Titration and p H Curves Titration and p











- Slides: 14

Titration and p. H Curves.

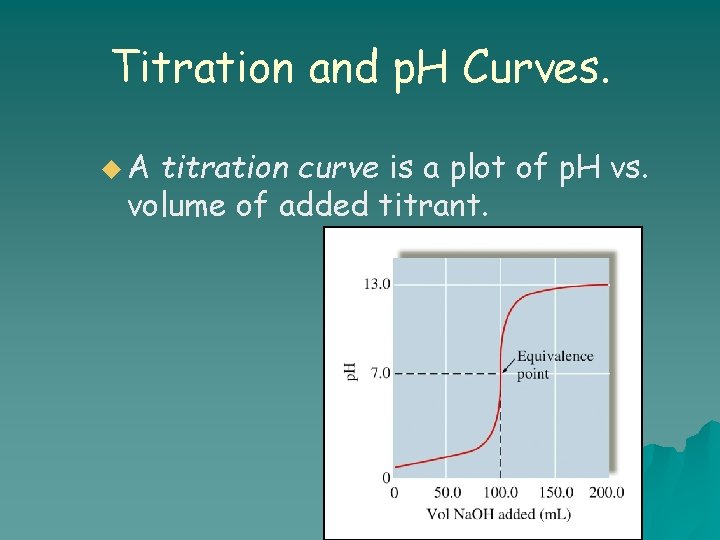
Titration and p. H Curves. u. A titration curve is a plot of p. H vs. volume of added titrant.

Parts of a titration u Titrant- acid or base of known concentration that is added to the substance being analyzed. u Analyte- the substance that is being analyzed, or your unknown.

u. Because Titrations titrations involve small concentrations, and m. L are of often used in titrations, and millimoles, or mmol. u. Molarity = mmol/m. L u. The equivalence point is when moles of titrant are equal to moles of analyte. u. All volumes in a titration are considered to be additive. u. Always label the equivalence point and for weak acid or base titrations the half equivalence point, where p. H = p. Ka.

Strong Acid-Strong Base Titration Curves. u Before the addition. u p. H is calculated directly from the initial concentration. u Additions before the equivalence point. u Construct a “stoichiometry” reaction table. Determine MOLES of acid in excess (not neutralized). u Divide MOLES by the TOTAL VOLUME to obtain [H 3 O+]. u Calculate the p. H.

Strong Acid-Strong Base Titration Curves. u. Additions at the equivalence point. u. The p. H ALWAYS is equal to 7. 00 when [H 3 O+] = [OH-]. u. Additions beyond the equivalence point. u. Construct a “stoichiometry” reaction table. Determine MOLES of base in excess (not neutralized). u Divide MOLES by the TOTAL VOLUME to obtain [OH-]. u Calculate the p. OH, then the p. H.

Problem u 50. 0 m. L of 0. 200 M HNO 3 are titrated with 0. 100 M Na. OH. u. Calculate the p. H after the additions of 0. 0, 10. 0, 20. 0, 50. 0, 100. 0, 150. 0, and 200. 0 m. L samples of Na. OH. u. Then, construct a titration curve and label it properly.




Strong Acid Strong Base Problem u 50. 0 m. L of 0. 500 M HCl are titrated with 0. 250 M Na. OH. u. Calculate the p. H after the additions of 0. 0, 10. 0, 20. 0, 50. 0, 100. 0, 150. 0, and 200. 0 m. L samples of Na. OH. u. Then, construct a titration curve and label it properly.



 Titration curve for arginine
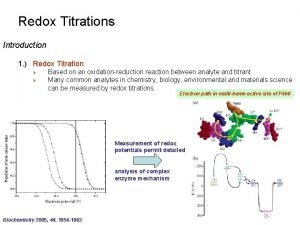
Titration curve for arginine Introduction to redox titration
Introduction to redox titration Amino acid titration curves
Amino acid titration curves Weak base strong acid titration curve
Weak base strong acid titration curve Advantages of conductometric titration
Advantages of conductometric titration Why do titration curves flatten out
Why do titration curves flatten out Why do titration curves flatten out
Why do titration curves flatten out Titration definition
Titration definition Amino acid titration curves
Amino acid titration curves Titration vs back titration
Titration vs back titration Titration vs back titration
Titration vs back titration Define redox titration
Define redox titration Vector function and space curves
Vector function and space curves Creating production possibilities schedules and curves
Creating production possibilities schedules and curves S and j curves
S and j curves