Isothermal Titration Calorimetry ITC Definitions and Principles An

- Slides: 20

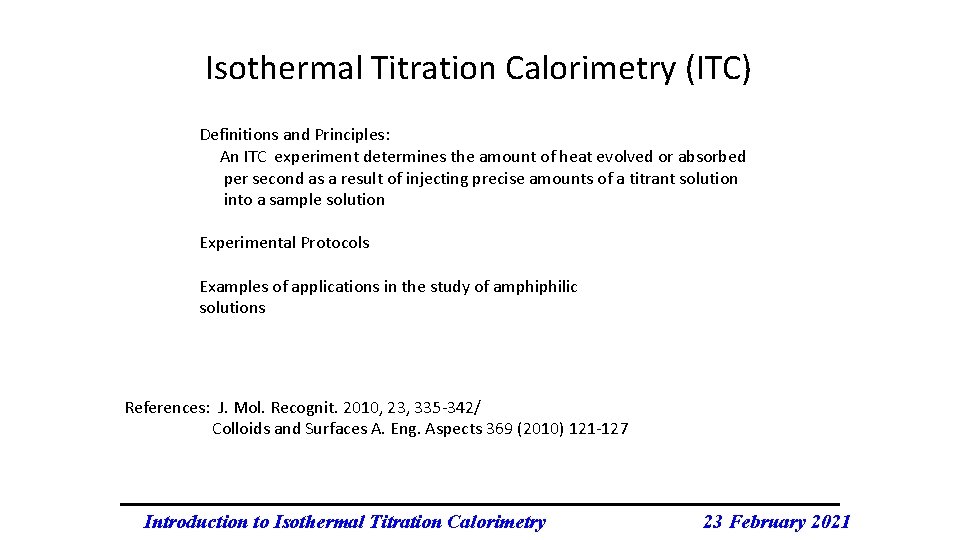
Isothermal Titration Calorimetry (ITC) Definitions and Principles: An ITC experiment determines the amount of heat evolved or absorbed per second as a result of injecting precise amounts of a titrant solution into a sample solution Experimental Protocols Examples of applications in the study of amphiphilic solutions References: J. Mol. Recognit. 2010, 23, 335 -342/ Colloids and Surfaces A. Eng. Aspects 369 (2010) 121 -127 Introduction to Isothermal Titration Calorimetry 23 February 2021

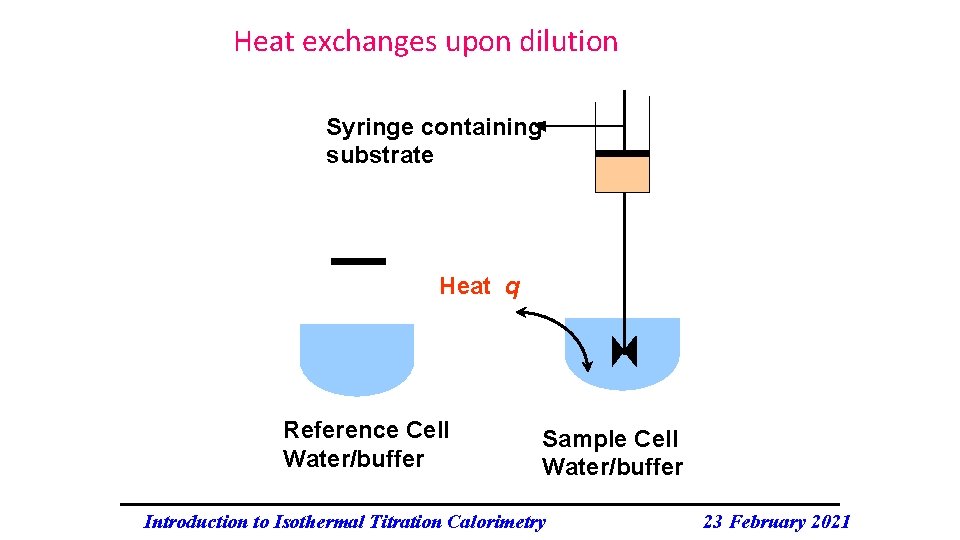
Heat exchanges upon dilution Syringe containing substrate Heat q Reference Cell Water/buffer Sample Cell Water/buffer Introduction to Isothermal Titration Calorimetry 23 February 2021

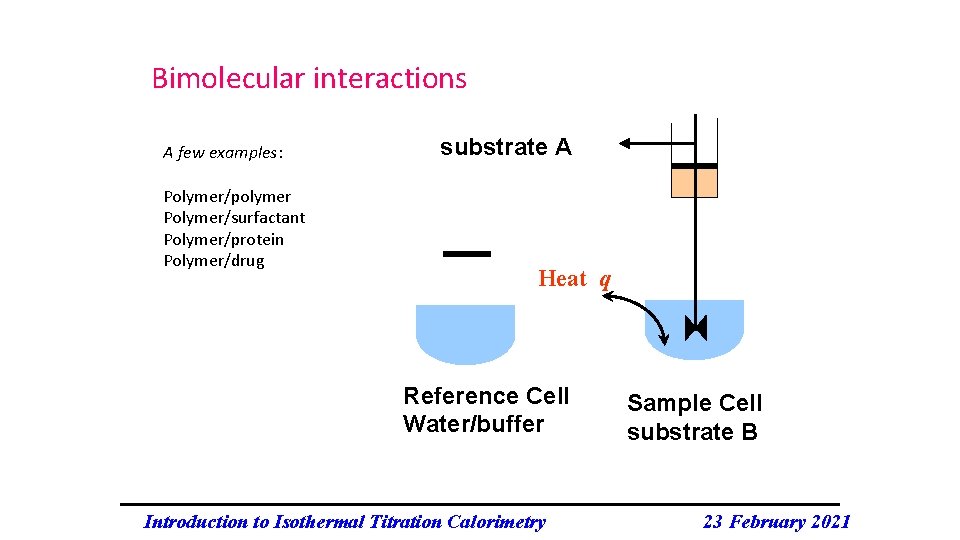
Bimolecular interactions A few examples: Polymer/polymer Polymer/surfactant Polymer/protein Polymer/drug substrate A Heat q Reference Cell Water/buffer Introduction to Isothermal Titration Calorimetry Sample Cell substrate B 23 February 2021

Quantities that can be measured upon dilution For amphiphilic polymers in aqueous solution: enthalpy of micellization critical association concentration dilution enthalpy For bimolecular interactions: Binding energy ‘ Binding stoechiometry (number of binding sites) Binding constant Also: changes in entropy, heat capacity. . Introduction to Isothermal Titration Calorimetry 23 February 2021

Isothermal Titration Calorimetry (ITC)

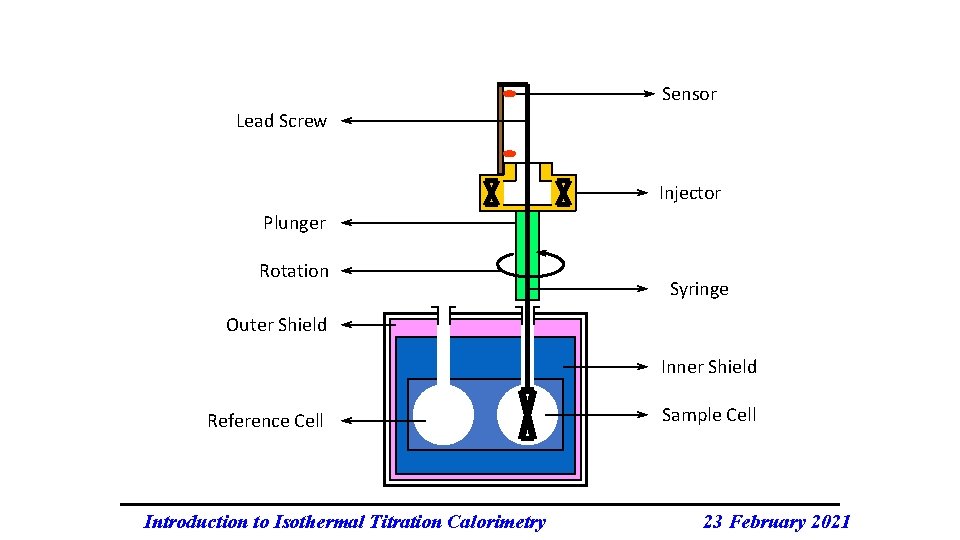
Sensor Lead Screw Injector Plunger Rotation Syringe Outer Shield Inner Shield Reference Cell Introduction to Isothermal Titration Calorimetry Sample Cell 23 February 2021

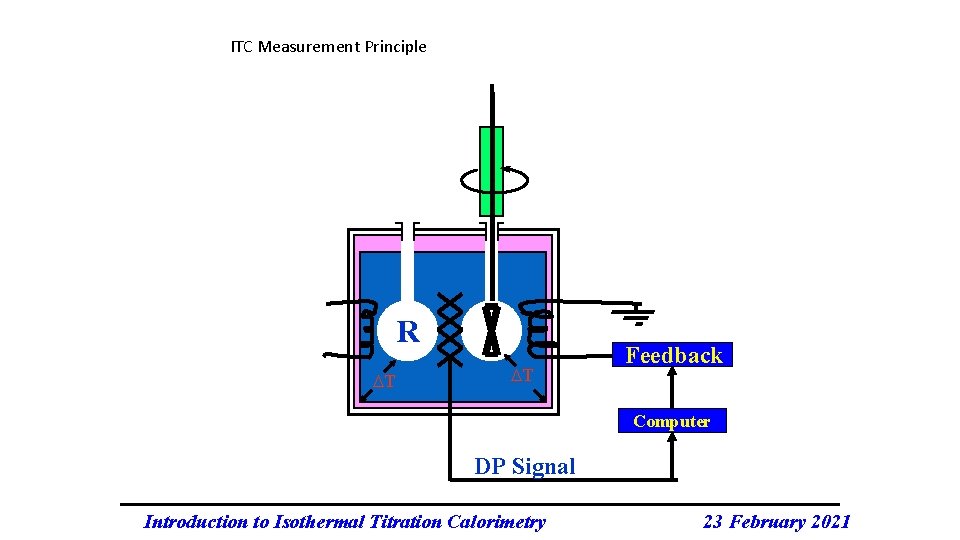
ITC Measurement Principle R T T Feedback Computer DP Signal Introduction to Isothermal Titration Calorimetry 23 February 2021

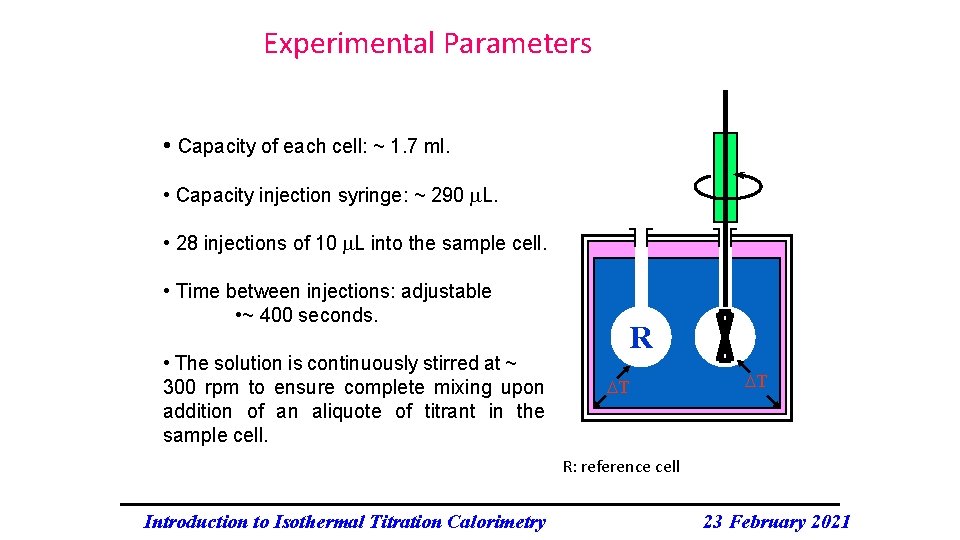
Experimental Parameters • Capacity of each cell: ~ 1. 7 ml. • Capacity injection syringe: ~ 290 L. • 28 injections of 10 L into the sample cell. • Time between injections: adjustable • ~ 400 seconds. • The solution is continuously stirred at ~ 300 rpm to ensure complete mixing upon addition of an aliquote of titrant in the sample cell. R T T R: reference cell Introduction to Isothermal Titration Calorimetry 23 February 2021

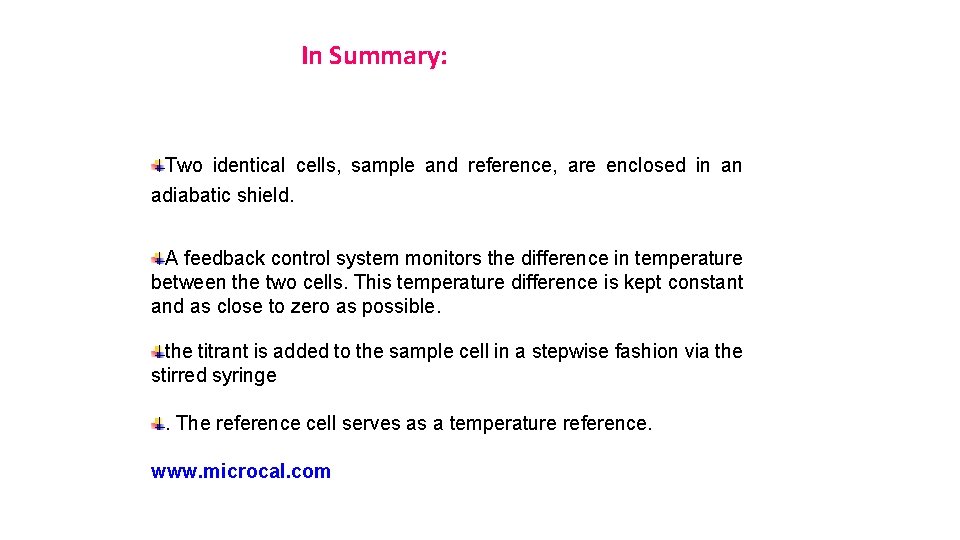
In Summary: Two identical cells, sample and reference, are enclosed in an adiabatic shield. A feedback control system monitors the difference in temperature between the two cells. This temperature difference is kept constant and as close to zero as possible. the titrant is added to the sample cell in a stepwise fashion via the stirred syringe. The reference cell serves as a temperature reference. www. microcal. com

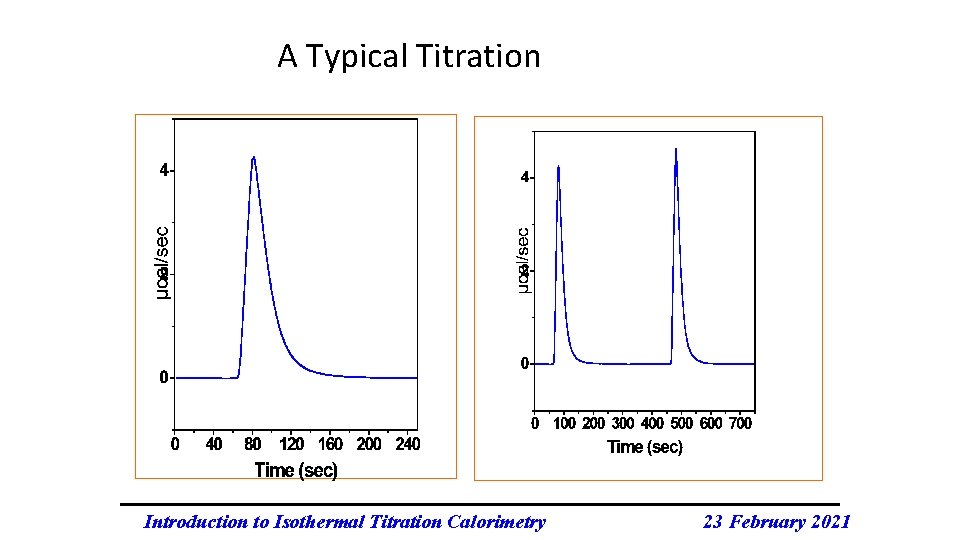
A Typical Titration Introduction to Isothermal Titration Calorimetry 23 February 2021

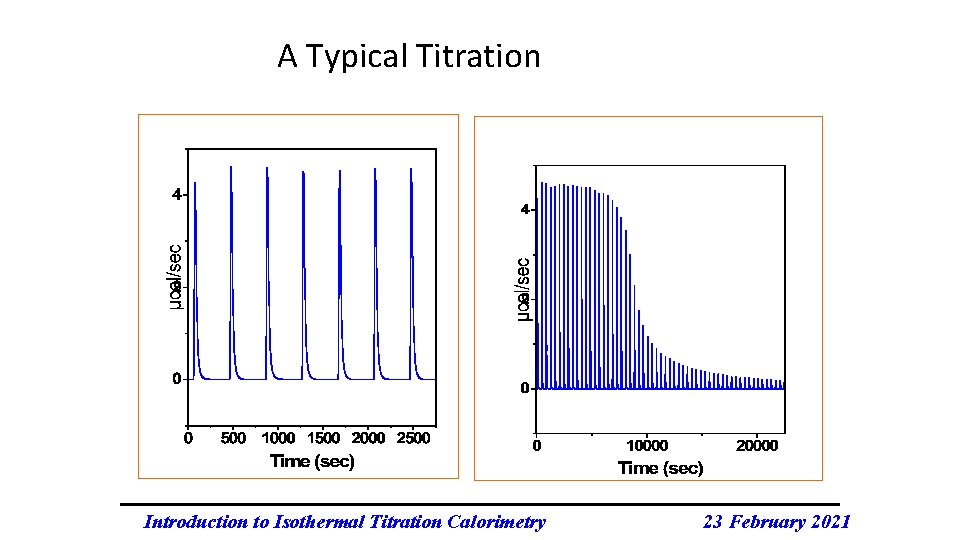
A Typical Titration Introduction to Isothermal Titration Calorimetry 23 February 2021

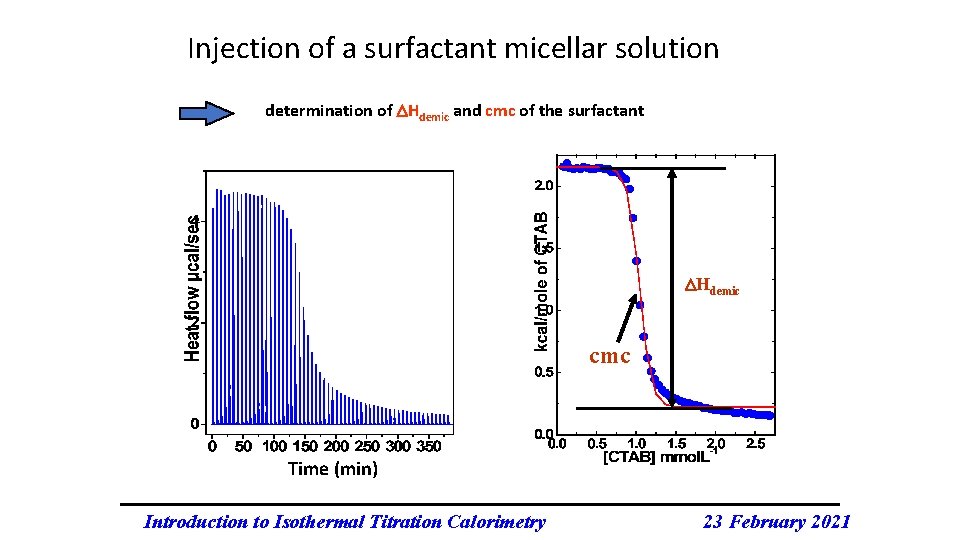
Injection of a surfactant micellar solution determination of Hdemic and cmc of the surfactant Hdemic cmc Time (min) Introduction to Isothermal Titration Calorimetry 23 February 2021

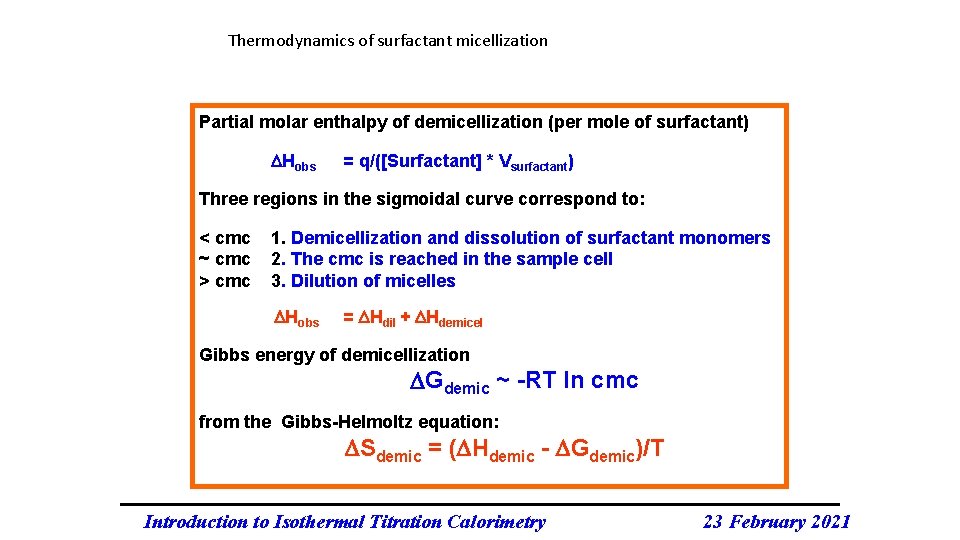
Thermodynamics of surfactant micellization Partial molar enthalpy of demicellization (per mole of surfactant) Hobs = q/([Surfactant] * Vsurfactant) Three regions in the sigmoidal curve correspond to: < cmc ~ cmc > cmc 1. Demicellization and dissolution of surfactant monomers 2. The cmc is reached in the sample cell 3. Dilution of micelles Hobs = Hdil + Hdemicel Gibbs energy of demicellization Gdemic ~ -RT ln cmc from the Gibbs-Helmoltz equation: Sdemic = ( Hdemic - Gdemic)/T Introduction to Isothermal Titration Calorimetry 23 February 2021

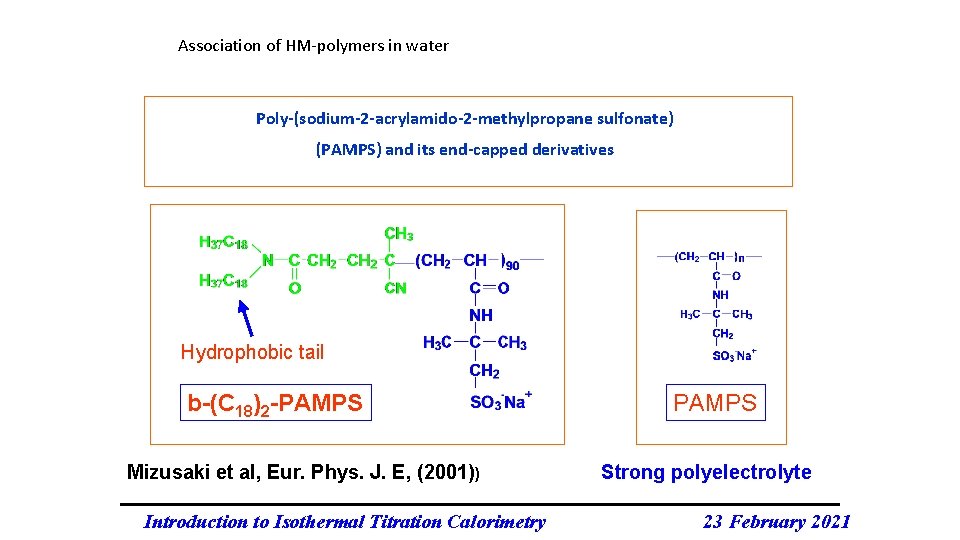
Association of HM-polymers in water Poly-(sodium-2 -acrylamido-2 -methylpropane sulfonate) (PAMPS) and its end-capped derivatives Hydrophobic tail b-(C 18)2 -PAMPS Mizusaki et al, Eur. Phys. J. E, (2001)) Introduction to Isothermal Titration Calorimetry PAMPS Strong polyelectrolyte 23 February 2021

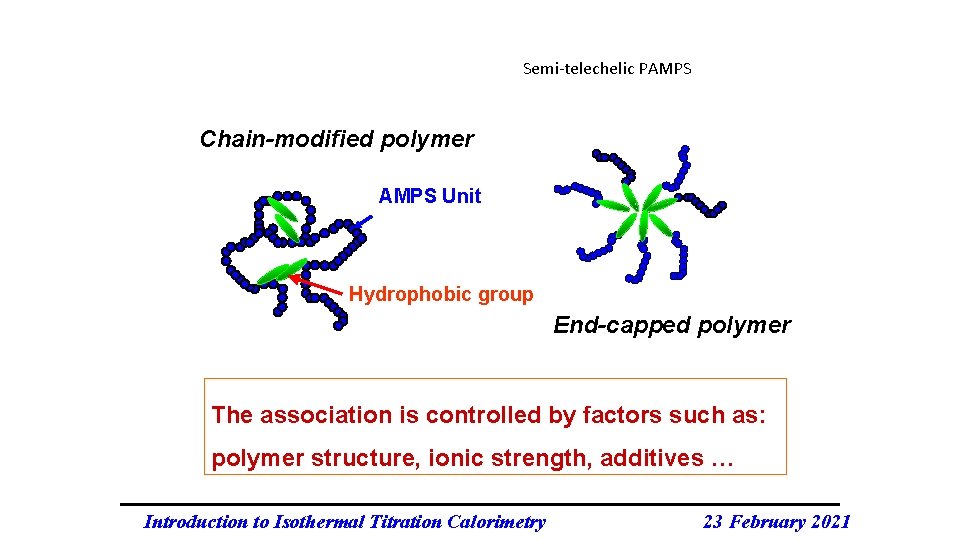
Semi-telechelic PAMPS Chain-modified polymer AMPS Unit Hydrophobic group End-capped polymer The association is controlled by factors such as: polymer structure, ionic strength, additives … Introduction to Isothermal Titration Calorimetry 23 February 2021

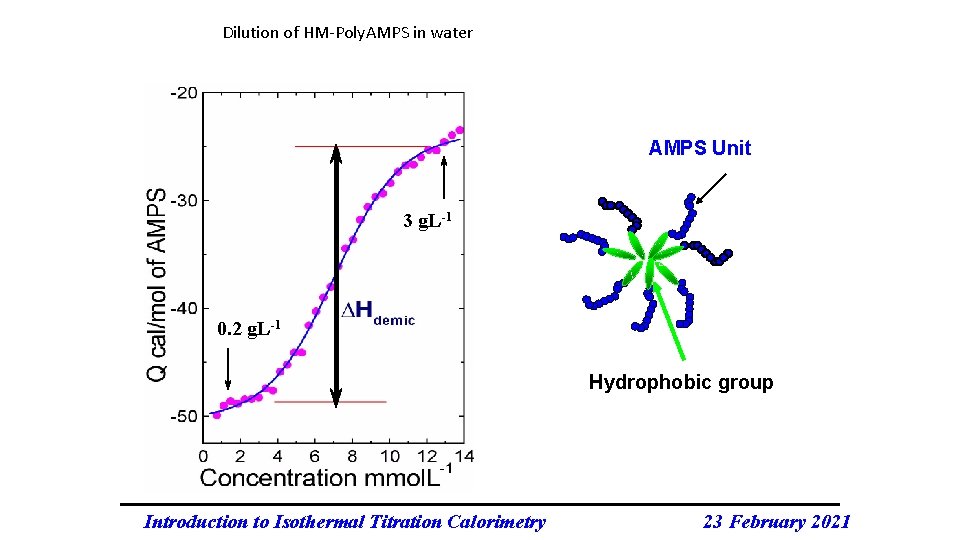
Dilution of HM-Poly. AMPS in water AMPS Unit 3 g. L-1 0. 2 g. L-1 Hydrophobic group Introduction to Isothermal Titration Calorimetry 23 February 2021

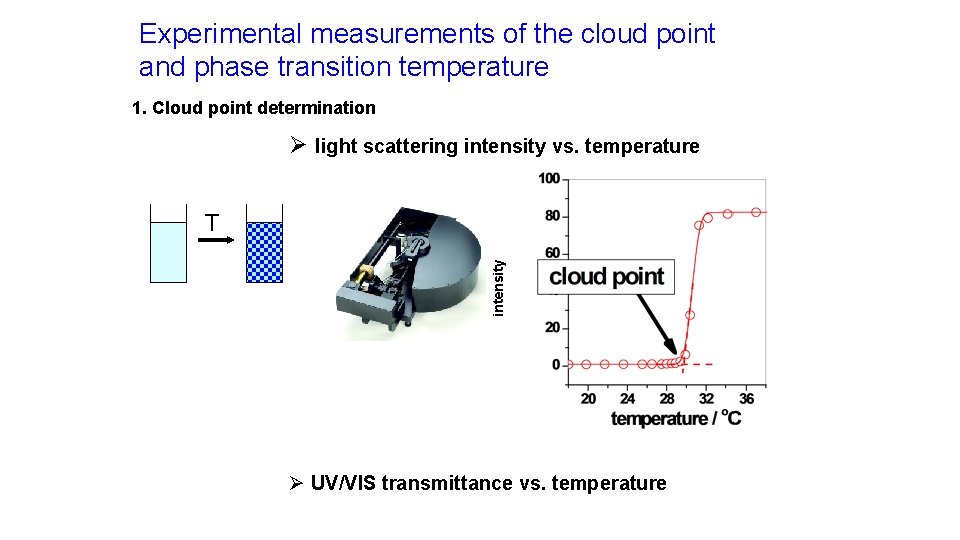
Experimental measurements of the cloud point and phase transition temperature 1. Cloud point determination Ø light scattering intensity vs. temperature intensity T Ø UV/VIS transmittance vs. temperature

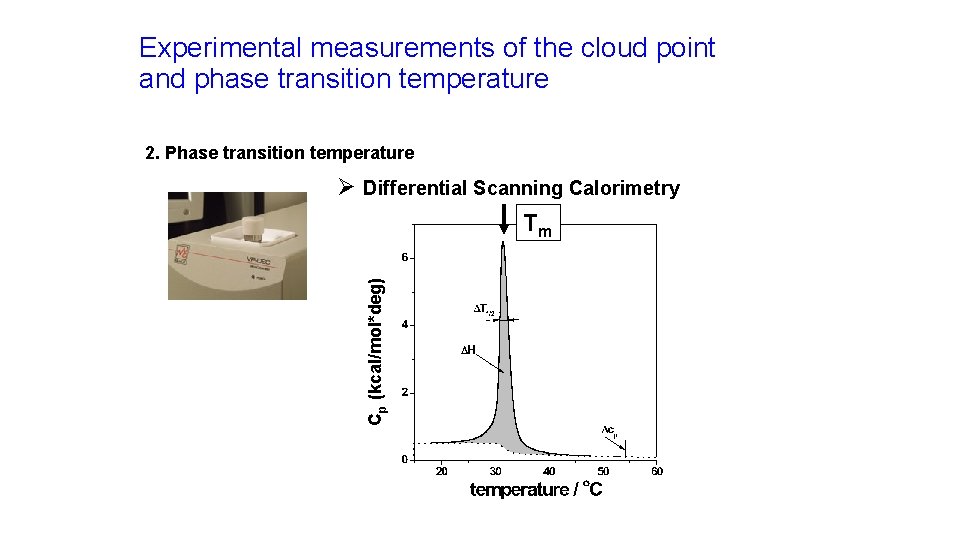
Experimental measurements of the cloud point and phase transition temperature 2. Phase transition temperature Cp (kcal/mol*deg) Ø Differential Scanning Calorimetry Tm

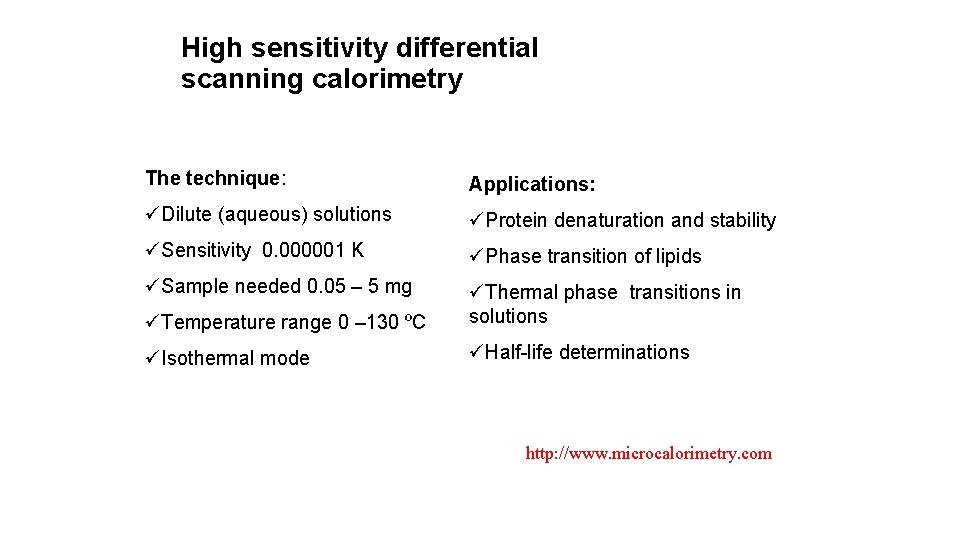
High sensitivity differential scanning calorimetry The technique: Applications: üDilute (aqueous) solutions üProtein denaturation and stability üSensitivity 0. 000001 K üPhase transition of lipids üSample needed 0. 05 – 5 mg üTemperature range 0 – 130 ºC üThermal phase transitions in solutions üIsothermal mode üHalf-life determinations http: //www. microcalorimetry. com

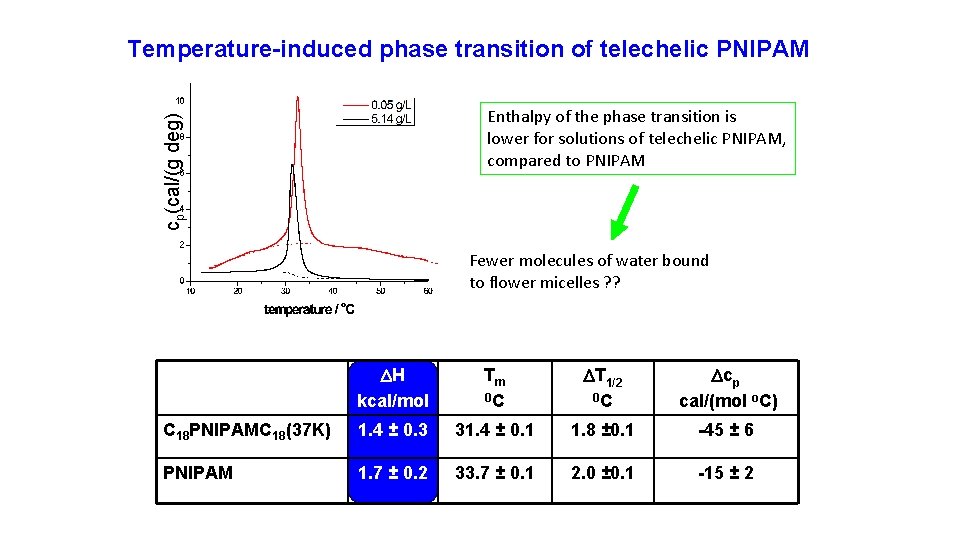
Temperature-induced phase transition of telechelic PNIPAM cp(cal/(g deg) Enthalpy of the phase transition is lower for solutions of telechelic PNIPAM, compared to PNIPAM Fewer molecules of water bound to flower micelles ? ? H kcal/mol Tm 0 C T 1/2 0 C cp cal/(mol o. C) C 18 PNIPAMC 18(37 K) 1. 4 ± 0. 3 31. 4 ± 0. 1 1. 8 ± 0. 1 -45 ± 6 PNIPAM 1. 7 ± 0. 2 33. 7 ± 0. 1 2. 0 ± 0. 1 -15 ± 2
 What is itc 01 02 03 and 04
What is itc 01 02 03 and 04 Isothermal calorimetry principle
Isothermal calorimetry principle Back titrations
Back titrations Precipitation titration calculations
Precipitation titration calculations Titration curve of permanganometric
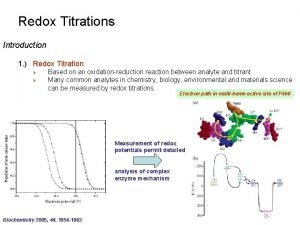
Titration curve of permanganometric Redox titration definition
Redox titration definition Thermodynamic property relations
Thermodynamic property relations Isothermal expansion of ideal gas
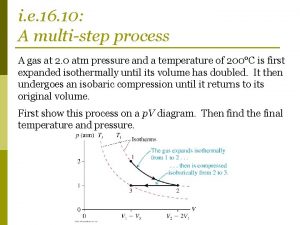
Isothermal expansion of ideal gas Non isothermal reactor design problems
Non isothermal reactor design problems Isobaric isothermal ensemble
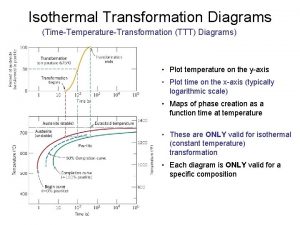
Isobaric isothermal ensemble What is ttt diagram
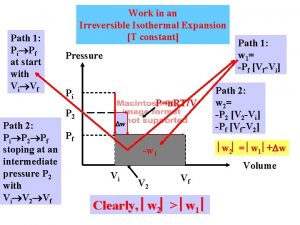
What is ttt diagram Isothermal work
Isothermal work Example of adiabatic process
Example of adiabatic process Isothermal reactor design
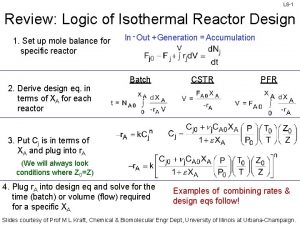
Isothermal reactor design L
L Katalisis
Katalisis Thomson
Thomson Isobaric process formula
Isobaric process formula Isothermal efficiency of reciprocating compressor
Isothermal efficiency of reciprocating compressor Isothermal process
Isothermal process Hess law constant heat summation
Hess law constant heat summation