ERT 316 REACTION ENGINEERING CHAPTER 2 CONVERSION REACTOR


![2. BATCH REACTOR DESIGN EQUATION Moles of A reacted [Moles of A reacted/consumed] = 2. BATCH REACTOR DESIGN EQUATION Moles of A reacted [Moles of A reacted/consumed] =](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-5.jpg)
![2. BATCH REACTOR DESIGN EQUATION Moles of A reacted NA NA 0 X [1] 2. BATCH REACTOR DESIGN EQUATION Moles of A reacted NA NA 0 X [1]](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-6.jpg)
![2. BATCH REACTOR DESIGN EQUATION Design Equation (in terms of conversion, X ): [3] 2. BATCH REACTOR DESIGN EQUATION Design Equation (in terms of conversion, X ): [3]](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-7.jpg)
![3. 1 CSTR Recall Design Equation for CSTR (Chapter 1); [1] Substituting into [1] 3. 1 CSTR Recall Design Equation for CSTR (Chapter 1); [1] Substituting into [1]](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-11.jpg)
![3. 2 PFR Recall Mole Balance for PFR (Chapter 1); [1] We know that 3. 2 PFR Recall Mole Balance for PFR (Chapter 1); [1] We know that](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-12.jpg)
![3. 2 PFR [4] Integrating [4] with limit V=0 when X=0; 13 3. 2 PFR [4] Integrating [4] with limit V=0 when X=0; 13](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-13.jpg)
 0. 0 5. 1 CSTR IN SERIES EXAMPLE 3 X [FA 0/-r. A](m 3) 0. 0](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-38.jpg)
 0. 0 0. 89 0. 1 1. EXAMPLE 3 X [FA 0/-r. A](m 3) 0. 0 0. 89 0. 1 1.](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-39.jpg)
 0. 0 5. 2 PFR IN SERIES EXAMPLE 4 X [FA 0/-r. A](m 3) 0. 0](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-42.jpg)
 0. 0 0. 89 0. 1 1. 08 0. X [FA 0/-r. A](m 3) 0. 0 0. 89 0. 1 1. 08 0.](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-43.jpg)
 0. 0 0. 89 0. 1 1. 08 0. X [FA 0/-r. A](m 3) 0. 0 0. 89 0. 1 1. 08 0.](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-44.jpg)
- Slides: 54

ERT 316: REACTION ENGINEERING CHAPTER 2 CONVERSION & REACTOR SIZING 1 Lecturer: Miss Anis Atikah Ahmad Email: anisatikah@unimap. edu. my Tel: +604 976 4160

OUTLINE Conversion Batch Reactor Design Equation Flow Reactors Design Equations CSTR PFR PBR Sizing Flow Reactors in Series Space Time Space Velocity 2

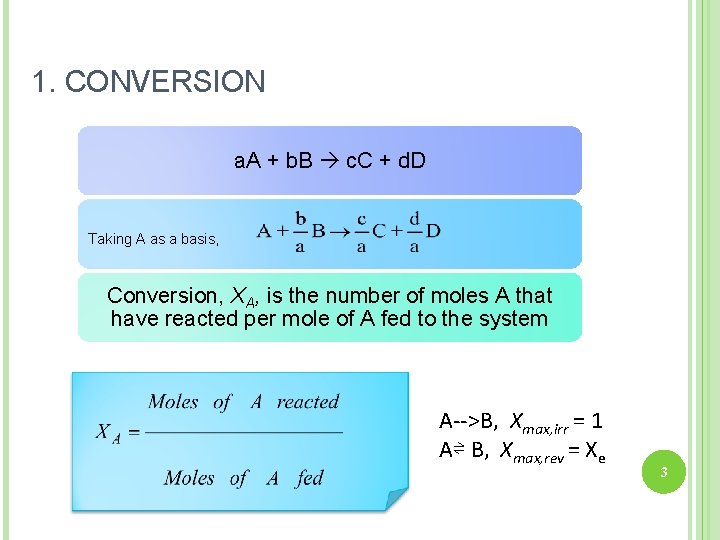
1. CONVERSION a. A + b. B c. C + d. D Taking A as a basis, Conversion, XA, is the number of moles A that have reacted per mole of A fed to the system A-->B, Xmax, irr = 1 A⇌ B, Xmax, rev = Xe 3

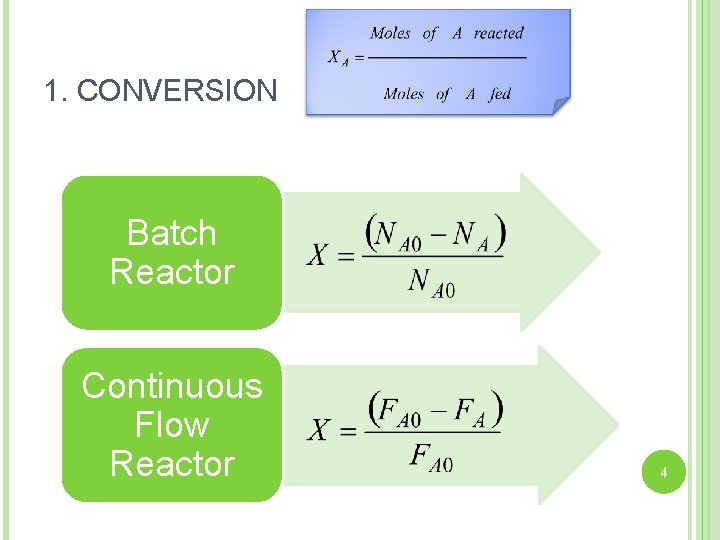
1. CONVERSION Batch Reactor Continuous Flow Reactor 4
![2 BATCH REACTOR DESIGN EQUATION Moles of A reacted Moles of A reactedconsumed 2. BATCH REACTOR DESIGN EQUATION Moles of A reacted [Moles of A reacted/consumed] =](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-5.jpg)
2. BATCH REACTOR DESIGN EQUATION Moles of A reacted [Moles of A reacted/consumed] = [Moles of A fed] · Moles of A reacted Moles of A fed · [Moles of A reacted/consumed] = [NA 0] [X] Moles of A that have Moles of A initially Moles of A in been consumed by fed to reactor at time t chemical reaction t = 0 [NA ] [NA 0 ] NA N A 0 [NA 0 X] (1 X)
![2 BATCH REACTOR DESIGN EQUATION Moles of A reacted NA NA 0 X 1 2. BATCH REACTOR DESIGN EQUATION Moles of A reacted NA NA 0 X [1]](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-6.jpg)
2. BATCH REACTOR DESIGN EQUATION Moles of A reacted NA NA 0 X [1] Differentiating wrt time; [2] Recall mole balance for batch reactor (Chapter 1); Rearranging and substituting into ; [2] [Design Equation in terms of conversion]
![2 BATCH REACTOR DESIGN EQUATION Design Equation in terms of conversion X 3 2. BATCH REACTOR DESIGN EQUATION Design Equation (in terms of conversion, X ): [3]](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-7.jpg)
2. BATCH REACTOR DESIGN EQUATION Design Equation (in terms of conversion, X ): [3] What is the time required to achieve a specific conversion? Integrating [3] with limits (t=0, X=0; t=t, X=X )

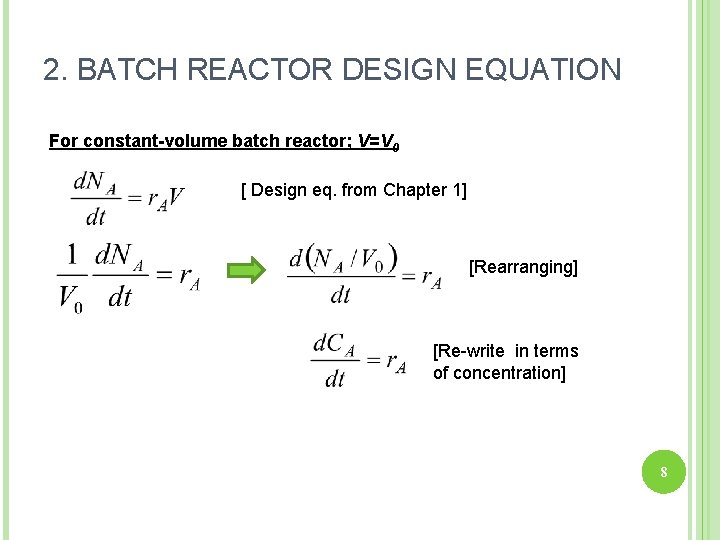
2. BATCH REACTOR DESIGN EQUATION For constant-volume batch reactor; V=V 0 [ Design eq. from Chapter 1] [Rearranging] [Re-write in terms of concentration] 8

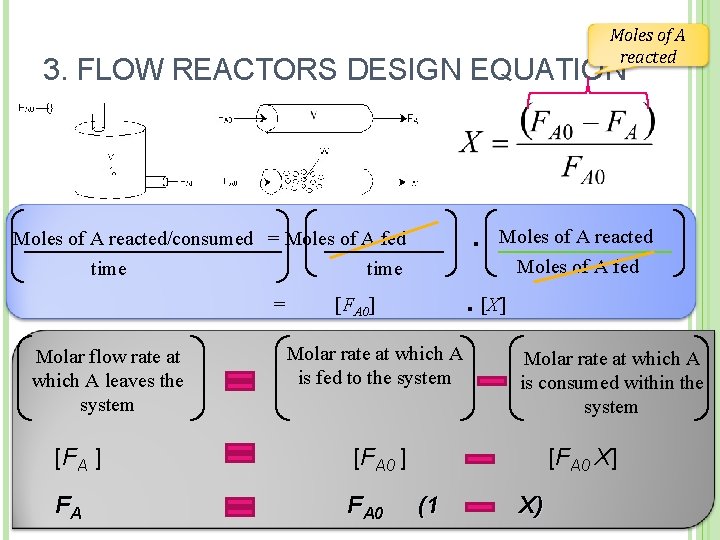
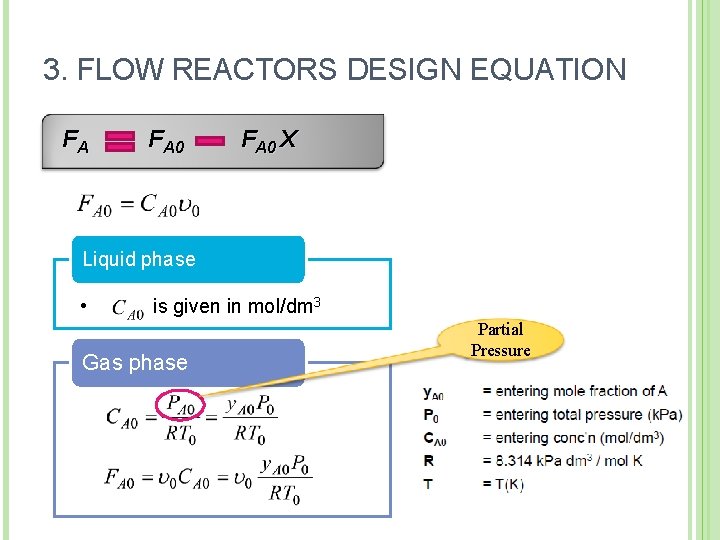
Moles of A reacted 3. FLOW REACTORS DESIGN EQUATION · Moles of A reacted/consumed = Moles of A fed time Moles of A reacted Moles of A fed · = [FA 0] [X] Molar rate at which A Molar flow rate at Molar rate at which A is fed to the system which A leaves the is consumed within the system [FA ] [FA 0 ] FA F A 0 [FA 0 X] (1 X)

3. FLOW REACTORS DESIGN EQUATION FA FA 0 X Liquid phase • is given in mol/dm 3 Gas phase Partial Pressure
![3 1 CSTR Recall Design Equation for CSTR Chapter 1 1 Substituting into 1 3. 1 CSTR Recall Design Equation for CSTR (Chapter 1); [1] Substituting into [1]](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-11.jpg)
3. 1 CSTR Recall Design Equation for CSTR (Chapter 1); [1] Substituting into [1] F F F X A Rearranging; A 0 11
![3 2 PFR Recall Mole Balance for PFR Chapter 1 1 We know that 3. 2 PFR Recall Mole Balance for PFR (Chapter 1); [1] We know that](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-12.jpg)
3. 2 PFR Recall Mole Balance for PFR (Chapter 1); [1] We know that [2] F F F X A A 0 Differentiating [2] wrt X [3] Substituting [3] into [1] 12 [4]
![3 2 PFR 4 Integrating 4 with limit V0 when X0 13 3. 2 PFR [4] Integrating [4] with limit V=0 when X=0; 13](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-13.jpg)
3. 2 PFR [4] Integrating [4] with limit V=0 when X=0; 13

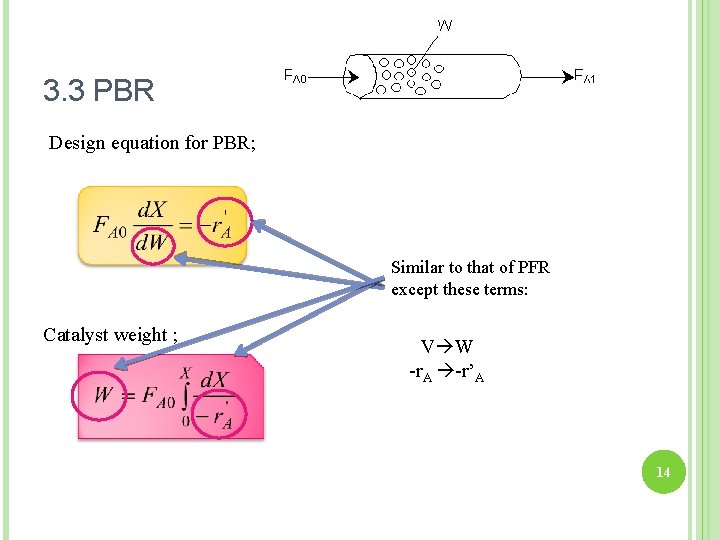
3. 3 PBR Design equation for PBR; Similar to that of PFR except these terms: Catalyst weight ; V W -r. A -r’A 14

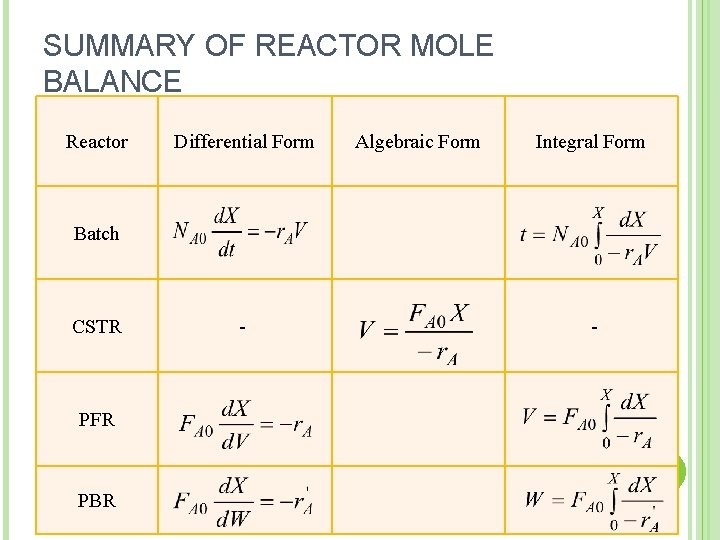
SUMMARY OF REACTOR MOLE BALANCE Reactor Differential Form Batch CSTR Algebraic Form Integral Form - - PFR PBR

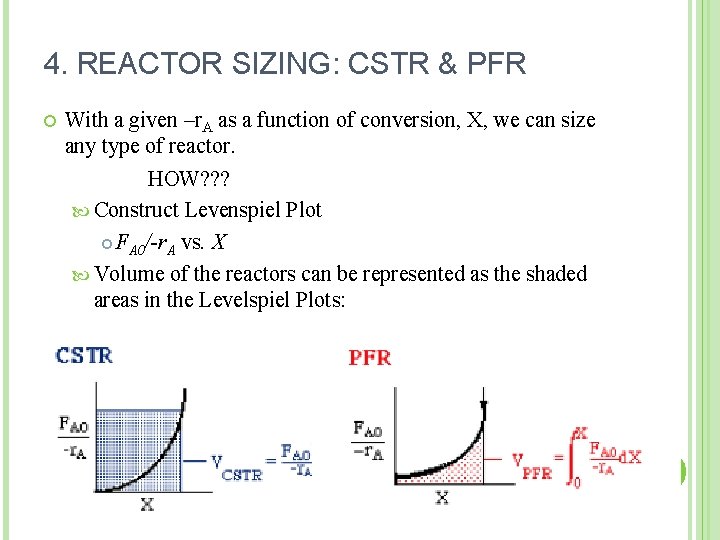
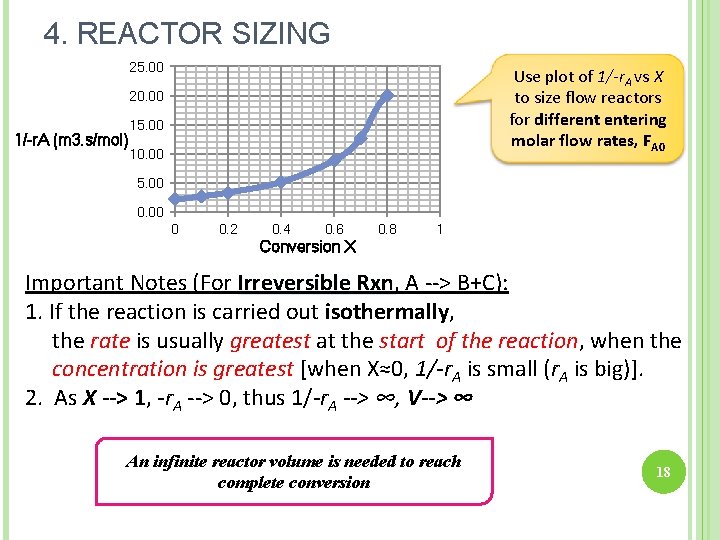
4. REACTOR SIZING: CSTR & PFR With a given –r. A as a function of conversion, X, we can size any type of reactor. HOW? ? ? Construct Levenspiel Plot FA 0/-r. A vs. X Volume of the reactors can be represented as the shaded areas in the Levelspiel Plots: 16

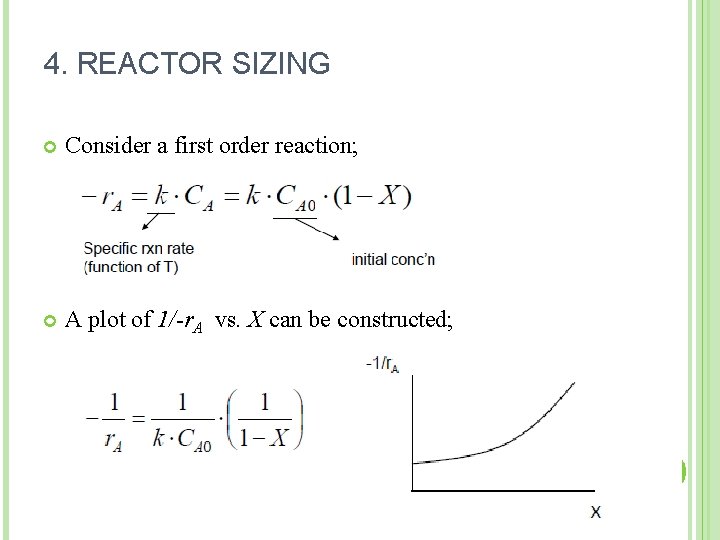
4. REACTOR SIZING Consider a first order reaction; A plot of 1/-r. A vs. X can be constructed; 17

1/-r. A (m 3. s/mol) Conversion X 18

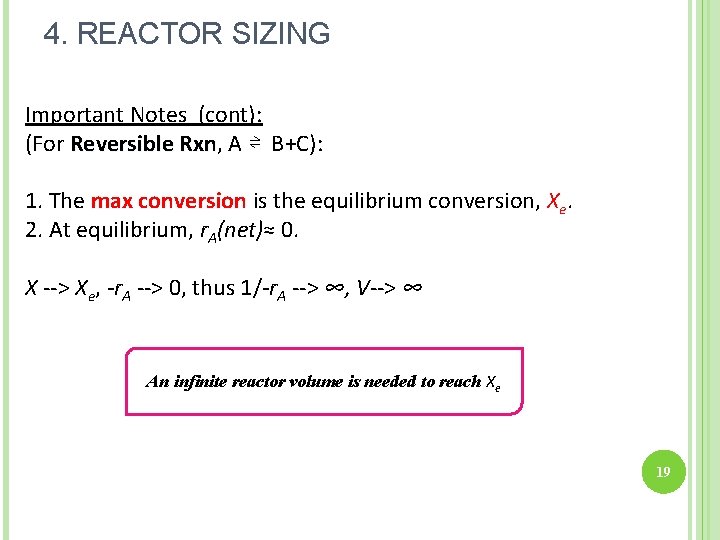
4. REACTOR SIZING Important Notes (cont): (For Reversible Rxn, Rxn A ⇌ B+C): 1. The max conversion is the equilibrium conversion, Xe. 2. At equilibrium, r. A(net)≈ 0. X --> Xe, -r. A --> 0, thus 1/-r. A --> ∞, V--> ∞ An infinite reactor volume is needed to reach Xe 19

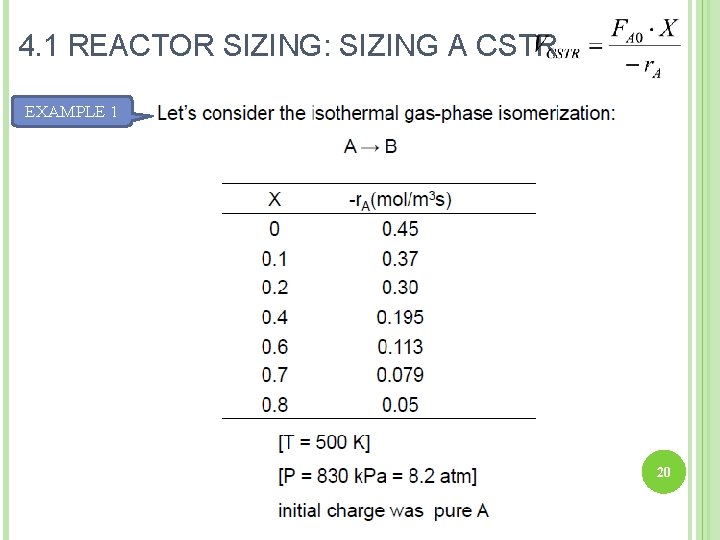
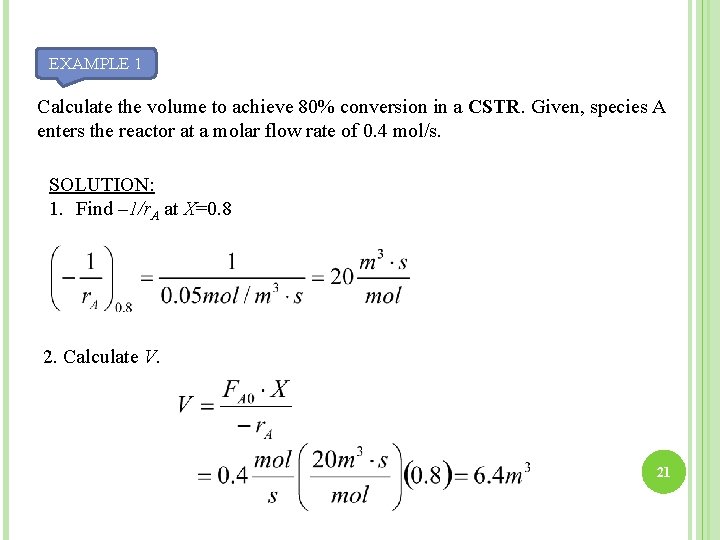
4. 1 REACTOR SIZING: SIZING A CSTR EXAMPLE 1 20

EXAMPLE 1 Calculate the volume to achieve 80% conversion in a CSTR. Given, species A enters the reactor at a molar flow rate of 0. 4 mol/s. SOLUTION: 1. Find – 1/r. A at X=0. 8 2. Calculate V. 21

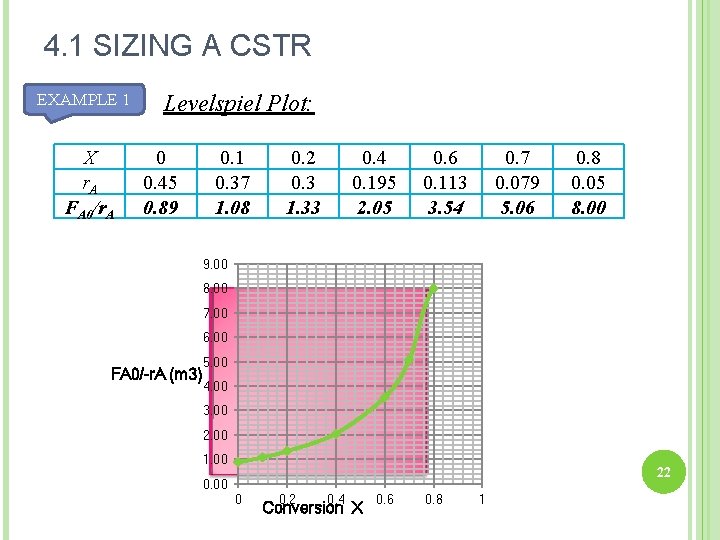
4. 1 SIZING A CSTR EXAMPLE 1 X r. A FA 0/r. A Levelspiel Plot: 0 0. 45 0. 89 0. 1 0. 37 1. 08 0. 2 0. 3 1. 33 0. 4 0. 195 2. 05 0. 6 0. 113 3. 54 0. 7 0. 079 5. 06 0. 8 0. 05 8. 00 FA 0/-r. A (m 3) 22 Conversion X

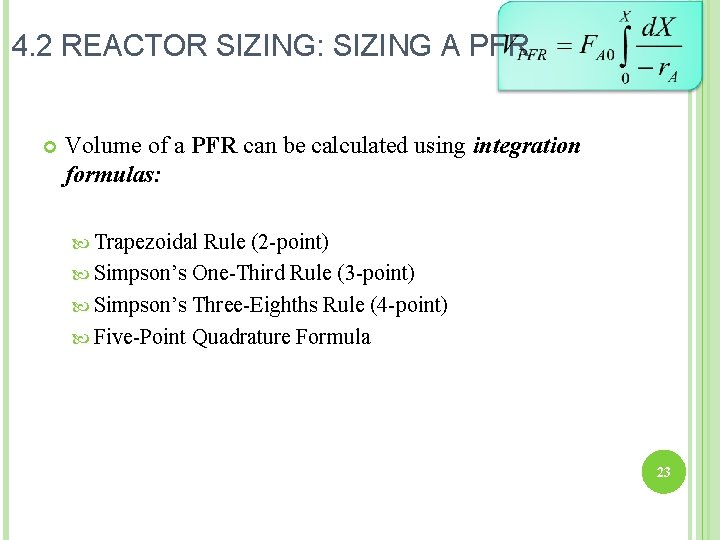
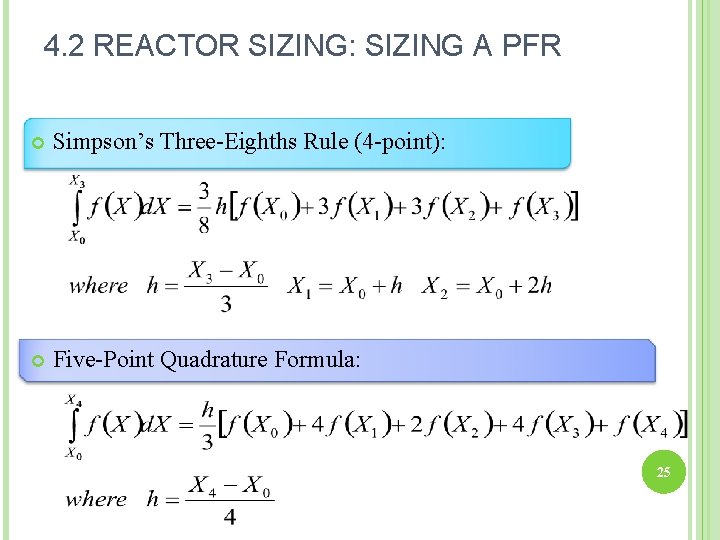
4. 2 REACTOR SIZING: SIZING A PFR Volume of a PFR can be calculated using integration formulas: Trapezoidal Rule (2 -point) Simpson’s One-Third Rule (3 -point) Simpson’s Three-Eighths Rule (4 -point) Five-Point Quadrature Formula 23

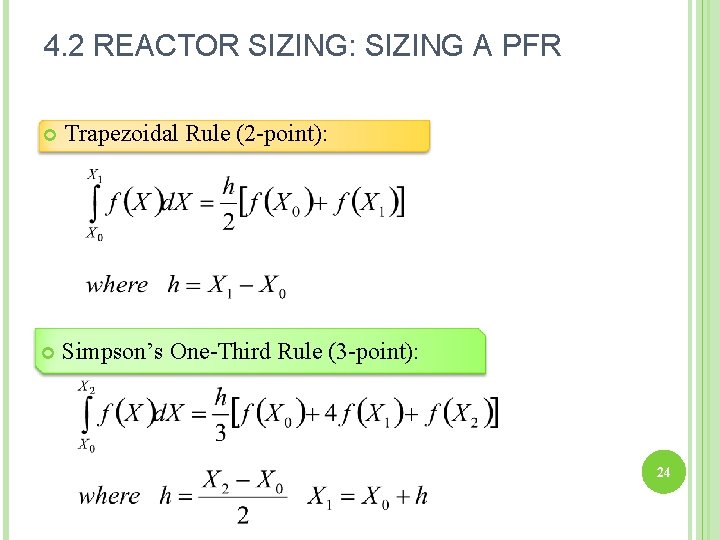
4. 2 REACTOR SIZING: SIZING A PFR Trapezoidal Rule (2 -point): Simpson’s One-Third Rule (3 -point): 24

4. 2 REACTOR SIZING: SIZING A PFR Simpson’s Three-Eighths Rule (4 -point): Five-Point Quadrature Formula: 25

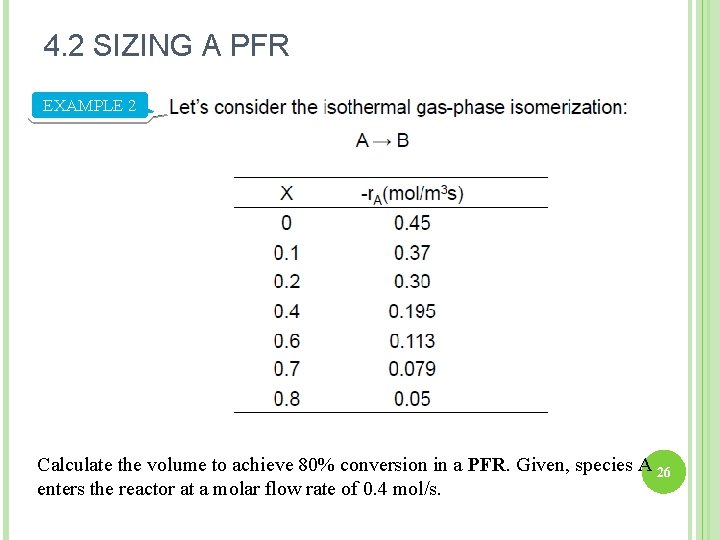
4. 2 SIZING A PFR EXAMPLE 2 Calculate the volume to achieve 80% conversion in a PFR. Given, species A 26 enters the reactor at a molar flow rate of 0. 4 mol/s.

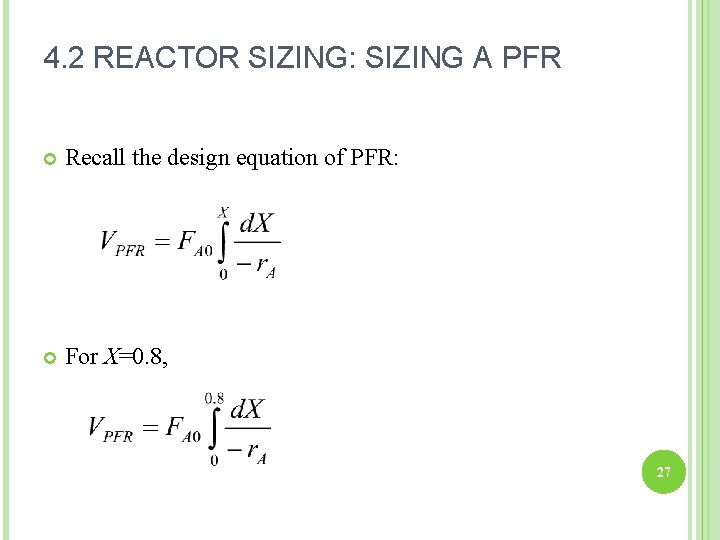
4. 2 REACTOR SIZING: SIZING A PFR Recall the design equation of PFR: For X=0. 8, 27

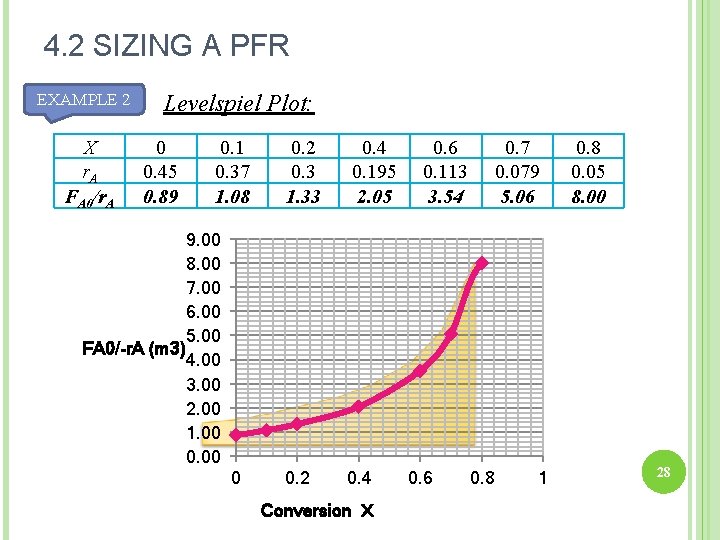
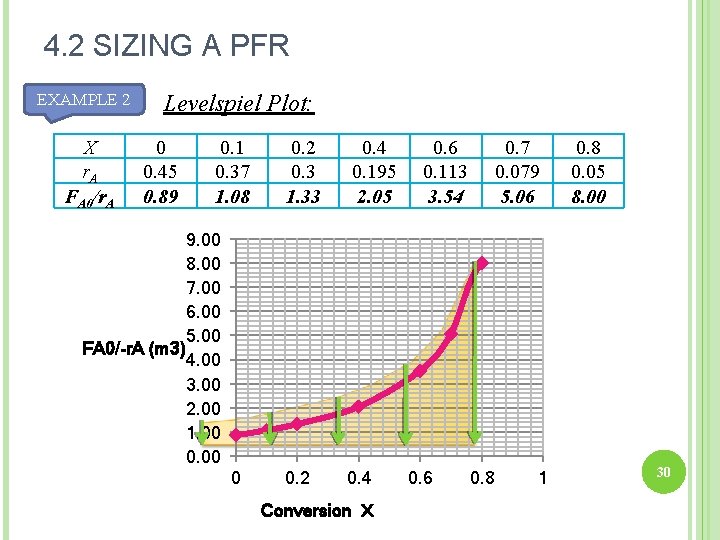
4. 2 SIZING A PFR EXAMPLE 2 X r. A FA 0/r. A Levelspiel Plot: 0 0. 45 0. 89 0. 1 0. 37 1. 08 0. 2 0. 3 1. 33 0. 4 0. 195 2. 05 0. 6 0. 113 3. 54 0. 7 0. 079 5. 06 9. 00 8. 00 7. 00 6. 00 5. 00 FA 0/-r. A (m 3) 4. 00 3. 00 2. 00 1. 00 0 0. 2 0. 4 Conversion X 0. 6 0. 8 1 0. 8 0. 05 8. 00 28

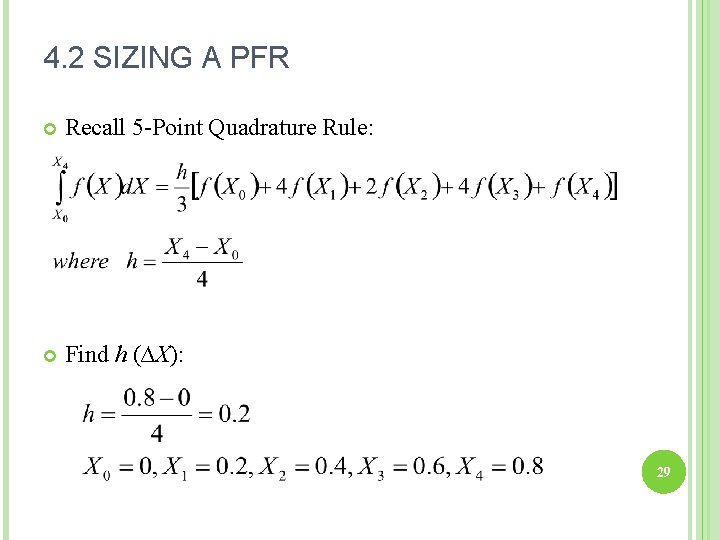
4. 2 SIZING A PFR Recall 5 -Point Quadrature Rule: Find h (∆X): 29

4. 2 SIZING A PFR EXAMPLE 2 X r. A FA 0/r. A Levelspiel Plot: 0 0. 45 0. 89 0. 1 0. 37 1. 08 0. 2 0. 3 1. 33 0. 4 0. 195 2. 05 0. 6 0. 113 3. 54 0. 7 0. 079 5. 06 9. 00 8. 00 7. 00 6. 00 5. 00 FA 0/-r. A (m 3) 4. 00 3. 00 2. 00 1. 00 0 0. 2 0. 4 Conversion X 0. 6 0. 8 1 0. 8 0. 05 8. 00 30

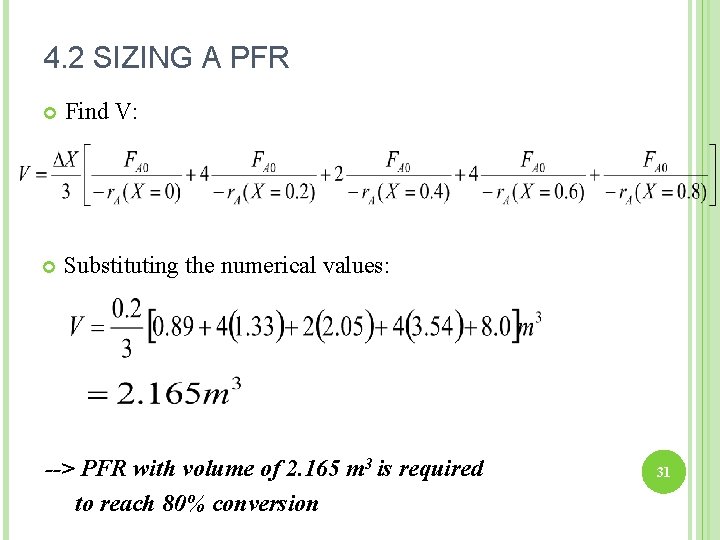
4. 2 SIZING A PFR Find V: Substituting the numerical values: --> PFR with volume of 2. 165 m 3 is required to reach 80% conversion 31

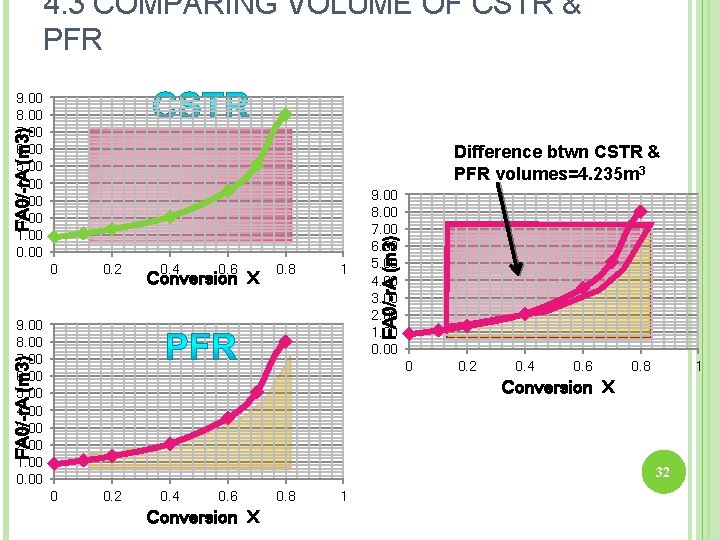
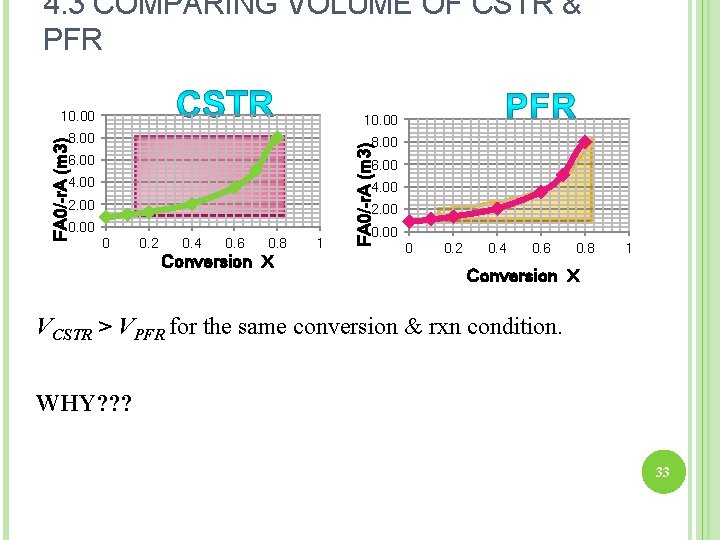
4. 3 COMPARING VOLUME OF CSTR & PFR FA 0/-r. A (m 3) 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0. 00 Difference btwn CSTR & PFR volumes=4. 235 m 3 0. 2 0. 4 0. 6 Conversion X 0. 8 1 FA 0/-r. A (m 3) 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0. 00 FA 0/-r. A (m 3) 0 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0 0. 2 0. 4 0. 6 0. 8 1 Conversion X 32 0 0. 2 0. 4 0. 6 Conversion X 0. 8 1

4. 3 COMPARING VOLUME OF CSTR & PFR 8. 00 6. 00 FA 0/-r. A (m 3) 10. 00 6. 00 4. 00 2. 00 0 0. 2 0. 4 0. 6 0. 8 Conversion X 1 0. 00 0 0. 2 0. 4 0. 6 0. 8 1 Conversion X VCSTR > VPFR for the same conversion & rxn condition. WHY? ? ? 33

5. REACTORS IN SERIES The exit stream of one reactor is fed to the next one 34

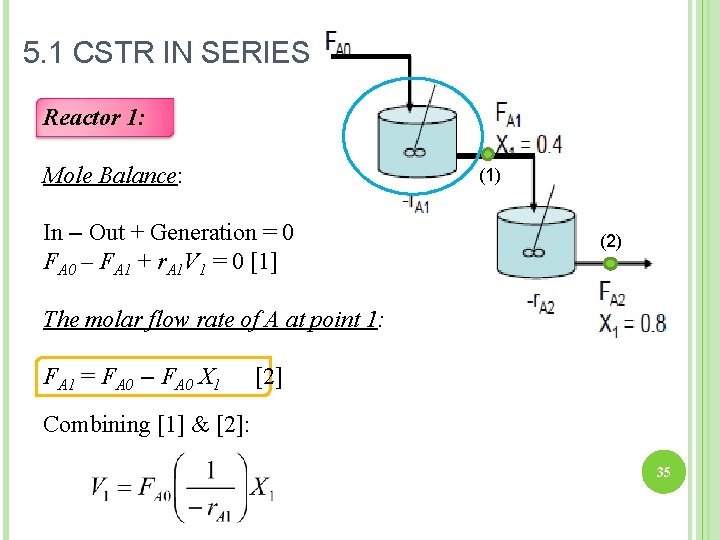
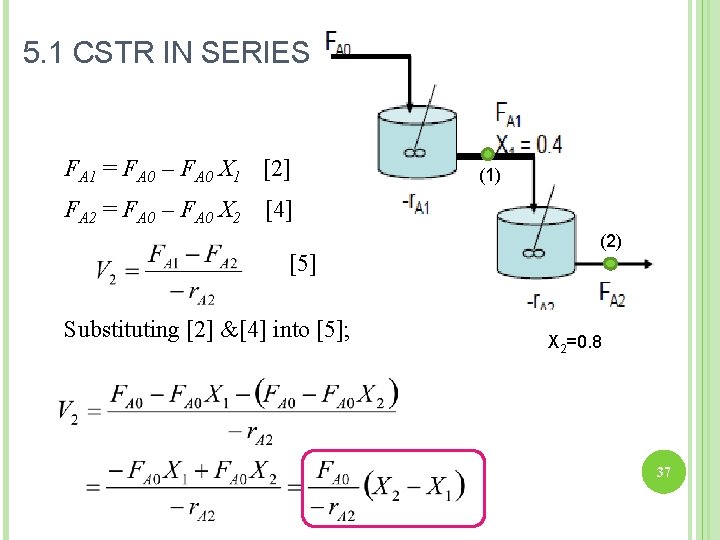
5. 1 CSTR IN SERIES Reactor 1: Mole Balance: (1) In – Out + Generation = 0 FA 0 – FA 1 + r. A 1 V 1 = 0 [1] (2) The molar flow rate of A at point 1: FA 1 = FA 0 – FA 0 X 1 [2] Combining [1] & [2]: 35

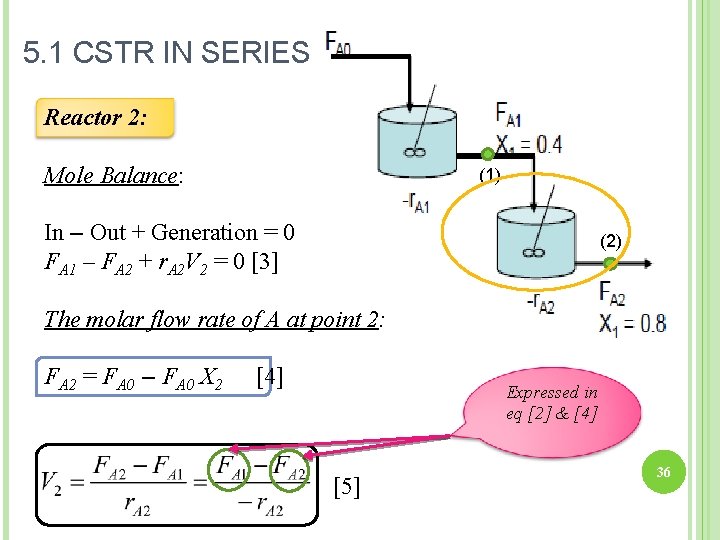
5. 1 CSTR IN SERIES Reactor 2: Mole Balance: (1) In – Out + Generation = 0 FA 1 – FA 2 + r. A 2 V 2 = 0 [3] (2) The molar flow rate of A at point 2: FA 2 = FA 0 – FA 0 X 2 [4] Expressed in eq [2] & [4] [5] 36

5. 1 CSTR IN SERIES FA 1 = FA 0 – FA 0 X 1 [2] (1) FA 2 = FA 0 – FA 0 X 2 [4] [5] Substituting [2] &[4] into [5]; (2) X 2=0. 8 37
 0. 0](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-38.jpg)
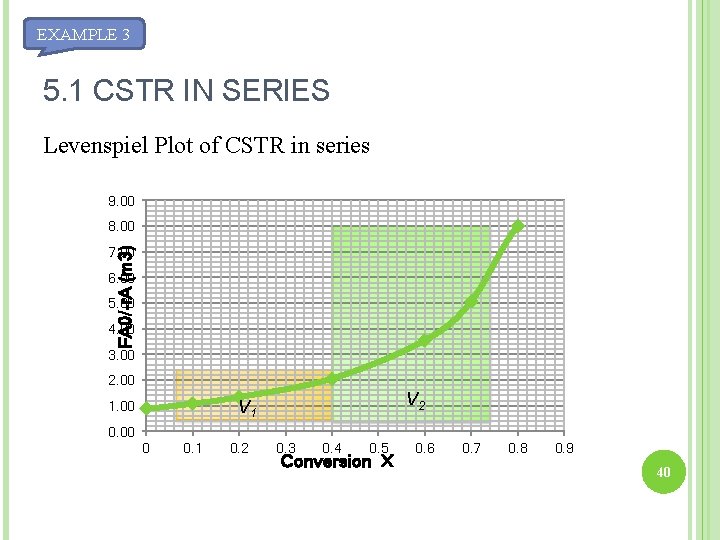
5. 1 CSTR IN SERIES EXAMPLE 3 X [FA 0/-r. A](m 3) 0. 0 0. 89 0. 1 1. 08 0. 2 1. 33 0. 4 2. 05 0. 6 3. 54 0. 7 5. 06 0. 8 8. 0 For the two CSTRs in series, 40% conversion is achieved in the first reactor. What is the volume of each of the two reactors necessary to achieve 80% overall conversion of entering species? 38
 0. 0 0. 89 0. 1 1.](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-39.jpg)
EXAMPLE 3 X [FA 0/-r. A](m 3) 0. 0 0. 89 0. 1 1. 08 0. 2 1. 33 0. 4 2. 05 0. 6 3. 54 0. 7 5. 06 0. 8 8. 0 For reactor 1, X = 0. 4 For reactor 2, X = 0. 8 Total V= (0. 82 + 3. 2)m 3 = 4. 02 m 3 39

EXAMPLE 3 5. 1 CSTR IN SERIES Levenspiel Plot of CSTR in series 9. 00 8. 00 FA 0/-r. A (m 3) 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 V 2 V 1 1. 00 0 0. 1 0. 2 0. 3 0. 4 0. 5 Conversion X 0. 6 0. 7 0. 8 0. 9 40

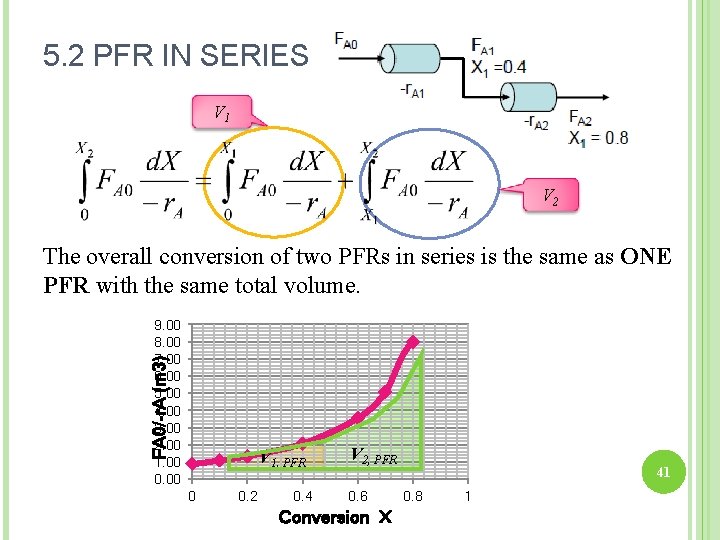
5. 2 PFR IN SERIES V 1 V 2 The overall conversion of two PFRs in series is the same as ONE PFR with the same total volume. FA 0/-r. A (m 3) 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0. 00 V 1, PFR 0 0. 2 0. 4 V 2, PFR 0. 6 Conversion X 41 0. 8 1
 0. 0](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-42.jpg)
5. 2 PFR IN SERIES EXAMPLE 4 X [FA 0/-r. A](m 3) 0. 0 0. 89 0. 1 1. 08 0. 2 1. 33 0. 4 2. 05 0. 6 3. 54 0. 7 5. 06 0. 8 8. 0 Calculate the reactor volume V 1 and V 2 for the plug-flow sequence shown below when the intermediate conversion is 40% & the final conversion is 80%. 42
 0. 0 0. 89 0. 1 1. 08 0.](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-43.jpg)
X [FA 0/-r. A](m 3) 0. 0 0. 89 0. 1 1. 08 0. 2 1. 33 0. 4 2. 05 0. 6 3. 54 0. 7 5. 06 0. 8 8. 0 Using Simpsons One-Third Rule; For reactor 1, ∆X=0. 2, X 0 = 0, X 1 = 0. 2, X 2 = 0. 4 43
 0. 0 0. 89 0. 1 1. 08 0.](https://slidetodoc.com/presentation_image_h/1849b8e335aafbe56c1c7cc636c6ee2c/image-44.jpg)
X [FA 0/-r. A](m 3) 0. 0 0. 89 0. 1 1. 08 0. 2 1. 33 0. 4 2. 05 0. 6 3. 54 0. 7 5. 06 0. 8 8. 0 For reactor 2, ∆X=0. 2, X 0 = 0. 4, X 1 = 0. 6, X 2 = 0. 8 Total volume; 44

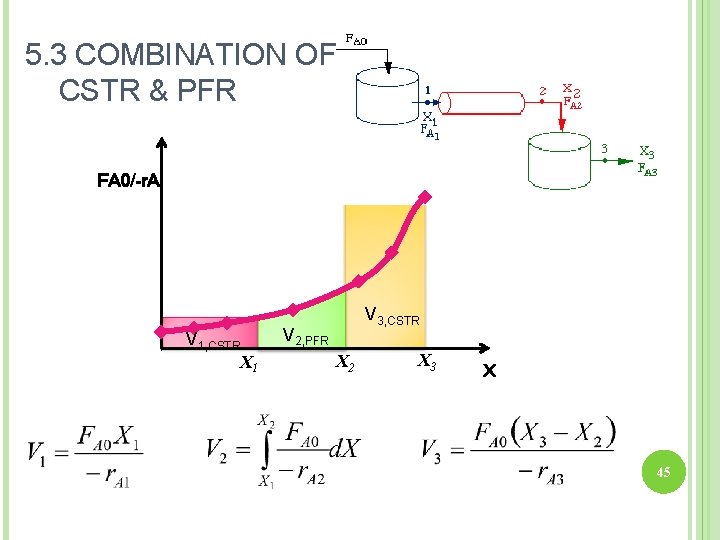
5. 3 COMBINATION OF CSTR & PFR FA 0/-r. A V 1, CSTR X 1 V 3, CSTR V 2, PFR X 2 X 3 X 45

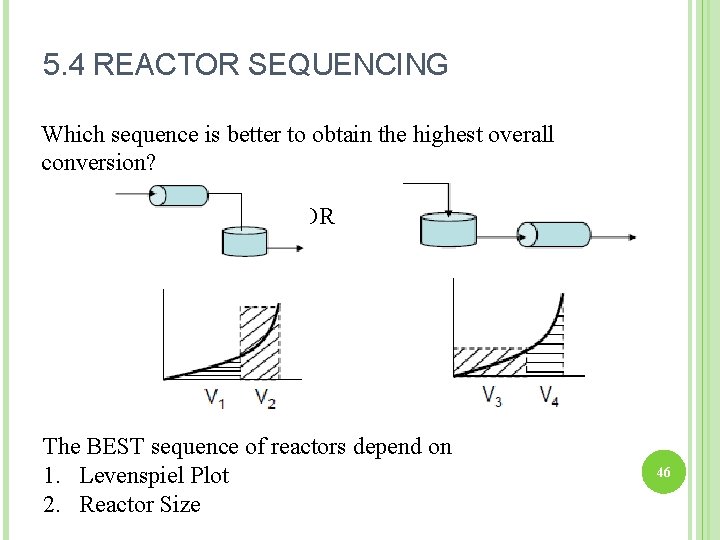
5. 4 REACTOR SEQUENCING Which sequence is better to obtain the highest overall conversion? OR The BEST sequence of reactors depend on 1. Levenspiel Plot 2. Reactor Size 46

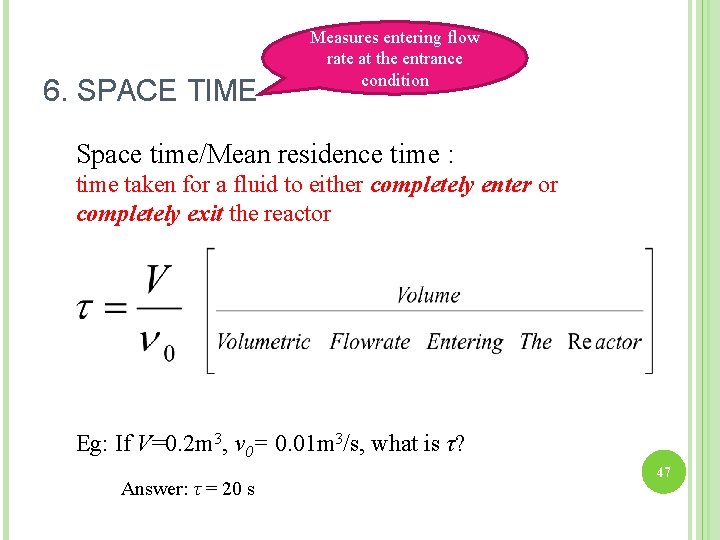
6. SPACE TIME Measures entering flow rate at the entrance condition Space time/Mean residence time : time taken for a fluid to either completely enter or completely exit the reactor Eg: If V=0. 2 m 3, v 0= 0. 01 m 3/s, what is τ? Answer: τ = 20 s 47

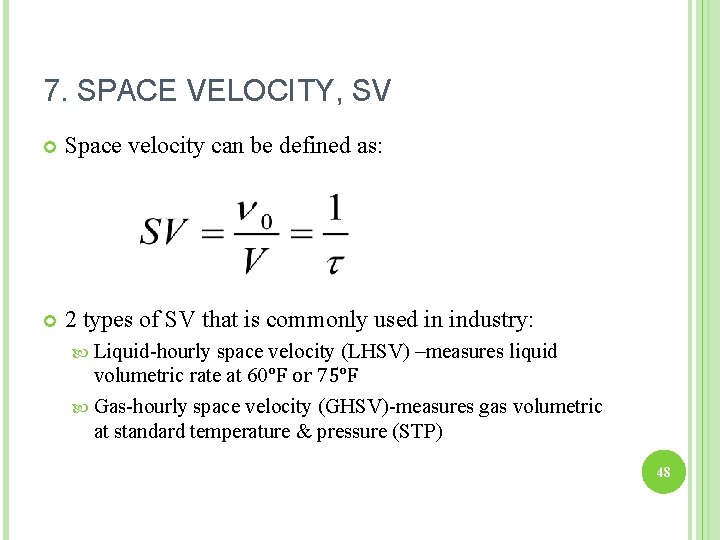
7. SPACE VELOCITY, SV Space velocity can be defined as: 2 types of SV that is commonly used in industry: Liquid-hourly space velocity (LHSV) –measures liquid volumetric rate at 60°F or 75°F Gas-hourly space velocity (GHSV)-measures gas volumetric at standard temperature & pressure (STP) 48

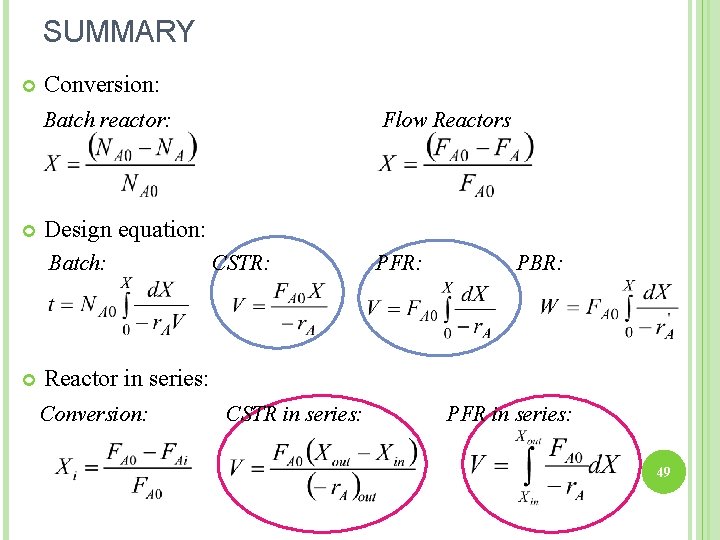
SUMMARY Conversion: Batch reactor: Design equation: Batch: Flow Reactors CSTR: PFR: PBR: Reactor in series: Conversion: CSTR in series: PFR in series: 49

EXERCISE The irreversible gas-phase non-elementary reaction A + 2 B --> C is to be carried out isothermally in a constant pressure batch reactor. The feed is at a temperature of 227°C, a pressure of 1013 k. Pa, and its composition is 30% A and 60% B. Laboratory data taken under identical conditions are as follows : -r. A (mol/dm 3. s) 0. 00001 0. 000005 0. 000002 0. 000001 X 0. 0 0. 15 0. 3 0. 6 (a) What is PFR volume necessary to achieve 30 % conversion for an 50 3 entering flow rate of 2 m /min ?

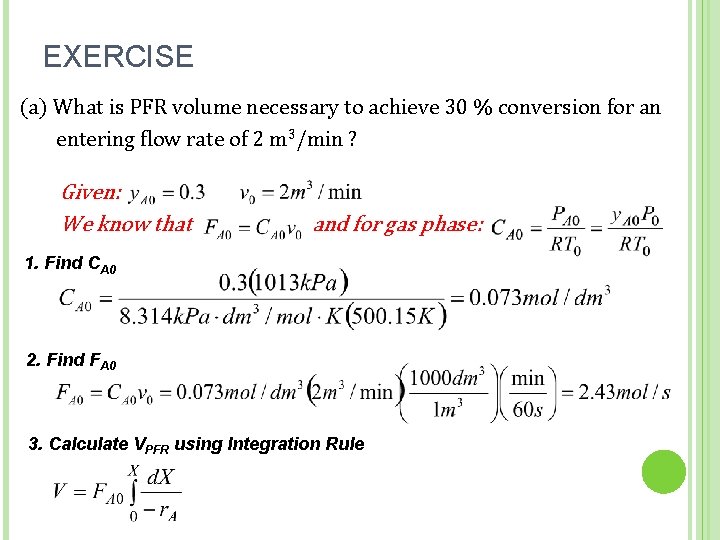
EXERCISE (a) What is PFR volume necessary to achieve 30 % conversion for an entering flow rate of 2 m 3/min ? Given: We know that and for gas phase: 1. Find CA 0 2. Find FA 0 51 3. Calculate VPFR using Integration Rule

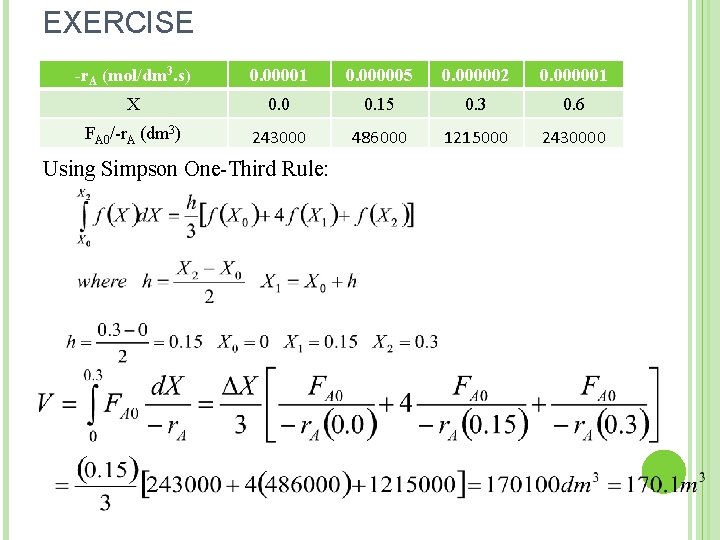
EXERCISE -r. A (mol/dm 3. s) 0. 00001 0. 000005 0. 000002 0. 000001 X 0. 0 0. 15 0. 3 0. 6 FA 0/-r. A (dm 3) 243000 486000 1215000 2430000 Using Simpson One-Third Rule:

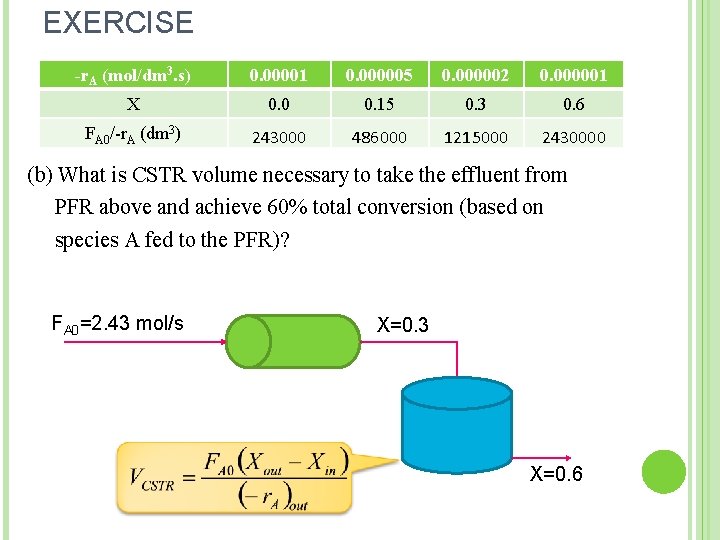
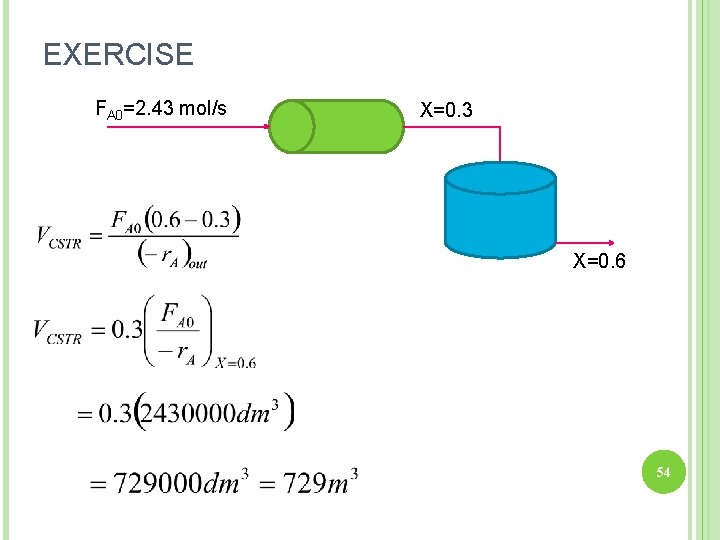
EXERCISE -r. A (mol/dm 3. s) 0. 00001 0. 000005 0. 000002 0. 000001 X 0. 0 0. 15 0. 3 0. 6 FA 0/-r. A (dm 3) 243000 486000 1215000 2430000 (b) What is CSTR volume necessary to take the effluent from PFR above and achieve 60% total conversion (based on species A fed to the PFR)? FA 0=2. 43 mol/s X=0. 3 X=0. 6

EXERCISE FA 0=2. 43 mol/s X=0. 3 X=0. 6 54