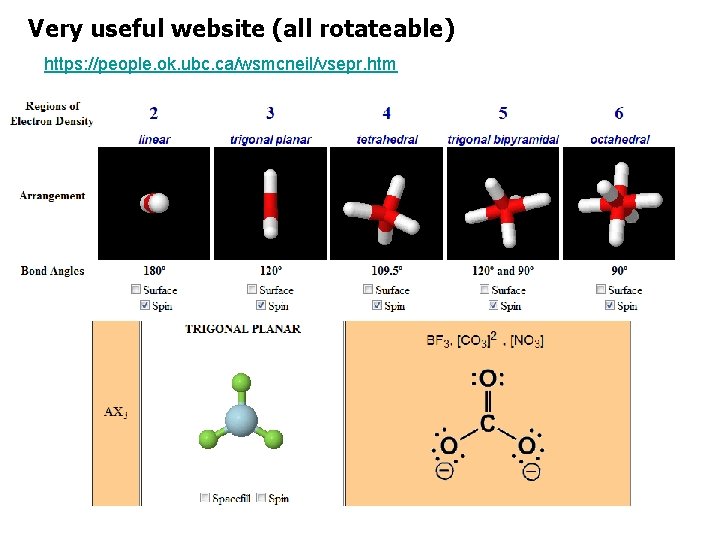
Very useful website (all rotateable) https: //people. ok. ubc. ca/wsmcneil/vsepr. htm

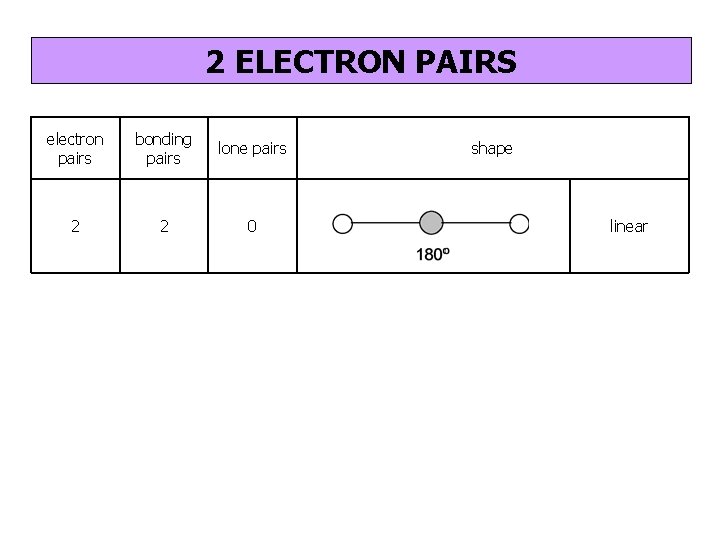
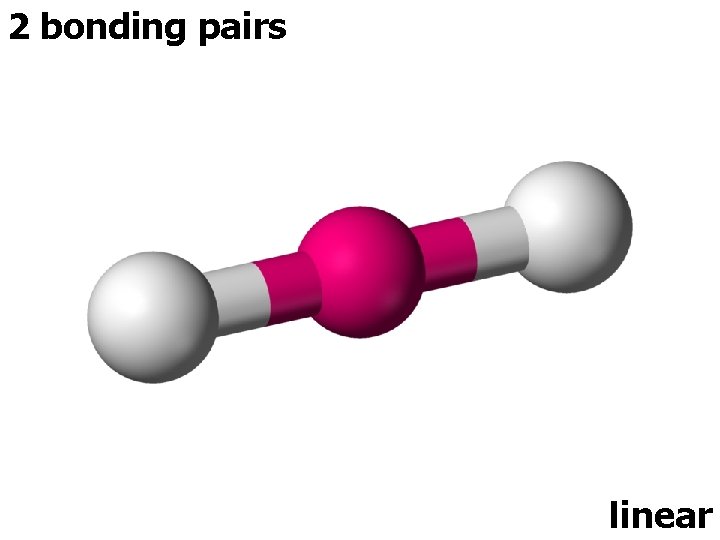
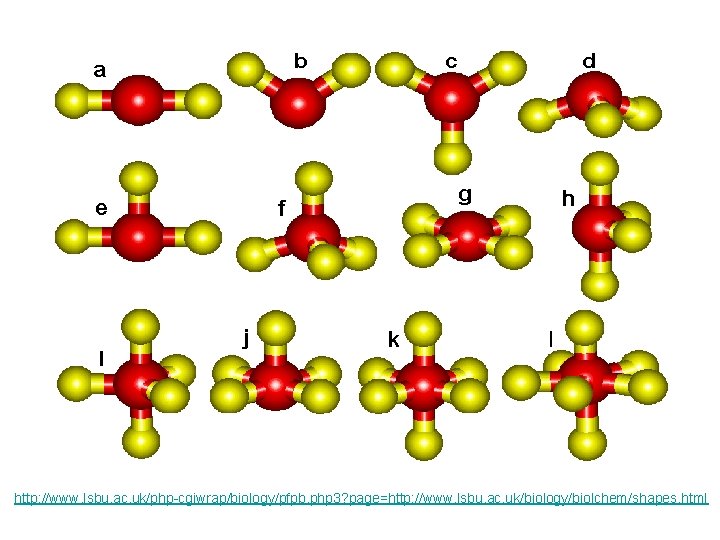
2 ELECTRON PAIRS electron pairs bonding pairs lone pairs 2 2 0 shape linear

2 bonding pairs linear

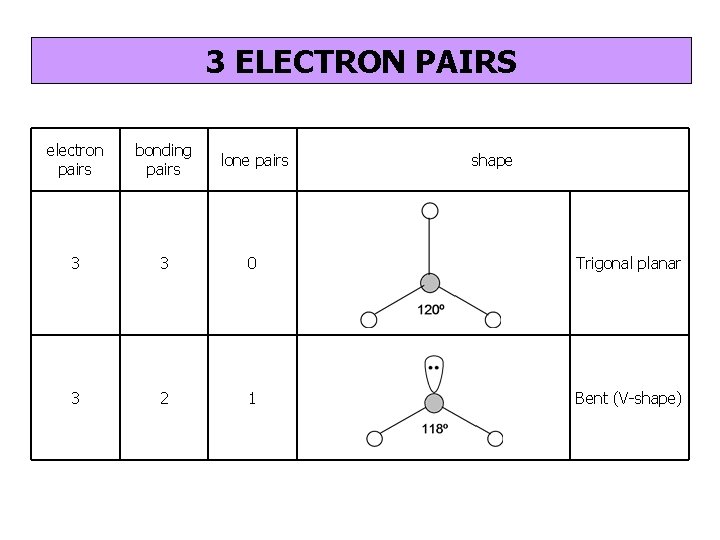
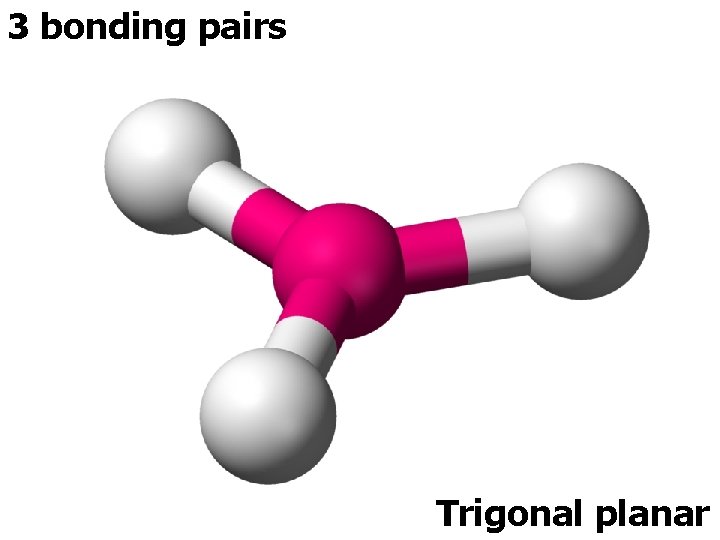
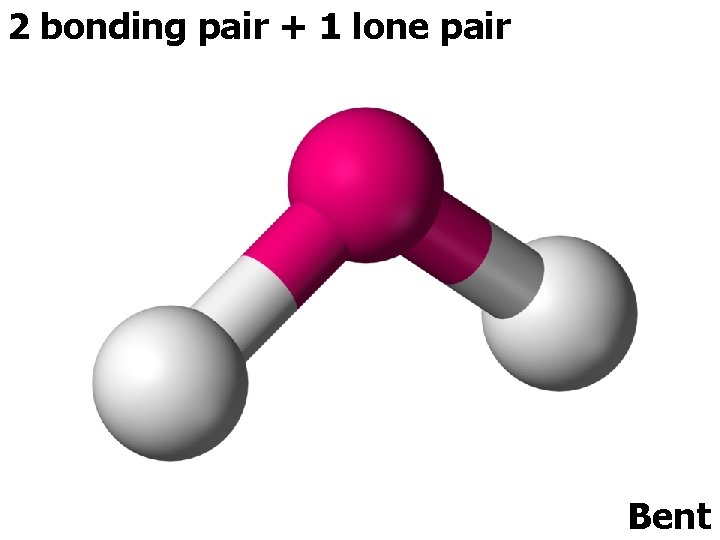
3 ELECTRON PAIRS electron pairs bonding pairs lone pairs 3 3 0 Trigonal planar 3 2 1 Bent (V-shape) shape

3 bonding pairs Trigonal planar

2 bonding pair + 1 lone pair Bent



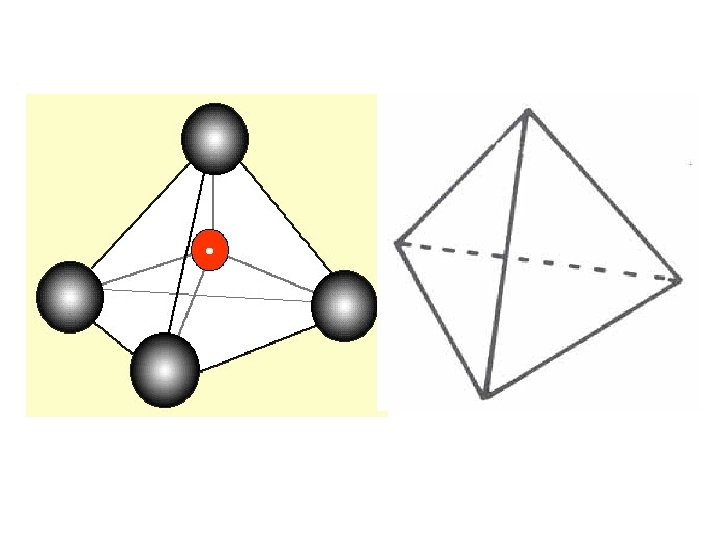
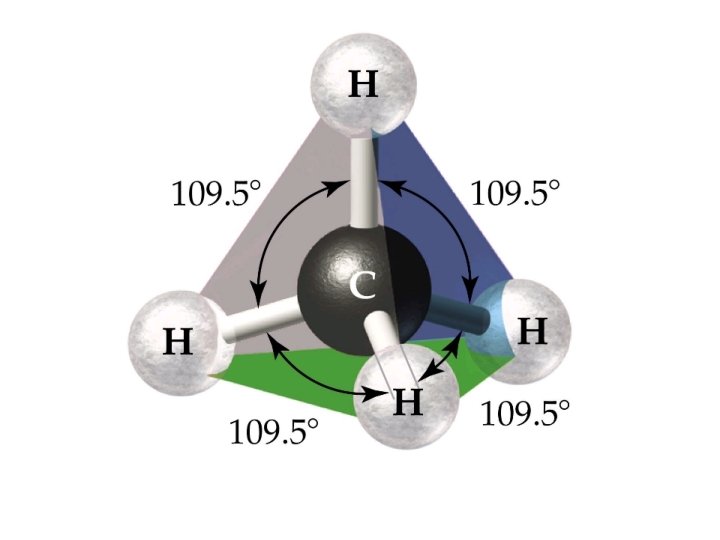
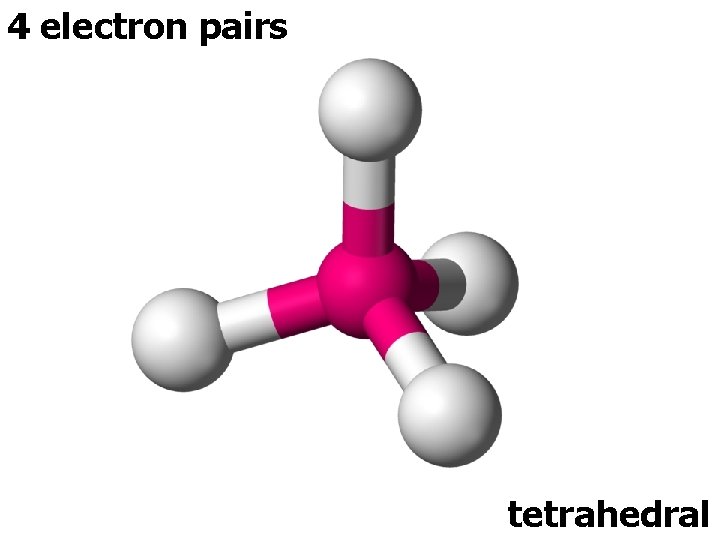
4 electron pairs tetrahedral


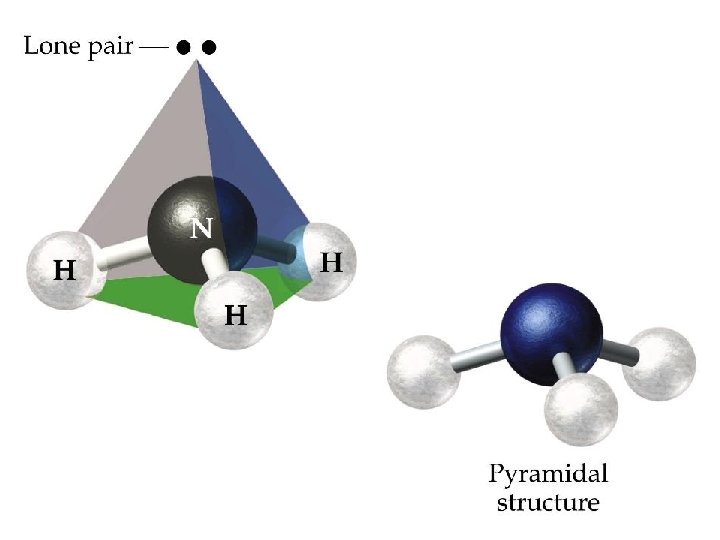
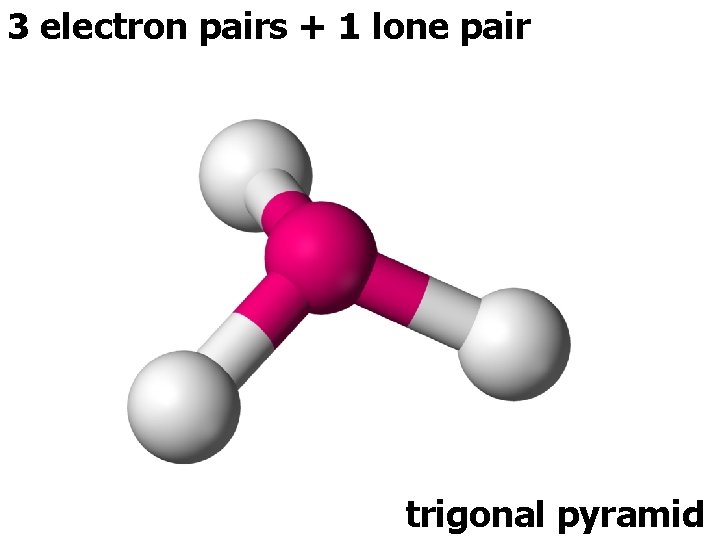
3 electron pairs + 1 lone pair trigonal pyramid


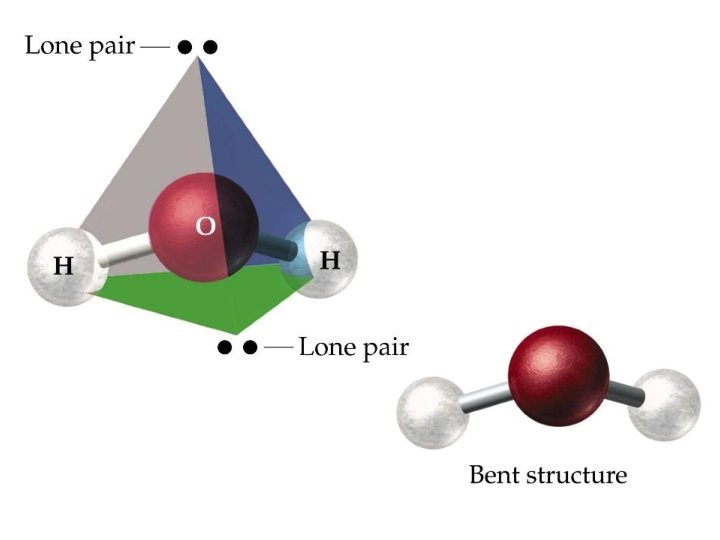
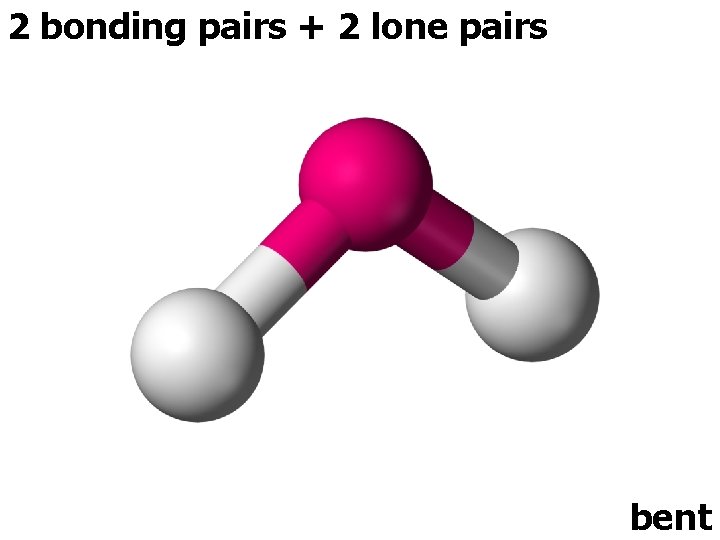
2 bonding pairs + 2 lone pairs bent

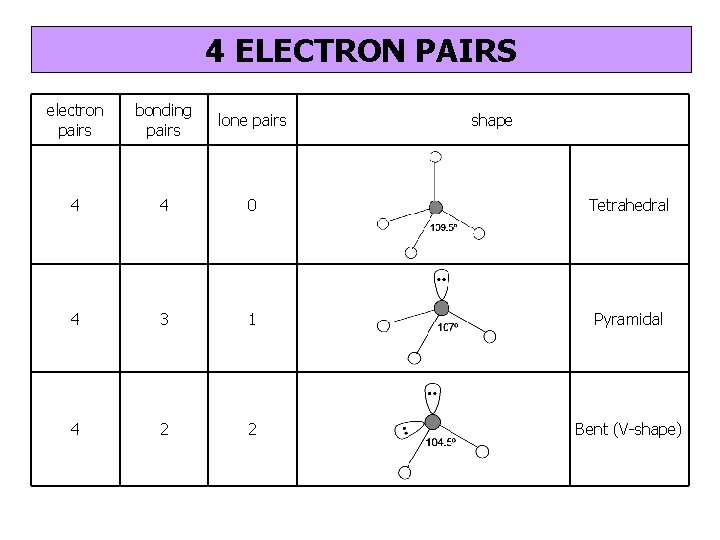
4 ELECTRON PAIRS electron pairs bonding pairs lone pairs 4 4 0 Tetrahedral 4 3 1 Pyramidal 4 2 2 Bent (V-shape) shape

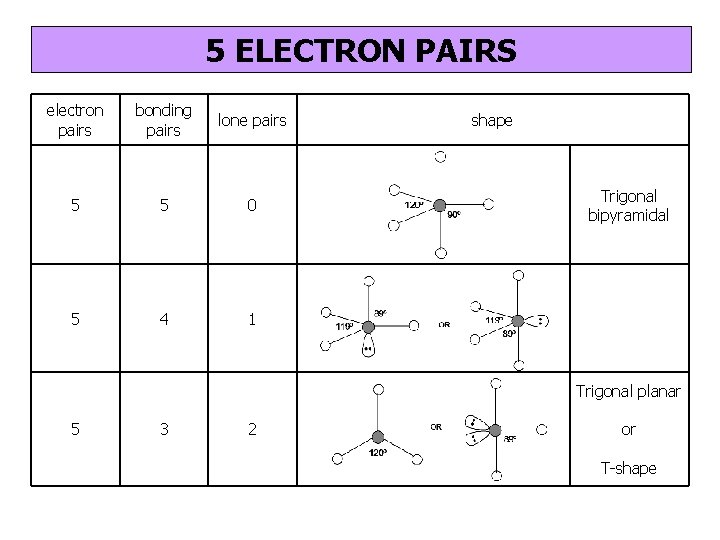
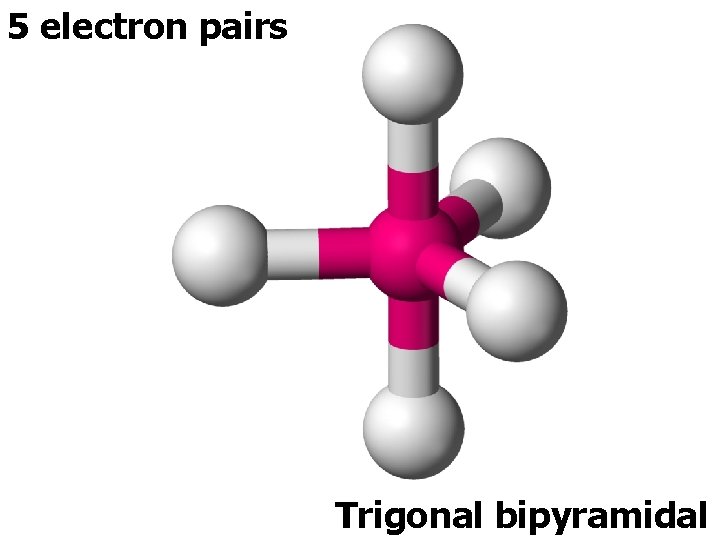
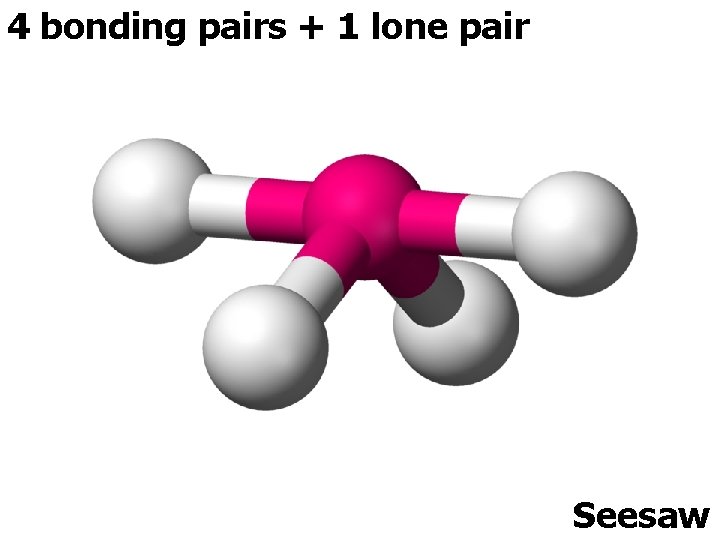
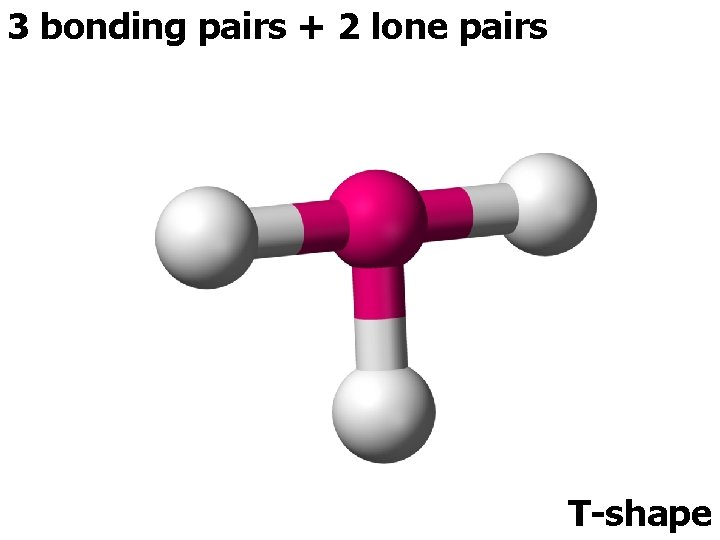
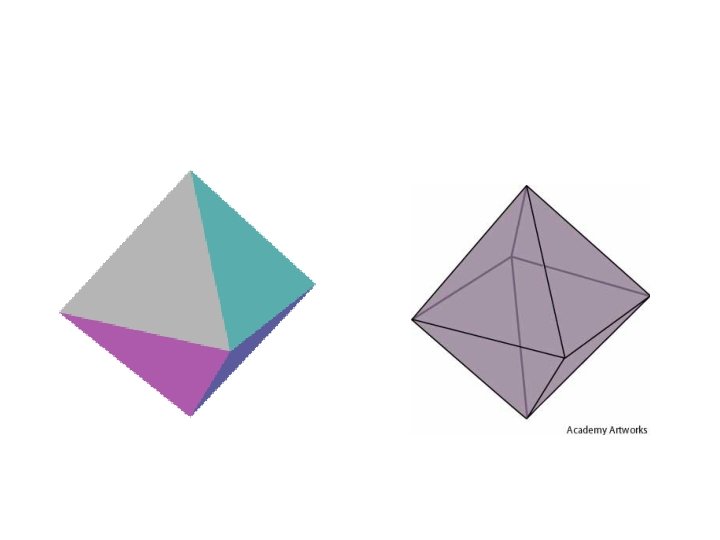
5 ELECTRON PAIRS electron pairs bonding pairs lone pairs 5 5 0 5 4 1 shape Trigonal bipyramidal Trigonal planar 5 3 2 or T-shape

5 electron pairs Trigonal bipyramidal

4 bonding pairs + 1 lone pair Seesaw

3 bonding pairs + 2 lone pairs T-shape

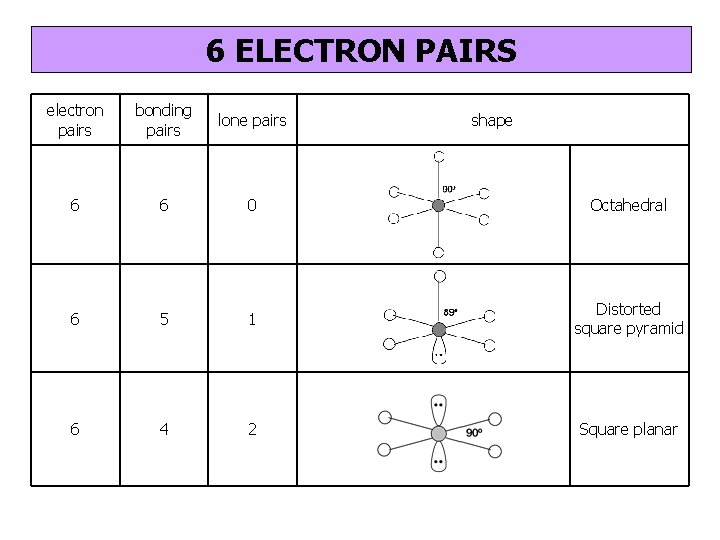
6 ELECTRON PAIRS electron pairs bonding pairs lone pairs 6 6 0 Octahedral 6 5 1 Distorted square pyramid 6 4 2 Square planar shape

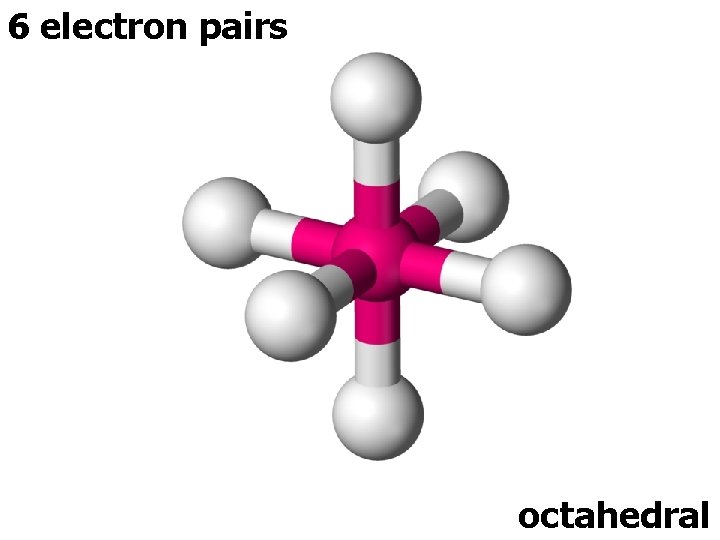
6 electron pairs octahedral

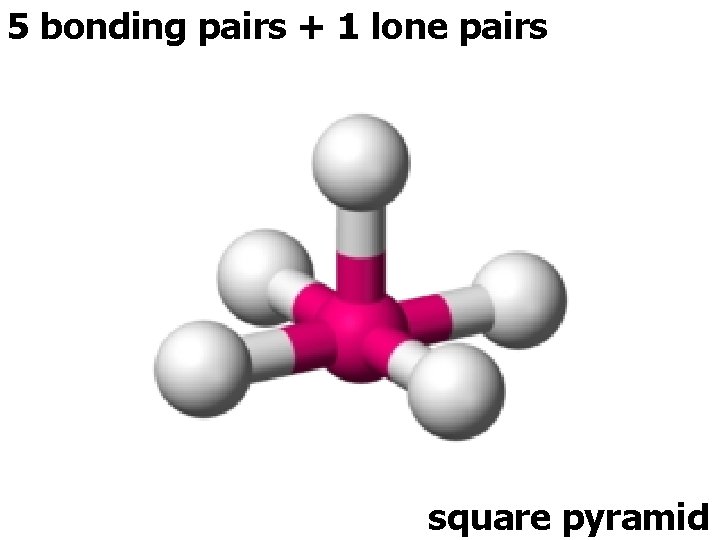
5 bonding pairs + 1 lone pairs square pyramid

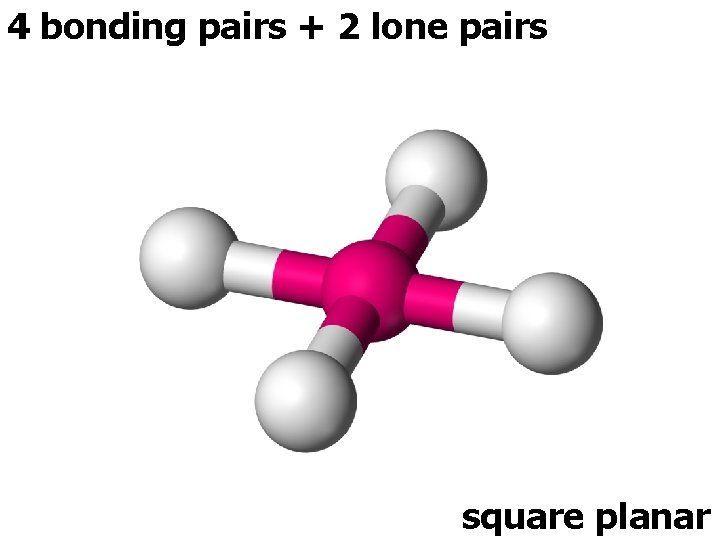
4 bonding pairs + 2 lone pairs square planar


http: //www. lsbu. ac. uk/php-cgiwrap/biology/pfpb. php 3? page=http: //www. lsbu. ac. uk/biology/biolchem/shapes. html

 Chemsheets as 1017 shapes of molecules
Chemsheets as 1017 shapes of molecules Io2f lewis structure
Io2f lewis structure Co2 steric number
Co2 steric number Chemsheets shapes of molecules answers
Chemsheets shapes of molecules answers Chemsheets shapes of molecules
Chemsheets shapes of molecules Chemsheets as 1018
Chemsheets as 1018 Chemsheets
Chemsheets Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Shapes of molecules a level chemistry
Shapes of molecules a level chemistry 4 electron domains 2 lone pairs
4 electron domains 2 lone pairs Chemistry geometric shapes
Chemistry geometric shapes Shapes of molecules a level
Shapes of molecules a level Co shape and polarity
Co shape and polarity Ift1025
Ift1025 Ift 1025
Ift 1025 Mit 1025
Mit 1025 Ift 1025
Ift 1025 1025 ce
1025 ce Java interface graphique
Java interface graphique Ift 1025
Ift 1025 Ift 1025
Ift 1025 Ift 1025
Ift 1025 Ift 1025
Ift 1025 Shapes that seem to follow no rules
Shapes that seem to follow no rules In order for john to hear jill air molecules
In order for john to hear jill air molecules How molecules are formed
How molecules are formed Van der waals equation partial derivative
Van der waals equation partial derivative Ozone molecular geometry
Ozone molecular geometry