Enthalpy is a measure of the total energy







- Slides: 16

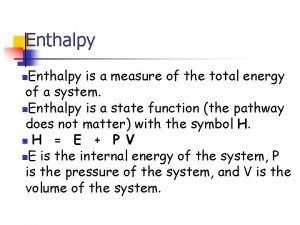
Enthalpy is a measure of the total energy of a system. n. Enthalpy is a state function (the pathway does not matter) with the symbol H. n H = E + P V n. E is the internal energy of the system, P is the pressure of the system, and V is the volume of the system. n

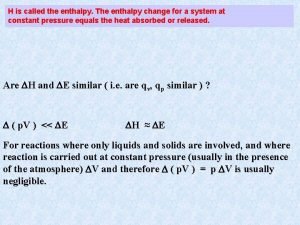
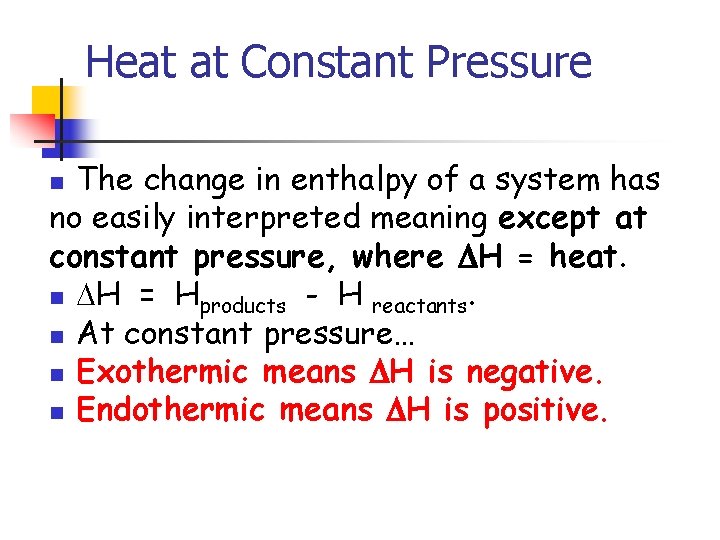
Heat at Constant Pressure The change in enthalpy of a system has no easily interpreted meaning except at constant pressure, where H = heat. n H = Hproducts - H reactants. n At constant pressure… n Exothermic means H is negative. n Endothermic means H is positive. n

Enthalpy n When 1 mole of methane is burned at constant pressure, 890 k. J of energy is released as heat. Calculate H for a process in which a 5. 8 g sample of methane is burned at constant pressure.

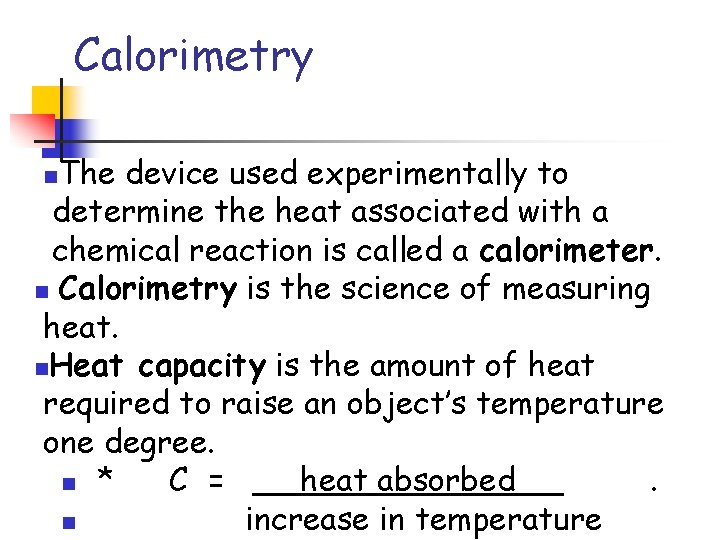
Calorimetry The device used experimentally to determine the heat associated with a chemical reaction is called a calorimeter. n Calorimetry is the science of measuring heat. n. Heat capacity is the amount of heat required to raise an object’s temperature one degree. n * C = heat absorbed. n increase in temperature n

Heat Capacity Specific heat capacity is the energy required to raise the temperature of one gram of a substance by one degree Celsius. units are J/o. C. g or J/K. g. Molar heat capacity is the energy required to raise the temperature of one mole of a substance one degree Celsius. units are J/o. C. mol or J/K. mol.

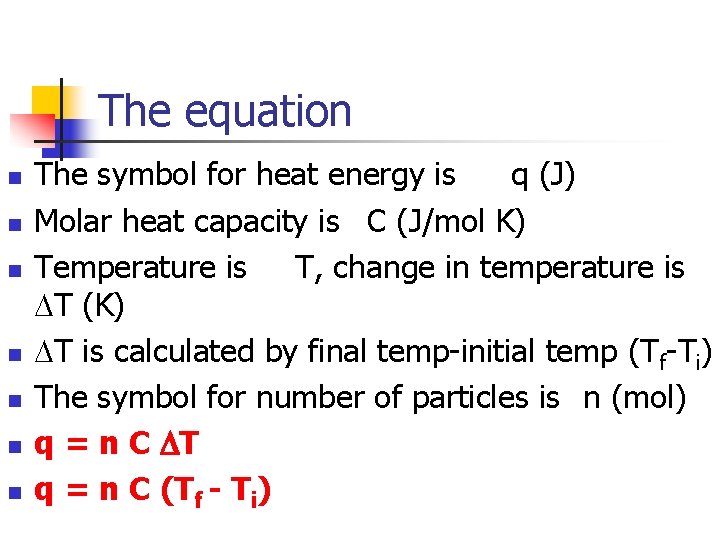
The equation n n n The symbol for heat energy is q (J) Molar heat capacity is C (J/mol K) Temperature is T, change in temperature is T (K) T is calculated by final temp-initial temp (Tf-Ti) The symbol for number of particles is n (mol) q = n C T q = n C (Tf - Ti)

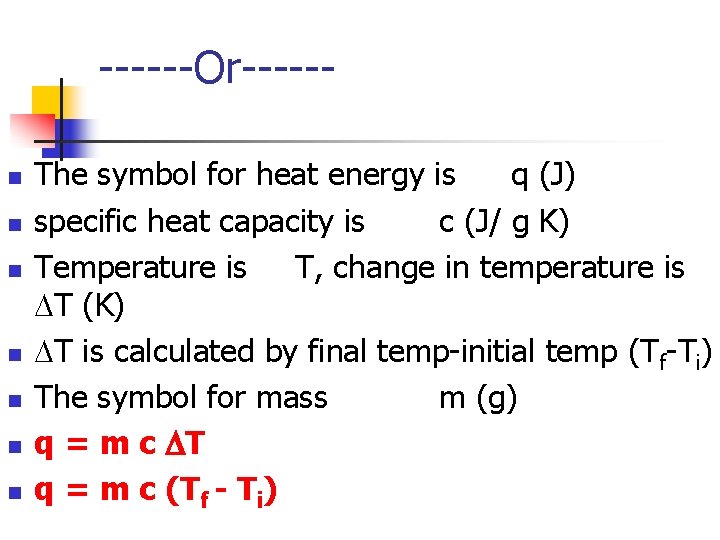
------Or-----n n n n The symbol for heat energy is q (J) specific heat capacity is c (J/ g K) Temperature is T, change in temperature is T (K) T is calculated by final temp-initial temp (Tf-Ti) The symbol for mass m (g) q = m c T q = m c (Tf - Ti)

Heat Capacity n n Some common values are found in Table 6. 1, p 251. * The higher the value, the longer it takes to heat an object and the longer it takes for that same object to cool.

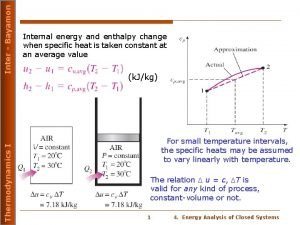
n n n If the pressure remains constant, the process is called constant-pressure calorimetry. Calorimetry experiments can also be carried out under conditions of constant volume. If V = 0, P V = 0, and E = qv

qlost = qgained n n n Sign convention Losing energy means the value is negative. Gaining energy means the value is positive. The absolute value of the energy transferred will be the same. However, losing will be negative, gaining will be positive

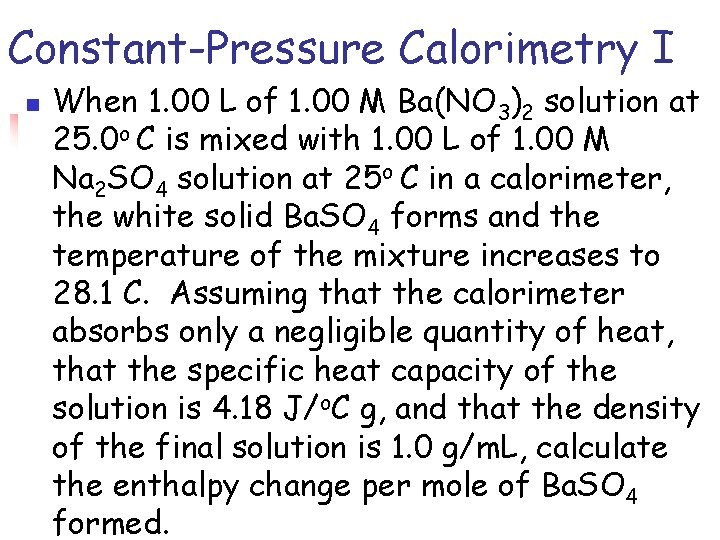
Constant-Pressure Calorimetry I n When 1. 00 L of 1. 00 M Ba(NO 3)2 solution at 25. 0 o C is mixed with 1. 00 L of 1. 00 M Na 2 SO 4 solution at 25 o C in a calorimeter, the white solid Ba. SO 4 forms and the temperature of the mixture increases to 28. 1 C. Assuming that the calorimeter absorbs only a negligible quantity of heat, that the specific heat capacity of the solution is 4. 18 J/o. C g, and that the density of the final solution is 1. 0 g/m. L, calculate the enthalpy change per mole of Ba. SO 4 formed.

Constant-Pressure Calorimetry II n One piece of copper jewelry placed at 100 o C has exactly twice the mass of another piece, which is at 40 o C. They are placed inside a calorimeter whose heat capacity is negligible. What is the final temperature inside the calorimeter? (c of copper = 0. 387 J/g K).

Constant-Pressure Calorimetry III n A. 5269 g of octane is placed in a bomb calorimeter known to have a heat capacity of 11. 3 k. J/C. The octane is ignited in the presence of gasoline. The temperature is increases by 2. 25 o C. What is the energy released per mole?

Enthalpy for phase changes n n n There are constant values for the enthalpy of a phase change. The energy required to go from solid to liquid is called the heat of fusion (Hfus). The energy required to go from liquid to gas is called heat of vaporization (Hvap). q=Hn For heating a substance, q = n. C T or q = mc T

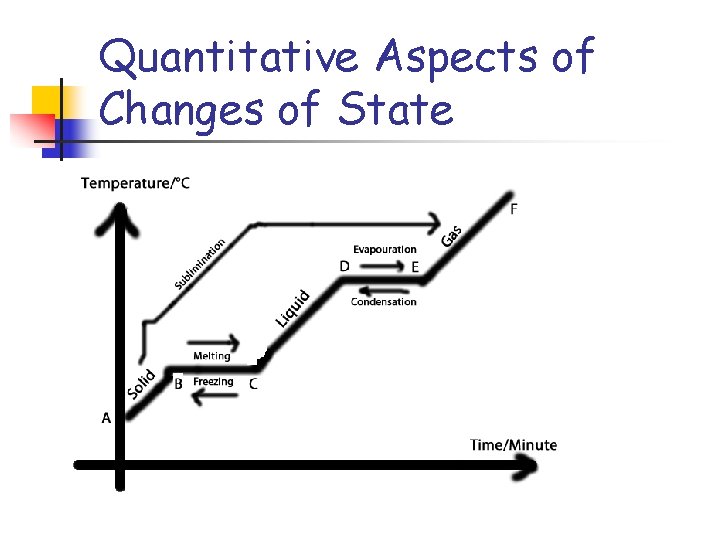
Quantitative Aspects of Changes of State n n The Heating-Cooling Curve.

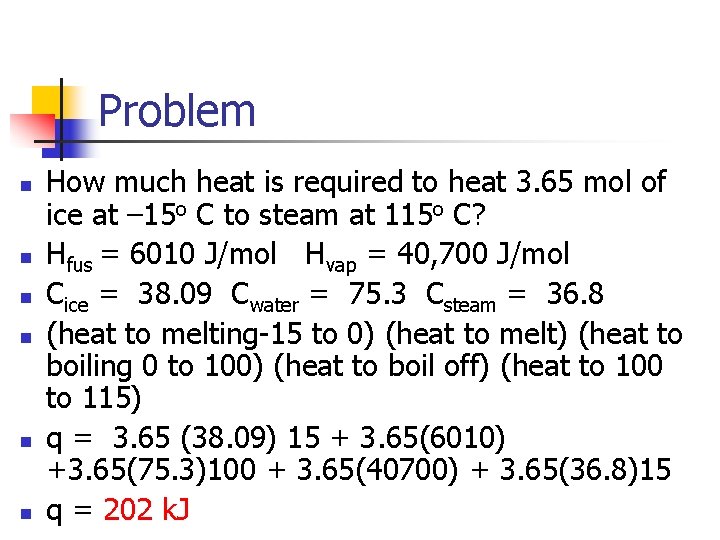
Problem n n n How much heat is required to heat 3. 65 mol of ice at – 15 o C to steam at 115 o C? Hfus = 6010 J/mol Hvap = 40, 700 J/mol Cice = 38. 09 Cwater = 75. 3 Csteam = 36. 8 (heat to melting-15 to 0) (heat to melt) (heat to boiling 0 to 100) (heat to boil off) (heat to 100 to 115) q = 3. 65 (38. 09) 15 + 3. 65(6010) +3. 65(75. 3)100 + 3. 65(40700) + 3. 65(36. 8)15 q = 202 k. J
 Enthalpy is a measure of
Enthalpy is a measure of What is delta h
What is delta h Ratio of useful energy to total input energy
Ratio of useful energy to total input energy Is measure for measure a comedy
Is measure for measure a comedy Barometer
Barometer Enthalpy change in a potential energy diagram
Enthalpy change in a potential energy diagram Cv and cp relationship
Cv and cp relationship When delta g is positive
When delta g is positive Enthalpy vs internal energy
Enthalpy vs internal energy First law of thermodynamics control mass
First law of thermodynamics control mass Relationship between entropy and free energy
Relationship between entropy and free energy Enthalpy entropy free energy
Enthalpy entropy free energy Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Formula de roe
Formula de roe Total revenues minus total costs equals
Total revenues minus total costs equals Total revenues minus total costs equals
Total revenues minus total costs equals