Chapter 12 Thermodynamic Property Relations Study Guide in

- Slides: 38

Chapter 12 Thermodynamic Property Relations Study Guide in Power. Point to accompany Thermodynamics: An Engineering Approach, 5 th edition by Yunus A. Çengel and Michael A. Boles

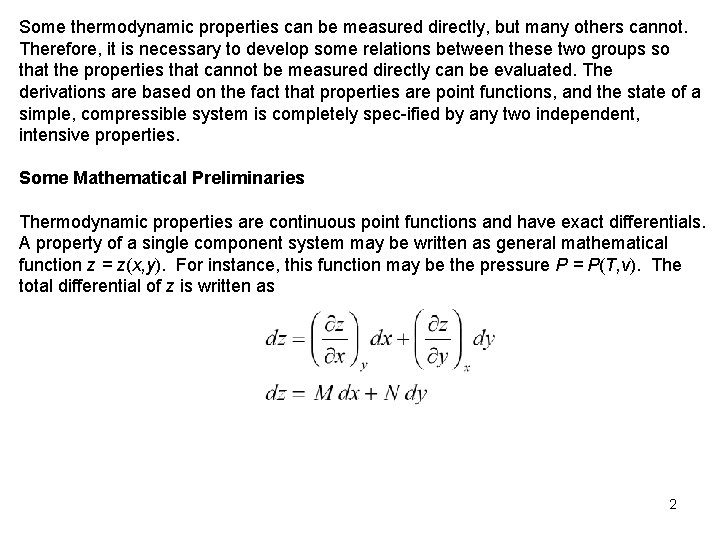
Some thermodynamic properties can be measured directly, but many others cannot. Therefore, it is necessary to develop some relations between these two groups so that the properties that cannot be measured directly can be evaluated. The derivations are based on the fact that properties are point functions, and the state of a simple, compressible system is completely spec ified by any two independent, intensive properties. Some Mathematical Preliminaries Thermodynamic properties are continuous point functions and have exact differentials. A property of a single component system may be written as general mathematical function z = z(x, y). For instance, this function may be the pressure P = P(T, v). The total differential of z is written as 2

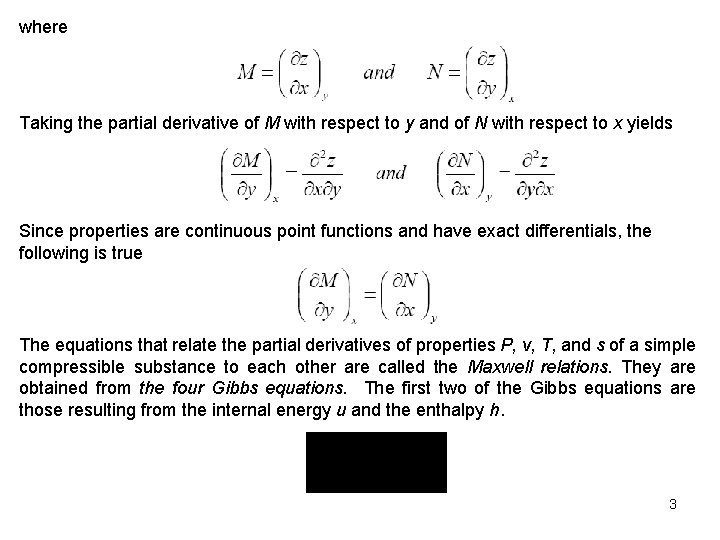
where Taking the partial derivative of M with respect to y and of N with respect to x yields Since properties are continuous point functions and have exact differentials, the following is true The equations that relate the partial derivatives of properties P, v, T, and s of a simple compressible substance to each other are called the Maxwell relations. They are obtained from the four Gibbs equations. The first two of the Gibbs equations are those resulting from the internal energy u and the enthalpy h. 3

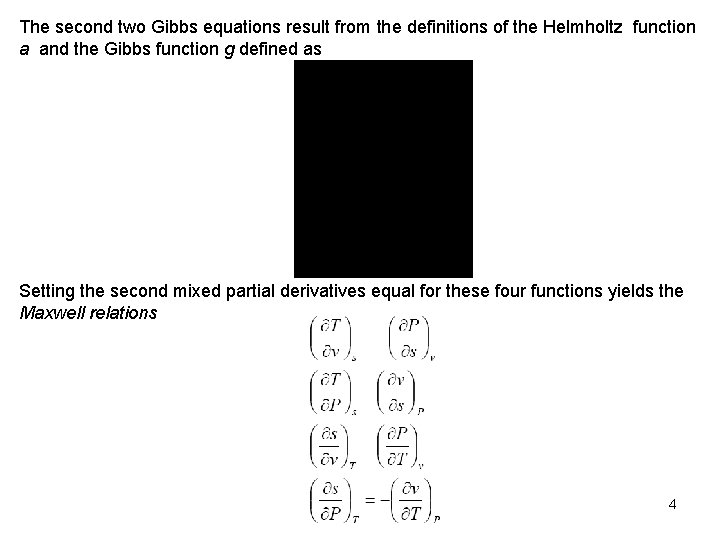
The second two Gibbs equations result from the definitions of the Helmholtz function a and the Gibbs function g defined as Setting the second mixed partial derivatives equal for these four functions yields the Maxwell relations 4

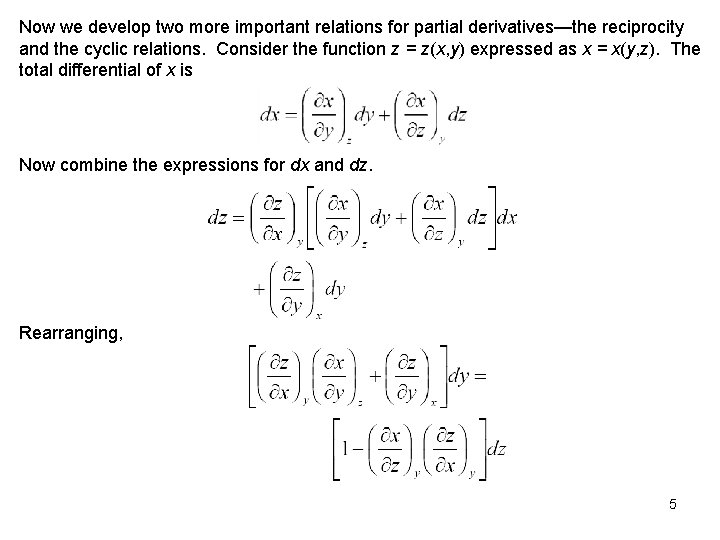
Now we develop two more important relations for partial derivatives—the reciprocity and the cyclic relations. Consider the function z = z(x, y) expressed as x = x(y, z). The total differential of x is Now combine the expressions for dx and dz. Rearranging, 5

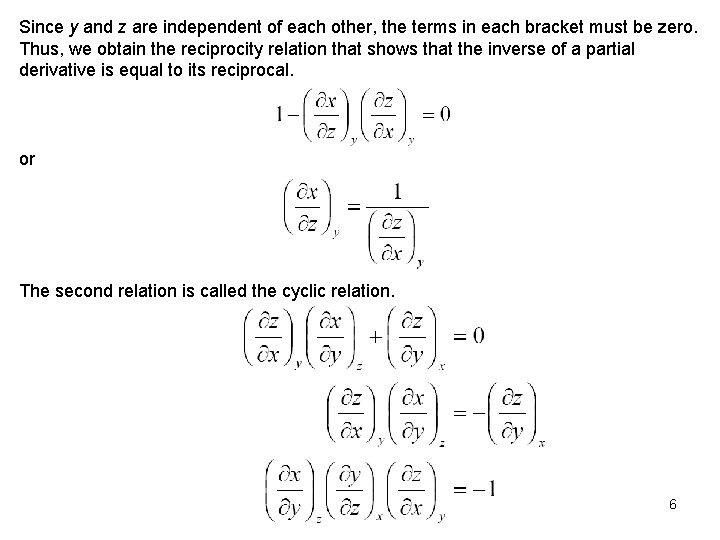
Since y and z are independent of each other, the terms in each bracket must be zero. Thus, we obtain the reciprocity relation that shows that the inverse of a partial derivative is equal to its reciprocal. or The second relation is called the cyclic relation. 6

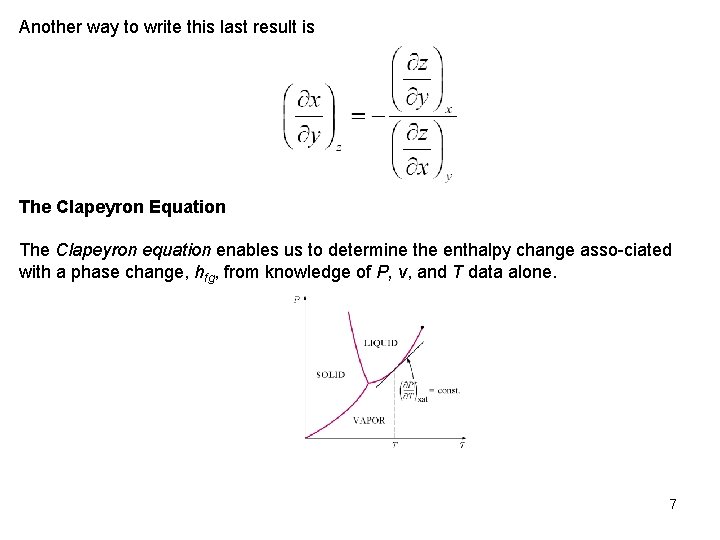
Another way to write this last result is The Clapeyron Equation The Clapeyron equation enables us to determine the enthalpy change asso ciated with a phase change, hfg, from knowledge of P, v, and T data alone. 7

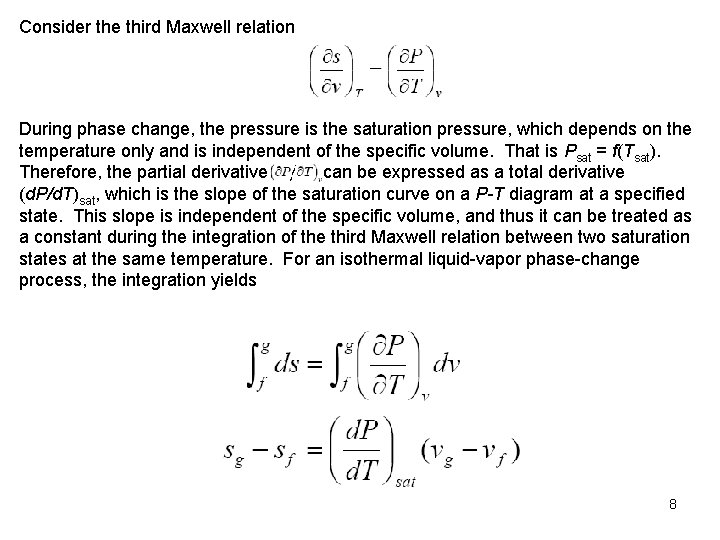
Consider the third Maxwell relation During phase change, the pressure is the saturation pressure, which depends on the temperature only and is independent of the specific volume. That is Psat = f(Tsat). Therefore, the partial derivative can be expressed as a total derivative (d. P/d. T)sat, which is the slope of the saturation curve on a P-T diagram at a specified state. This slope is independent of the specific volume, and thus it can be treated as a constant during the integration of the third Maxwell relation between two saturation states at the same temperature. For an isothermal liquid vapor phase change process, the integration yields 8

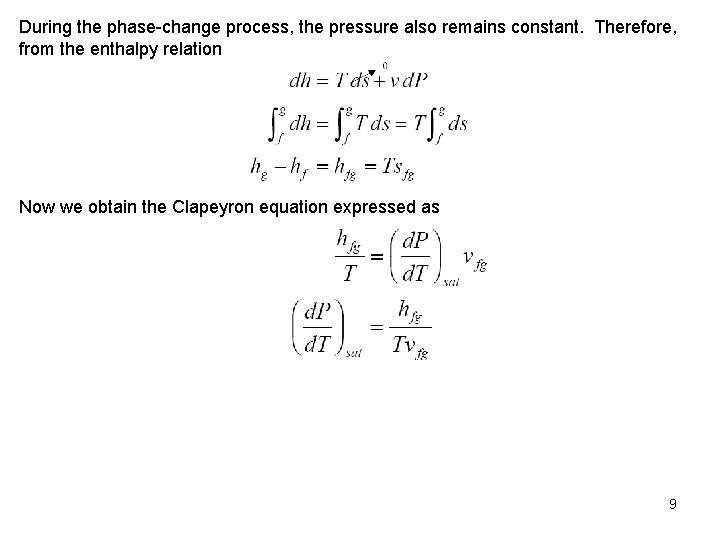
During the phase change process, the pressure also remains constant. Therefore, from the enthalpy relation Now we obtain the Clapeyron equation expressed as 9

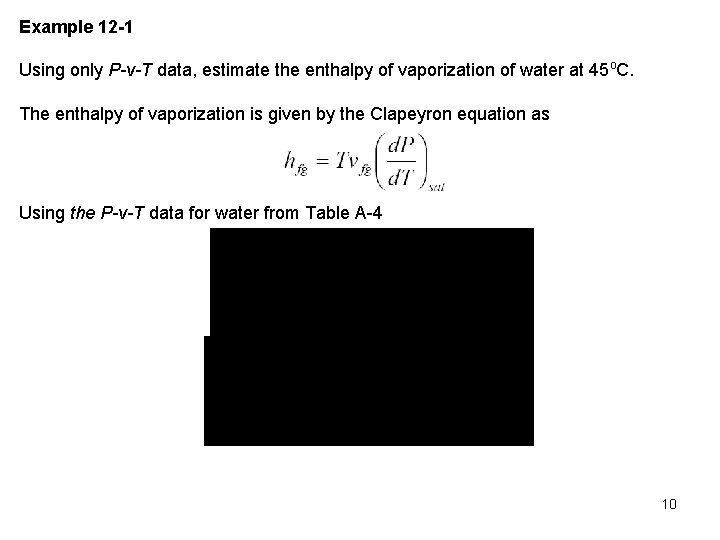
Example 12 -1 Using only P-v-T data, estimate the enthalpy of vaporization of water at 45 o. C. The enthalpy of vaporization is given by the Clapeyron equation as Using the P-v-T data for water from Table A 4 10

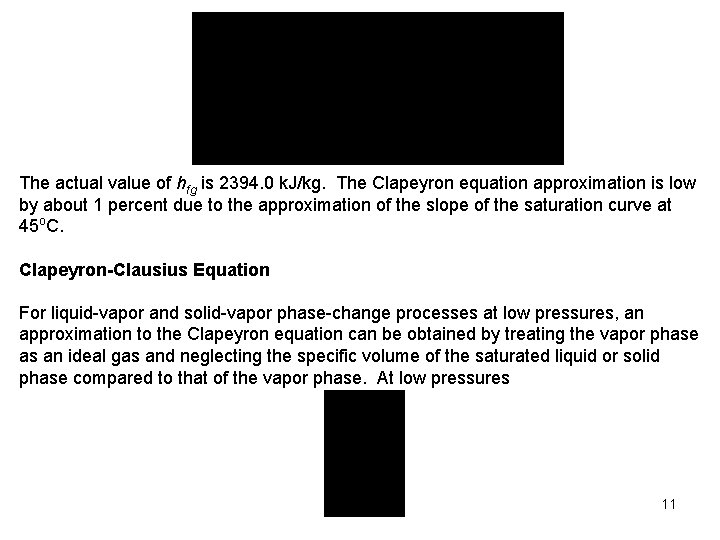
The actual value of hfg is 2394. 0 k. J/kg. The Clapeyron equation approximation is low by about 1 percent due to the approximation of the slope of the saturation curve at 45 o. C. Clapeyron-Clausius Equation For liquid vapor and solid vapor phase change processes at low pressures, an approximation to the Clapeyron equation can be obtained by treating the vapor phase as an ideal gas and neglecting the specific volume of the saturated liquid or solid phase compared to that of the vapor phase. At low pressures 11

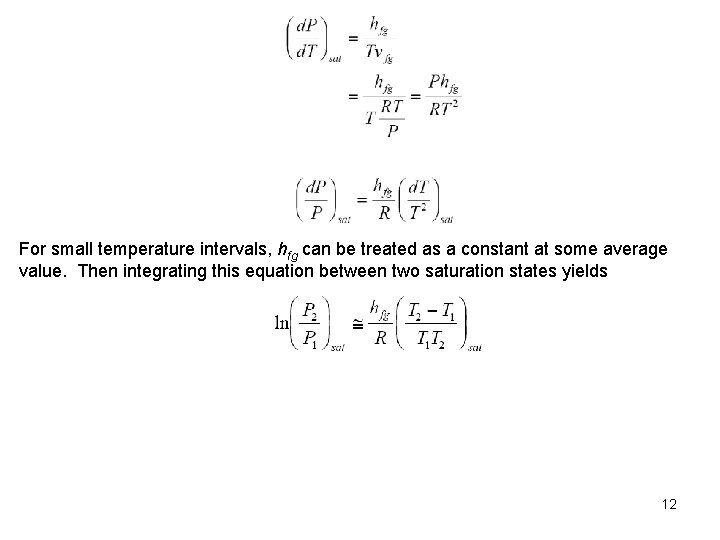
For small temperature intervals, hfg can be treated as a constant at some average value. Then integrating this equation between two saturation states yields 12

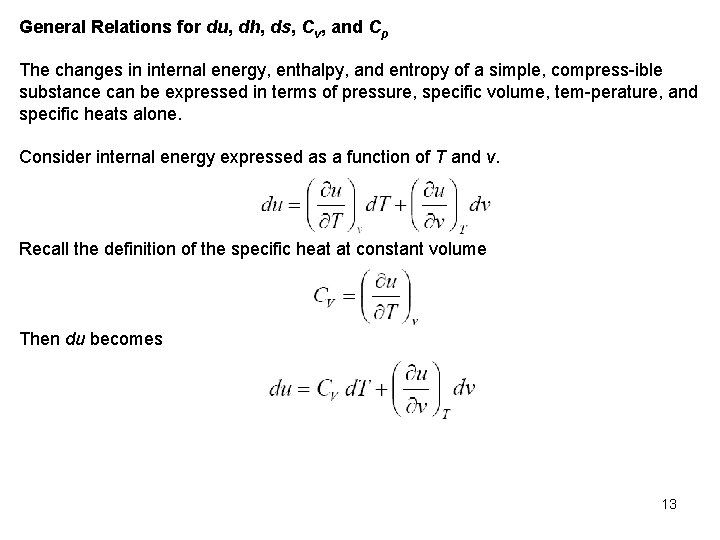
General Relations for du, dh, ds, Cv, and Cp The changes in internal energy, enthalpy, and entropy of a simple, compress ible substance can be expressed in terms of pressure, specific volume, tem perature, and specific heats alone. Consider internal energy expressed as a function of T and v. Recall the definition of the specific heat at constant volume Then du becomes 13

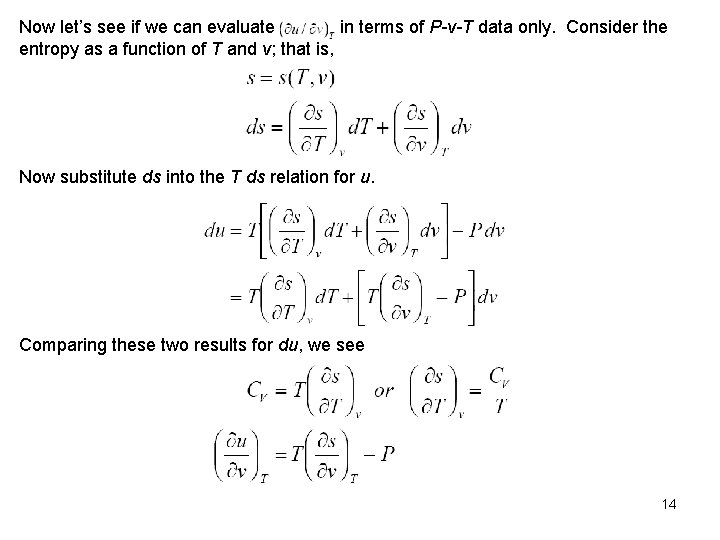
Now let’s see if we can evaluate in terms of P-v-T data only. Consider the entropy as a function of T and v; that is, Now substitute ds into the T ds relation for u. Comparing these two results for du, we see 14

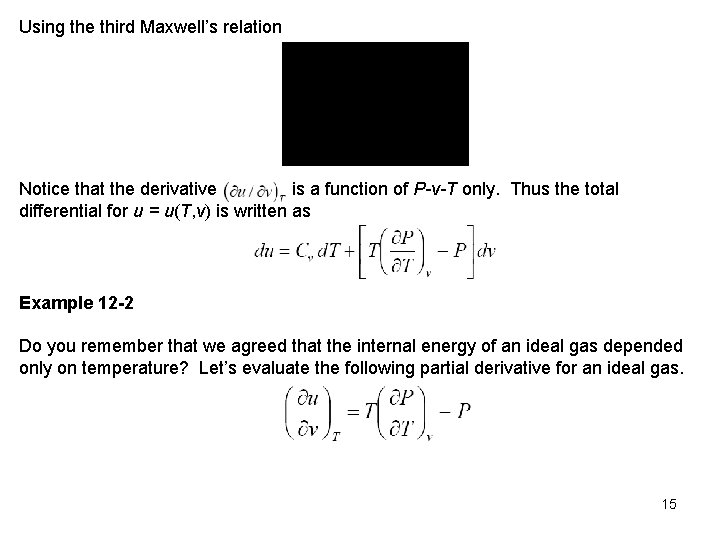
Using the third Maxwell’s relation Notice that the derivative is a function of P-v-T only. Thus the total differential for u = u(T, v) is written as Example 12 -2 Do you remember that we agreed that the internal energy of an ideal gas depended only on temperature? Let’s evaluate the following partial derivative for an ideal gas. 15

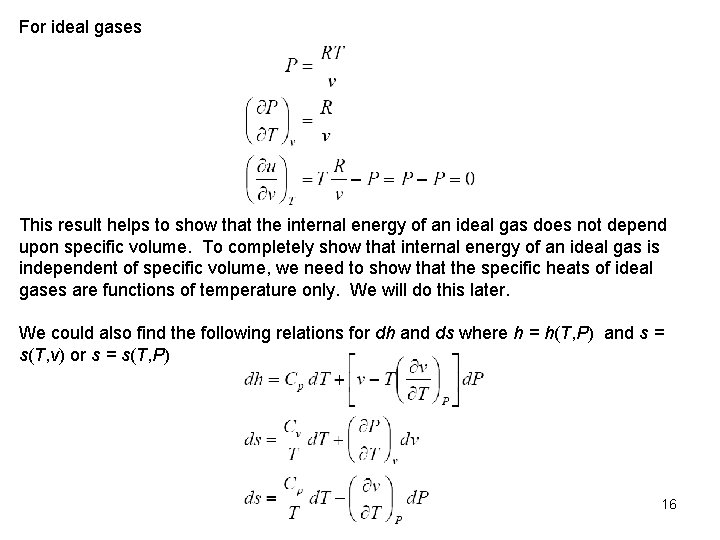
For ideal gases This result helps to show that the internal energy of an ideal gas does not depend upon specific volume. To completely show that internal energy of an ideal gas is independent of specific volume, we need to show that the specific heats of ideal gases are functions of temperature only. We will do this later. We could also find the following relations for dh and ds where h = h(T, P) and s = s(T, v) or s = s(T, P) 16

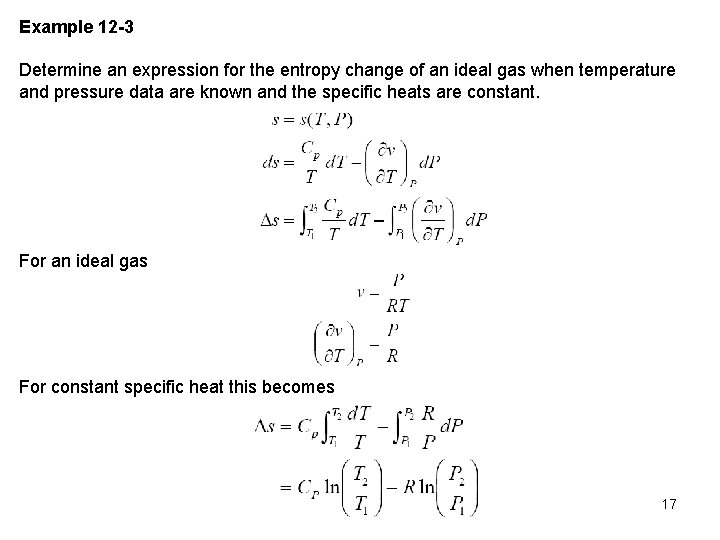
Example 12 -3 Determine an expression for the entropy change of an ideal gas when temperature and pressure data are known and the specific heats are constant. For an ideal gas For constant specific heat this becomes 17

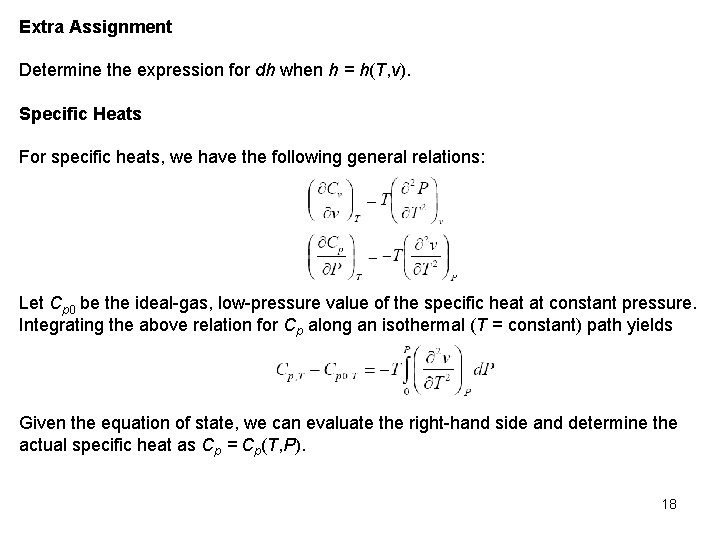
Extra Assignment Determine the expression for dh when h = h(T, v). Specific Heats For specific heats, we have the following general relations: Let Cp 0 be the ideal gas, low pressure value of the specific heat at constant pressure. Integrating the above relation for Cp along an isothermal (T = constant) path yields Given the equation of state, we can evaluate the right hand side and determine the actual specific heat as Cp = Cp(T, P). 18

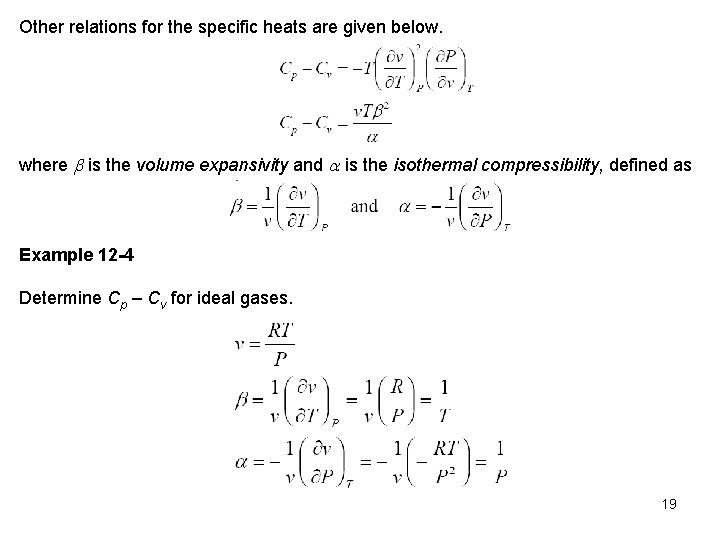
Other relations for the specific heats are given below. where is the volume expansivity and is the isothermal compressibility, defined as Example 12 -4 Determine Cp – Cv for ideal gases. 19

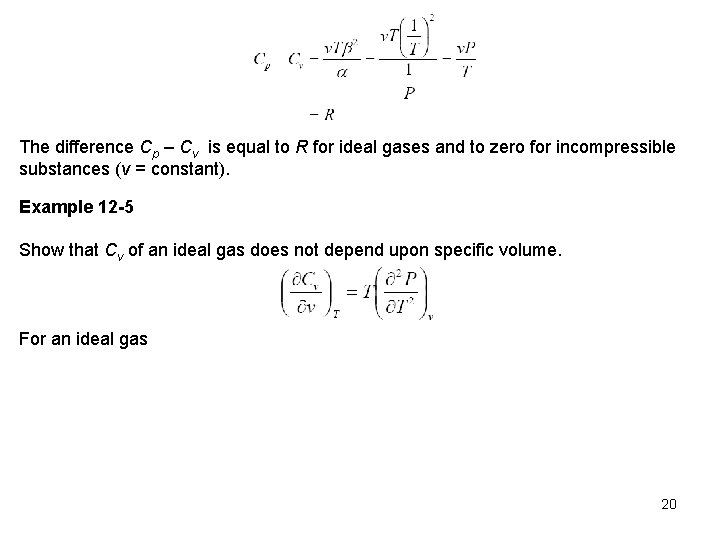
The difference Cp – Cv is equal to R for ideal gases and to zero for incompressible substances (v = constant). Example 12 -5 Show that Cv of an ideal gas does not depend upon specific volume. For an ideal gas 20

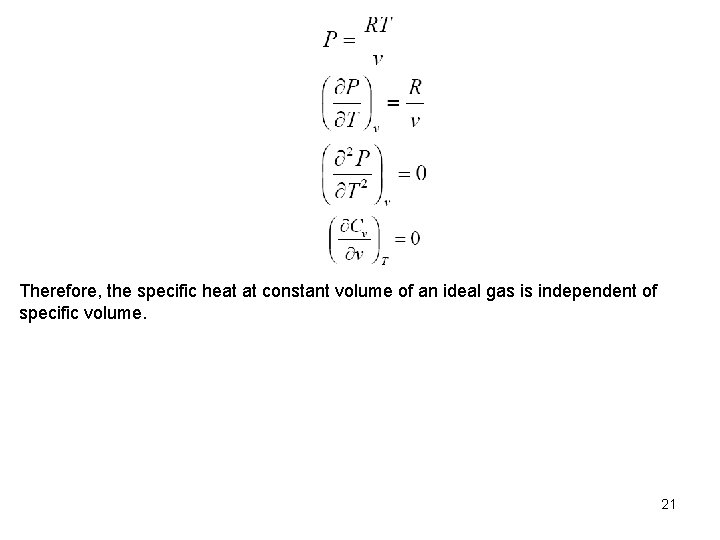
Therefore, the specific heat at constant volume of an ideal gas is independent of specific volume. 21

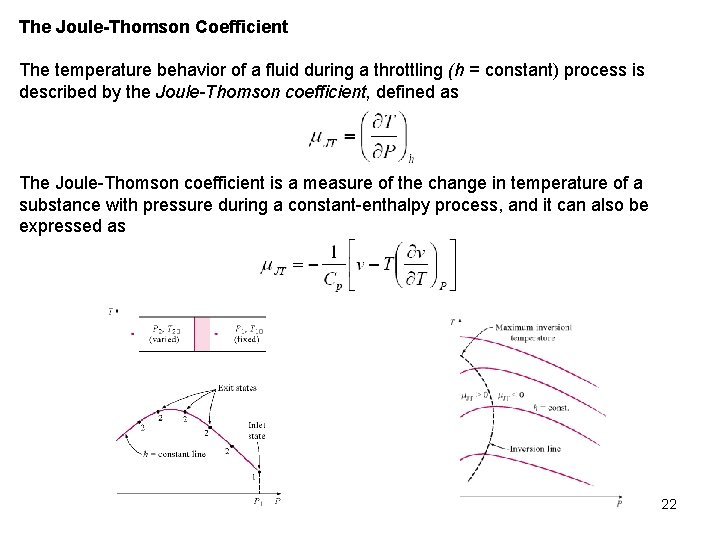
The Joule-Thomson Coefficient The temperature behavior of a fluid during a throttling (h = constant) process is described by the Joule-Thomson coefficient, defined as The Joule Thomson coefficient is a measure of the change in temperature of a substance with pressure during a constant enthalpy process, and it can also be expressed as 22

Example For You To Do Take a moment to determine the Joule Thomson coefficient for an ideal gas. What is the enthalpy change of an ideal gas during an isothermal process? Enthalpy, Internal Energy, and Entropy Changes for Real Gases The enthalpy, internal energy, and entropy changes of real gases can be determined accurately by utilizing generalized enthalpy or entropy departure charts to account for the deviation from the ideal gas behavior. Considering the enthalpy a function of T and P, h = h(T, P), we found dh to be To integrate this relation to obtain the expression for the enthalpy change of a real gas, we need the equation of state data, the P-v-T relation, and Cp data. Here we use the generalized compressibility charts and the compressibility factor, Figure A 15 a, to supply the equation of state data. Let’s integrate the dh equation between two states from T 1, P 1 to T 2, P 2. 23

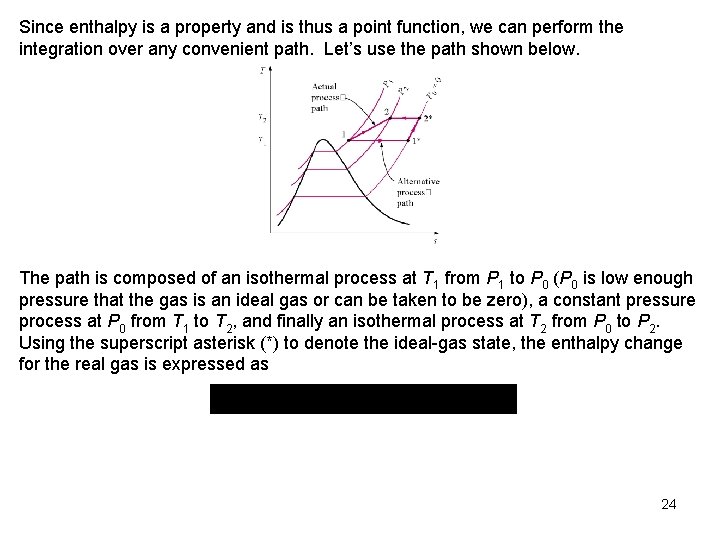
Since enthalpy is a property and is thus a point function, we can perform the integration over any convenient path. Let’s use the path shown below. The path is composed of an isothermal process at T 1 from P 1 to P 0 (P 0 is low enough pressure that the gas is an ideal gas or can be taken to be zero), a constant pressure process at P 0 from T 1 to T 2, and finally an isothermal process at T 2 from P 0 to P 2. Using the superscript asterisk (*) to denote the ideal gas state, the enthalpy change for the real gas is expressed as 24

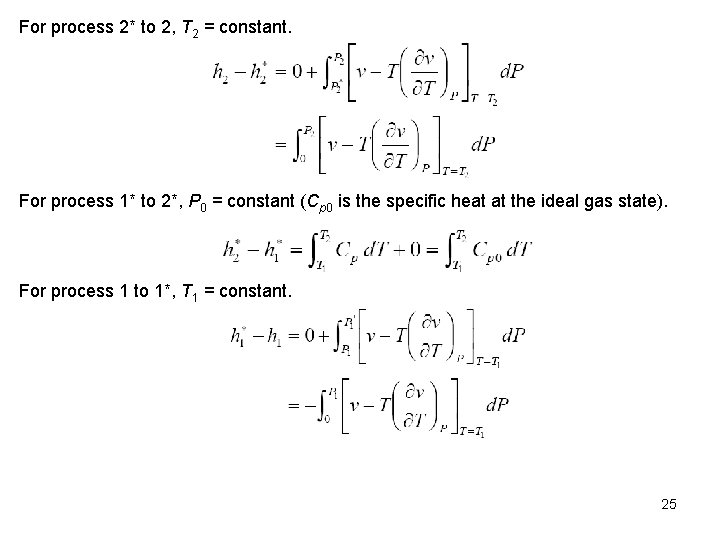
For process 2* to 2, T 2 = constant. For process 1* to 2*, P 0 = constant (Cp 0 is the specific heat at the ideal gas state). For process 1 to 1*, T 1 = constant. 25

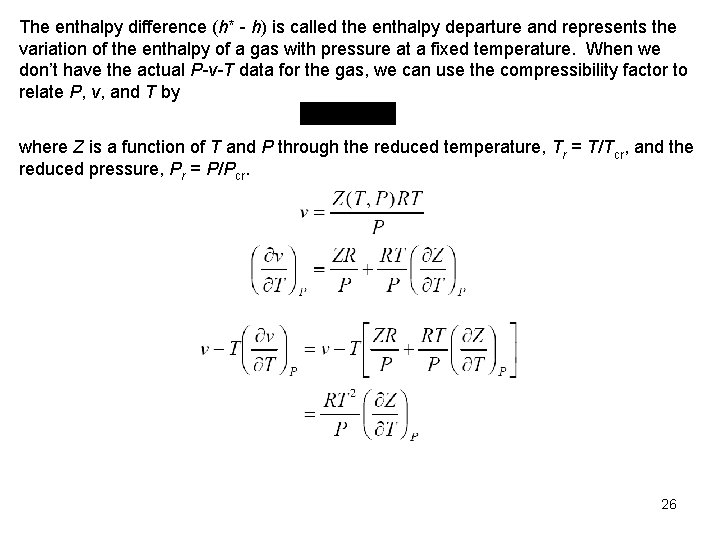
The enthalpy difference (h* h) is called the enthalpy departure and represents the variation of the enthalpy of a gas with pressure at a fixed temperature. When we don’t have the actual P-v-T data for the gas, we can use the compressibility factor to relate P, v, and T by where Z is a function of T and P through the reduced temperature, Tr = T/Tcr, and the reduced pressure, Pr = P/Pcr. 26

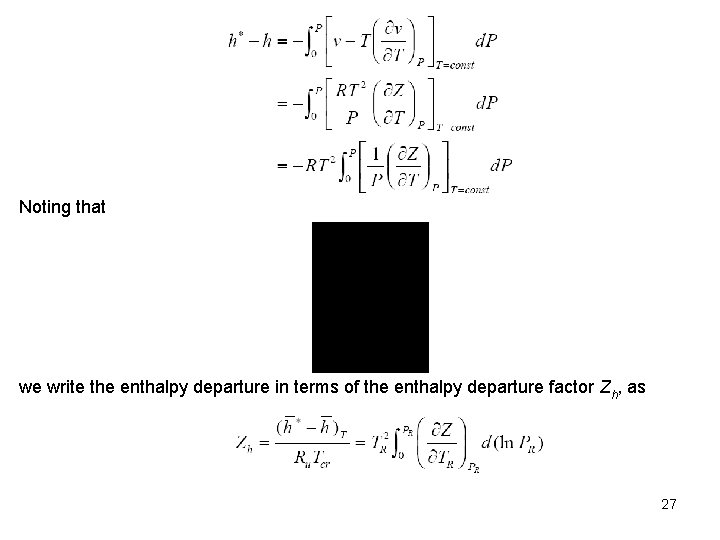
Noting that we write the enthalpy departure in terms of the enthalpy departure factor Zh, as 27

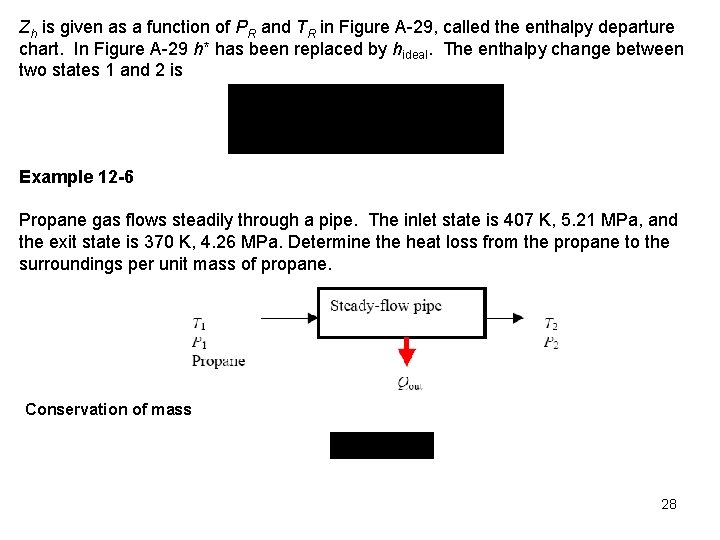
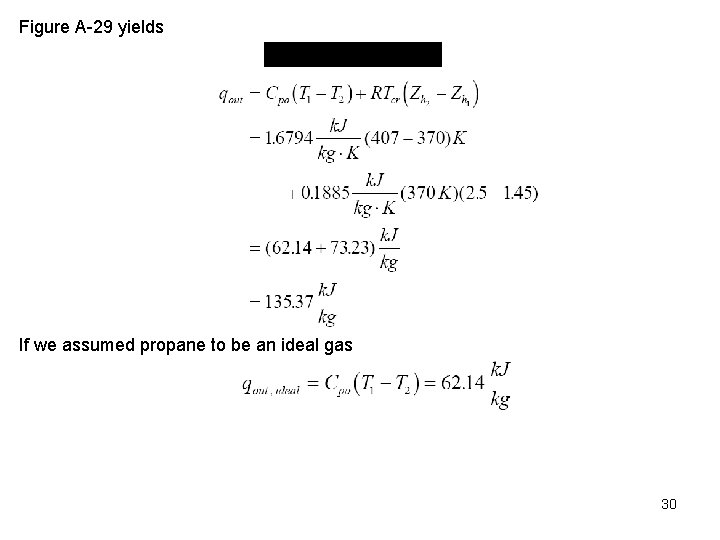
Zh is given as a function of PR and TR in Figure A 29, called the enthalpy departure chart. In Figure A 29 h* has been replaced by hideal. The enthalpy change between two states 1 and 2 is Example 12 -6 Propane gas flows steadily through a pipe. The inlet state is 407 K, 5. 21 MPa, and the exit state is 370 K, 4. 26 MPa. Determine the heat loss from the propane to the surroundings per unit mass of propane. Conservation of mass 28

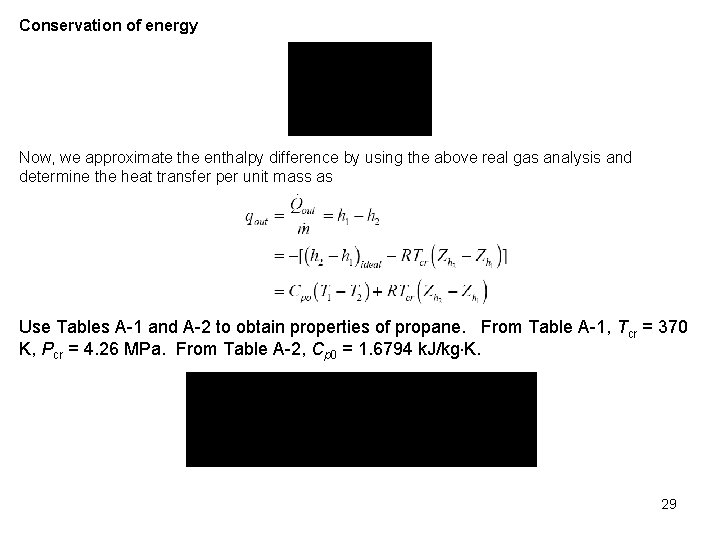
Conservation of energy Now, we approximate the enthalpy difference by using the above real gas analysis and determine the heat transfer per unit mass as Use Tables A 1 and A 2 to obtain properties of propane. From Table A 1, Tcr = 370 K, Pcr = 4. 26 MPa. From Table A 2, Cp 0 = 1. 6794 k. J/kg K. 29

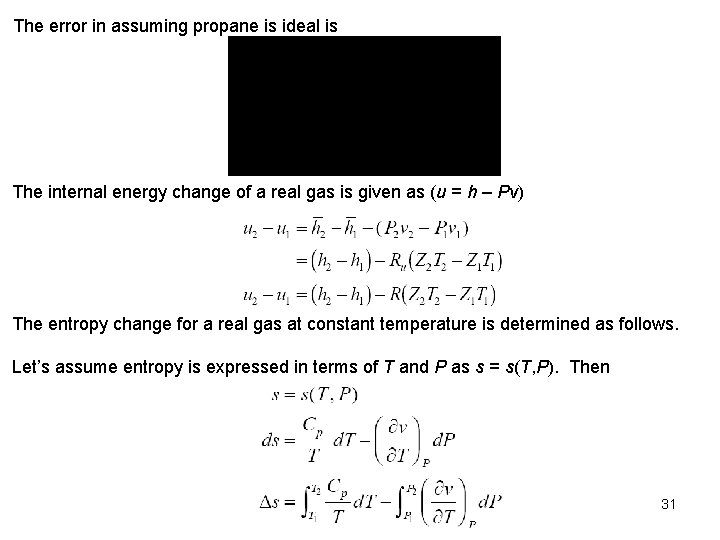
Figure A 29 yields If we assumed propane to be an ideal gas 30

The error in assuming propane is ideal is The internal energy change of a real gas is given as (u = h – Pv) The entropy change for a real gas at constant temperature is determined as follows. Let’s assume entropy is expressed in terms of T and P as s = s(T, P). Then 31

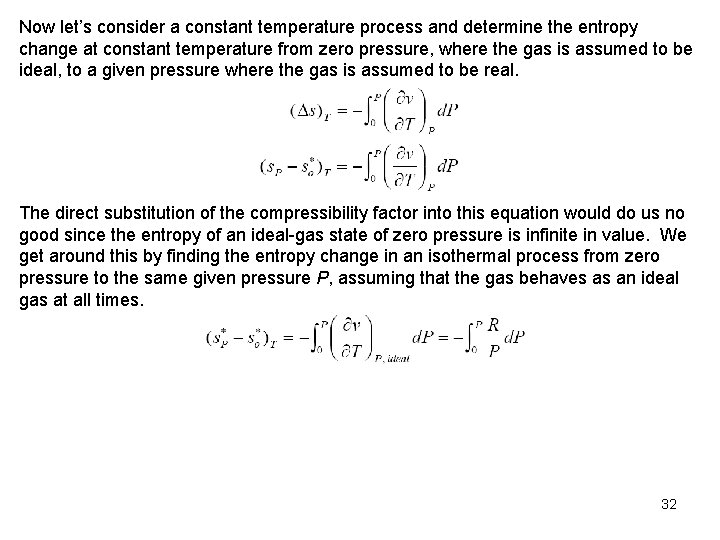
Now let’s consider a constant temperature process and determine the entropy change at constant temperature from zero pressure, where the gas is assumed to be ideal, to a given pressure where the gas is assumed to be real. The direct substitution of the compressibility factor into this equation would do us no good since the entropy of an ideal gas state of zero pressure is infinite in value. We get around this by finding the entropy change in an isothermal process from zero pressure to the same given pressure P, assuming that the gas behaves as an ideal gas at all times. 32

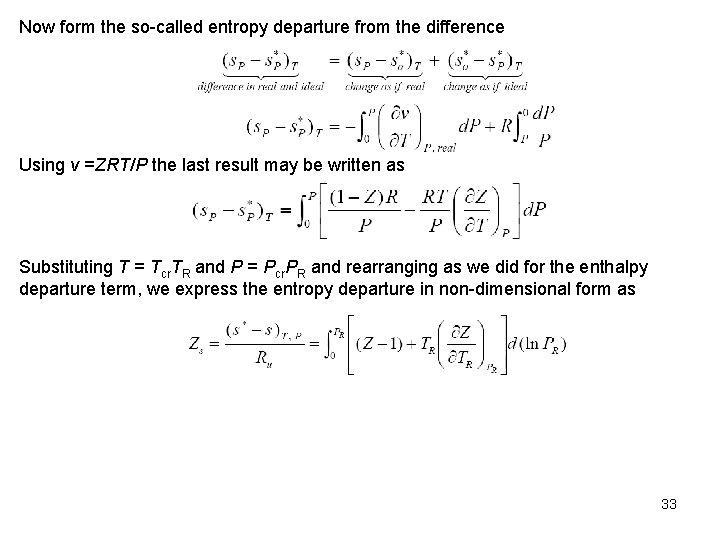
Now form the so called entropy departure from the difference Using v =ZRT/P the last result may be written as Substituting T = Tcr. TR and P = Pcr. PR and rearranging as we did for the enthalpy departure term, we express the entropy departure in non dimensional form as 33

Zs is called the entropy departure factor and is found in Table A 30, called the entropy departure chart. In Table A 30 s* is replaced by sideal. The entropy change during a process 1 2 is given as Note: The concept for finding the entropy change using the entropy departure charts is different than that used to find the enthalpy change. The entropy change between two states is the ideal gas change between the two states plus two correction factors, one at each state—the entropy departures, to account for nonideal gas behavior at each state. 34

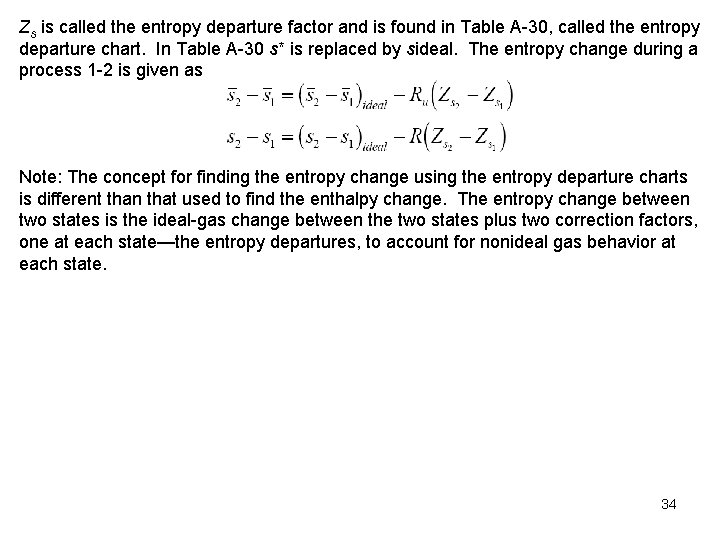
Example 12 -7 Carbon dioxide gas is compressed reversibly and adiabatically from 0. 1 MPa and 220 K to 4. 0 MPa. Find the final temperature for the process. Since the process is reversible and adiabatic, the process is also isentropic; therefore, or using the real gas results for entropy change Use Tables A 1 and A 20 to obtain properties of carbon dioxide. From Table A 1, Tcr = 304. 2 K, Pcr = 7. 39 MPa. 35

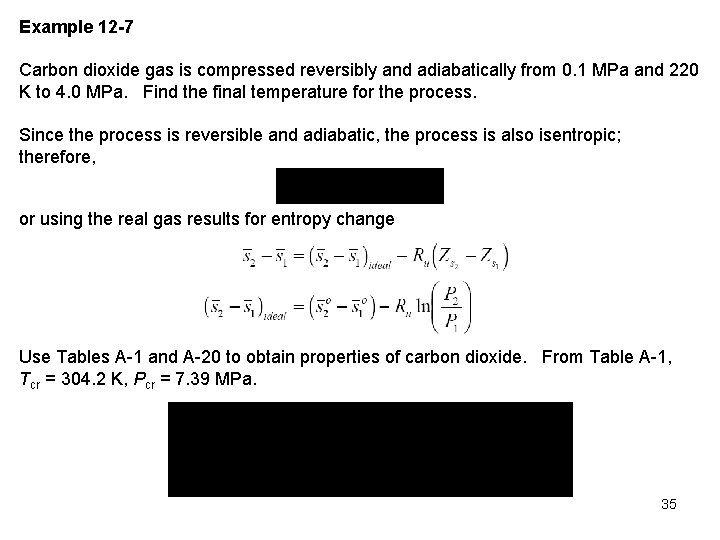
Figure A 15 a yields (state 1 is an ideal gas state) Table A 20 yields Assuming ideal gas behavior with constant specific heats for an isentropic process 36

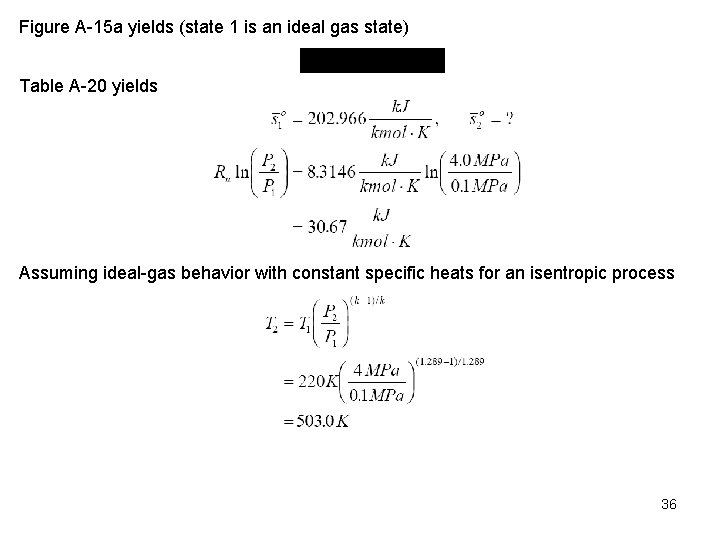
Guess T 2 = 500 K Figure A 15 a yields (state 1 is an ideal gas state) Table A 20 yields 37

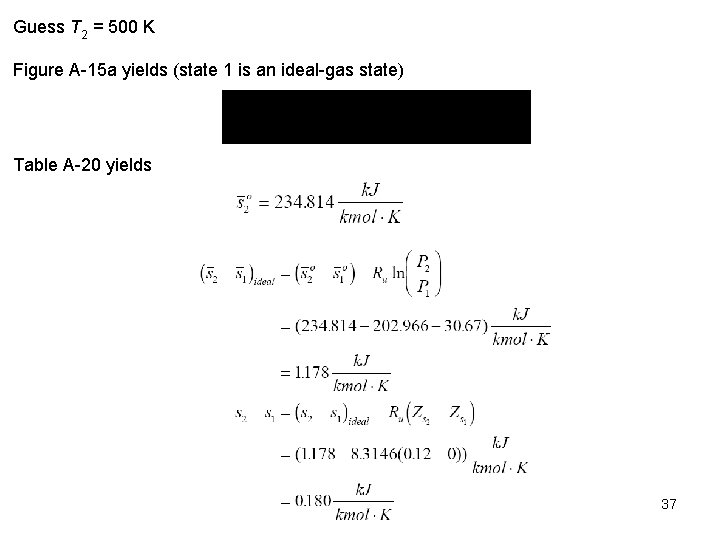
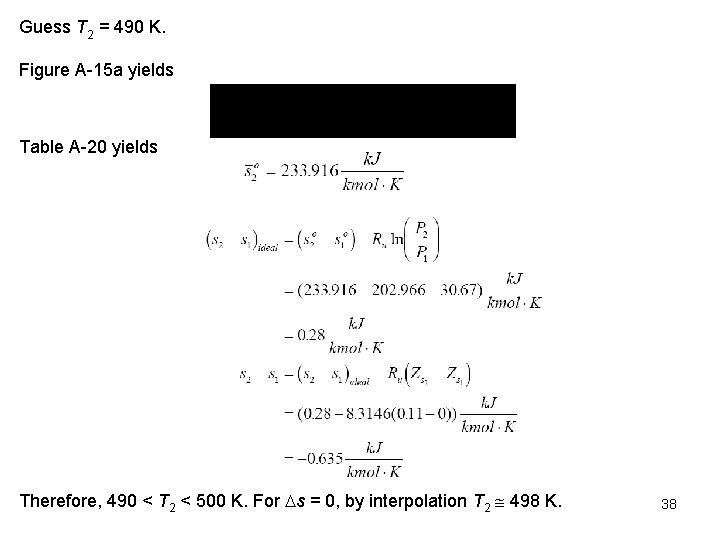
Guess T 2 = 490 K. Figure A 15 a yields Table A 20 yields Therefore, 490 < T 2 < 500 K. For s = 0, by interpolation T 2 498 K. 38