Chapter 4 Isothermal Reactor Design Overview Chapter 1



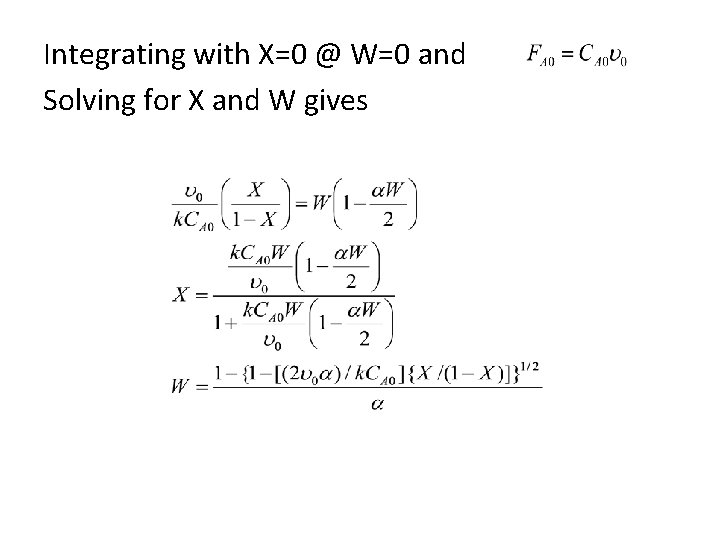
- Slides: 16

Chapter 4 Isothermal Reactor Design

Overview • Chapter 1 and 2 focus on mole balances on reactors to predict the volume • Chapter 3 focuses on reactions • Cahpter 4 combine previous chapters to obtain optimum reactor design

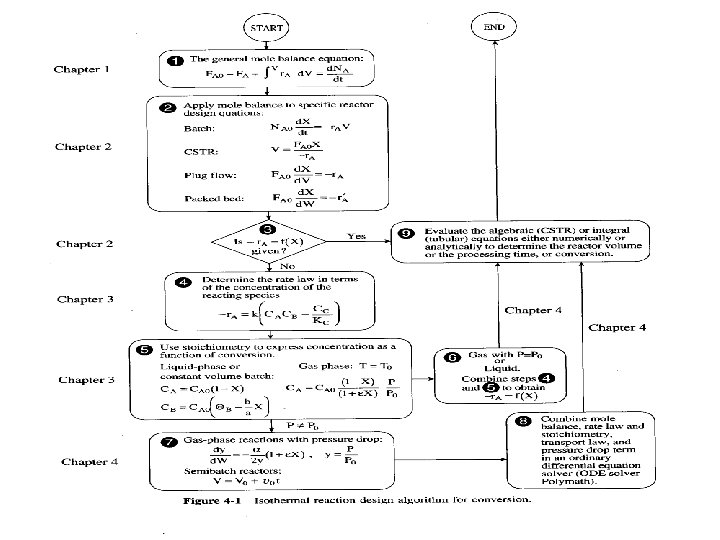
Design Algorithm 1. Mole balance (reactor type) 2. Reaction rate law (reaction type, orders) 3. Stoichiometry (reaction coefficients) 4. Combine steps 1, 2 and 3 5. Evaluate (integrate) either Analytically Graphically Numerically Polymath


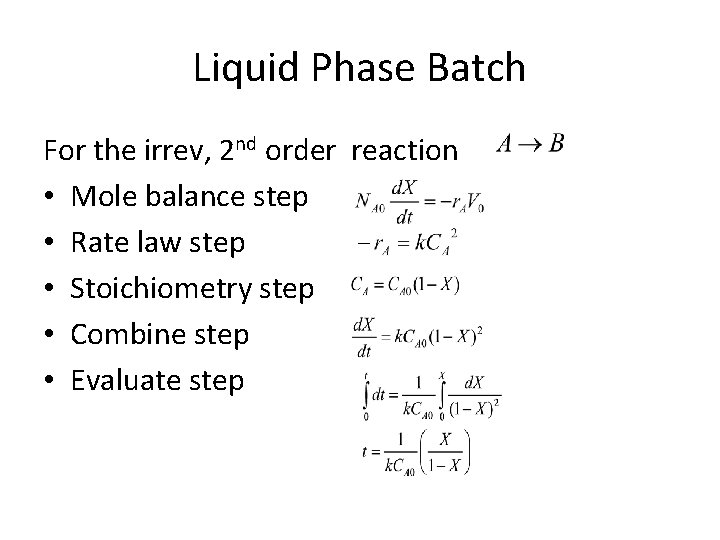
Liquid Phase Batch For the irrev, 2 nd order reaction • Mole balance step • Rate law step • Stoichiometry step • Combine step • Evaluate step

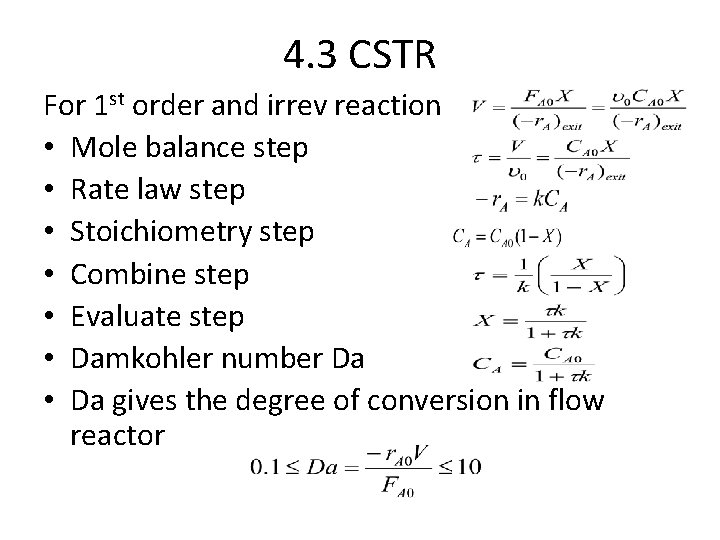
4. 3 CSTR For 1 st order and irrev reaction • Mole balance step • Rate law step • Stoichiometry step • Combine step • Evaluate step • Damkohler number Da • Da gives the degree of conversion in flow reactor

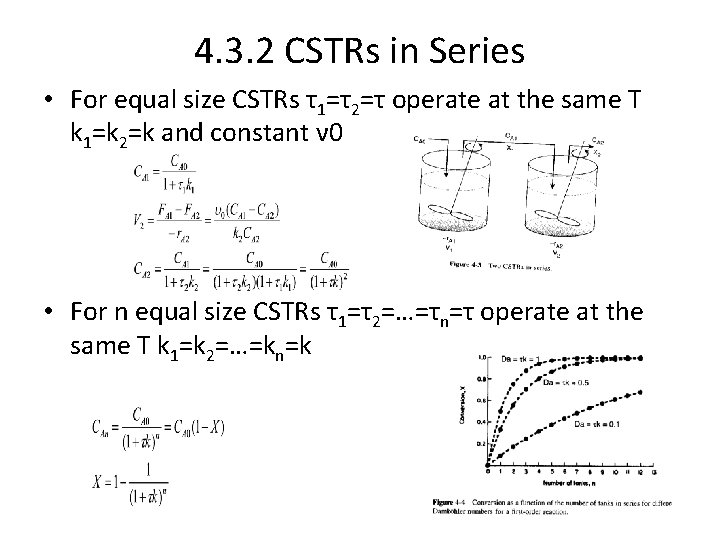
4. 3. 2 CSTRs in Series • For equal size CSTRs τ1=τ2=τ operate at the same T k 1=k 2=k and constant ν 0 • For n equal size CSTRs τ1=τ2=…=τn=τ operate at the same T k 1=k 2=…=kn=k

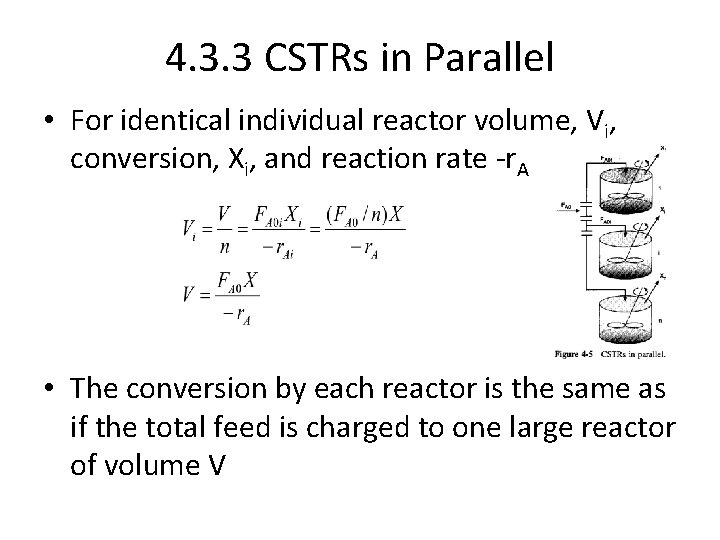
4. 3. 3 CSTRs in Parallel • For identical individual reactor volume, Vi, conversion, Xi, and reaction rate -r. Ai • The conversion by each reactor is the same as if the total feed is charged to one large reactor of volume V

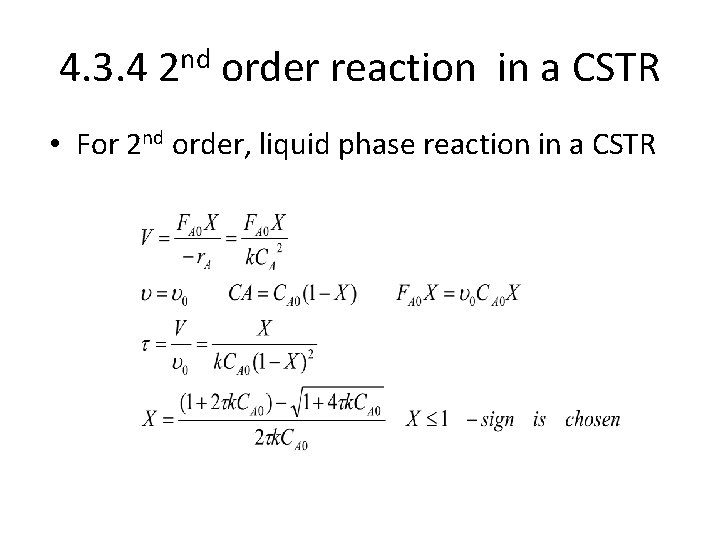
4. 3. 4 2 nd order reaction in a CSTR • For 2 nd order, liquid phase reaction in a CSTR

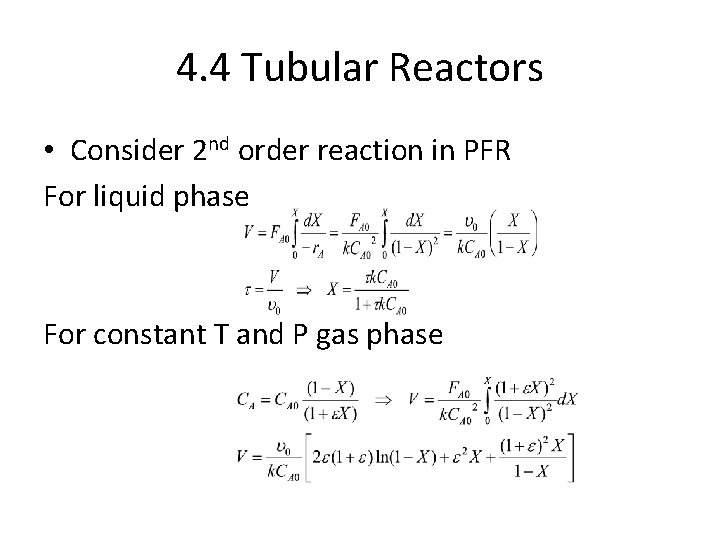
4. 4 Tubular Reactors • Consider 2 nd order reaction in PFR For liquid phase For constant T and P gas phase

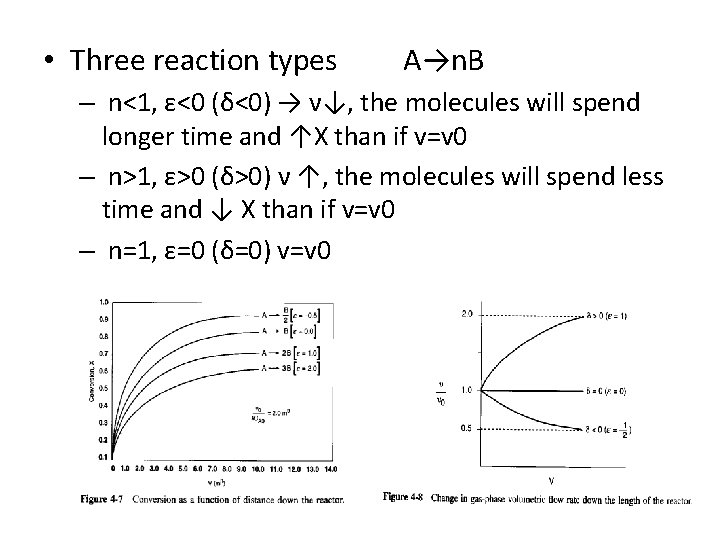
• Three reaction types A→n. B – n<1, ε<0 (δ<0) → ν↓, the molecules will spend longer time and ↑X than if v=v 0 – n>1, ε>0 (δ>0) ν ↑, the molecules will spend less time and ↓ X than if v=v 0 – n=1, ε=0 (δ=0) v=v 0

4. 5 Pressure Drop in Reactors • For liquid phase reactions the pressure drop can be ignored because the effect of pressure on the concs is small. • For gas phase reactions the conc. of the reacting species is directly proportional to the total pressure • Accounting for the pressure drop is a key factor in the proper reactor operation

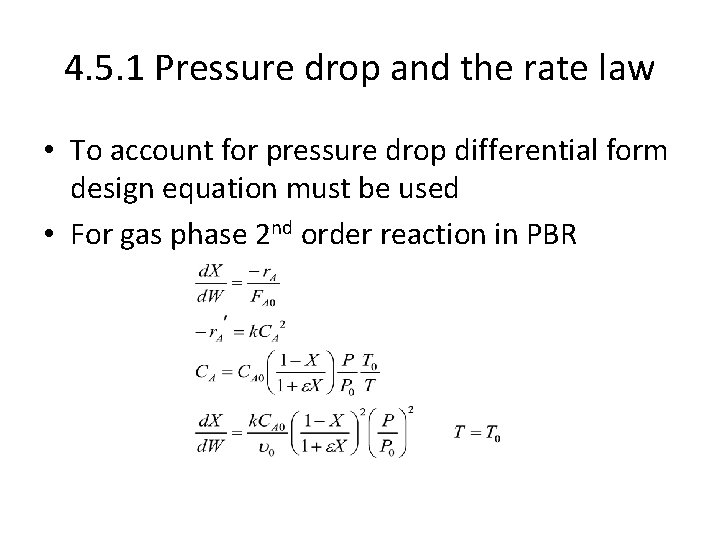
4. 5. 1 Pressure drop and the rate law • To account for pressure drop differential form design equation must be used • For gas phase 2 nd order reaction in PBR

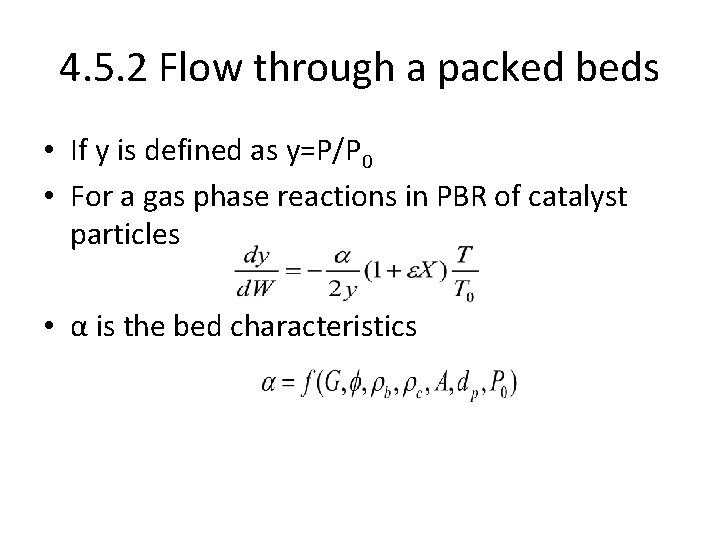
4. 5. 2 Flow through a packed beds • If y is defined as y=P/P 0 • For a gas phase reactions in PBR of catalyst particles • α is the bed characteristics

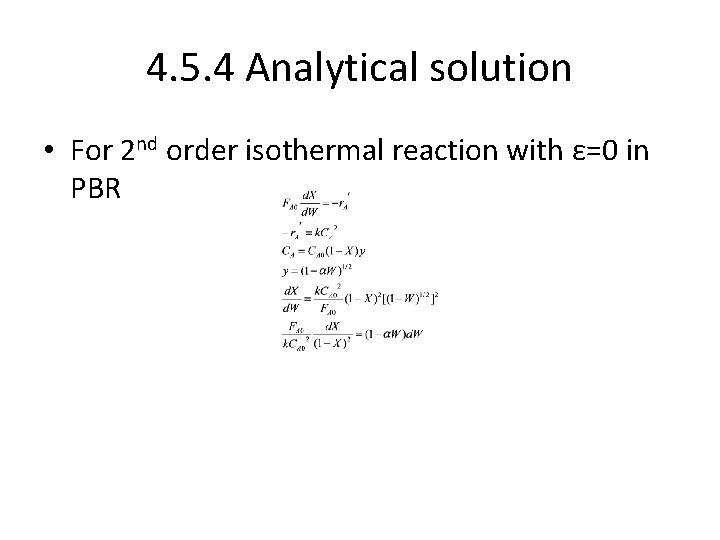
4. 5. 4 Analytical solution • For 2 nd order isothermal reaction with ε=0 in PBR
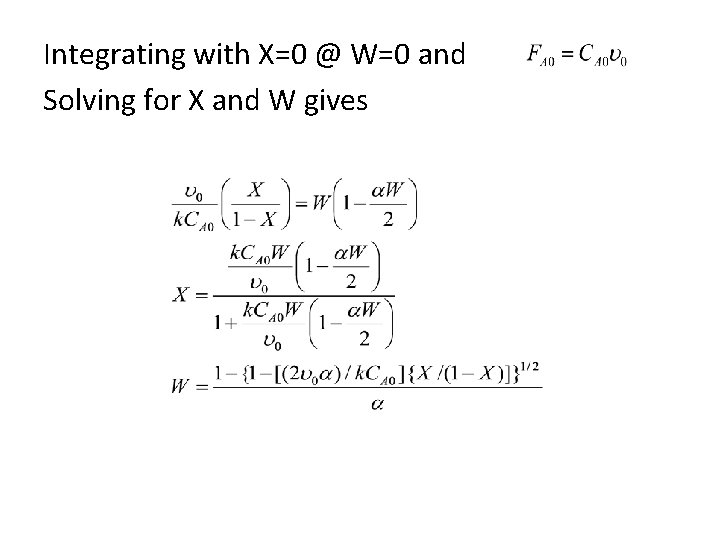
Integrating with X=0 @ W=0 and Solving for X and W gives