CHEMSHEETS PERIODICITY www chemsheets co uk AS 1055

- Slides: 20

CHEMSHEETS PERIODICITY © www. chemsheets. co. uk AS 1055 3 -July-2015

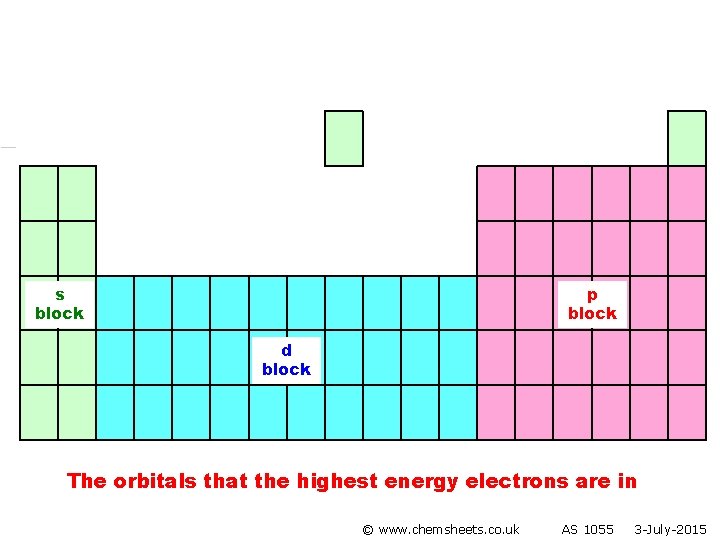
s block p block d block The orbitals that the highest energy electrons are in © www. chemsheets. co. uk AS 1055 3 -July-2015

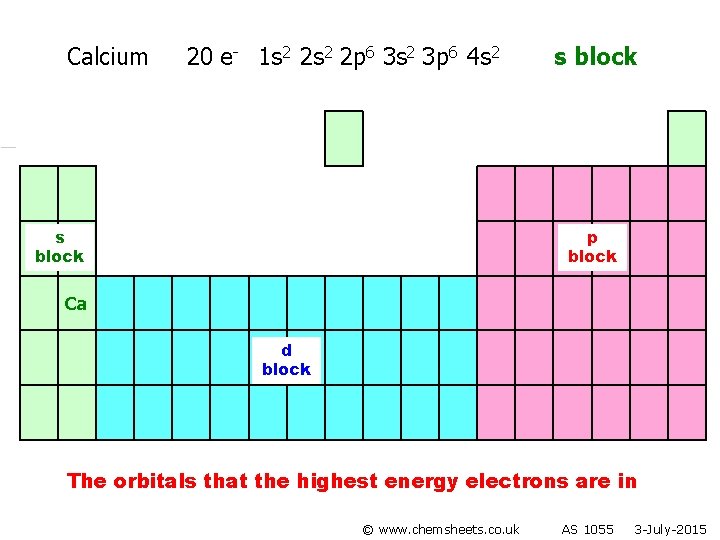
Calcium 20 e- 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 s block p block Ca d block The orbitals that the highest energy electrons are in © www. chemsheets. co. uk AS 1055 3 -July-2015

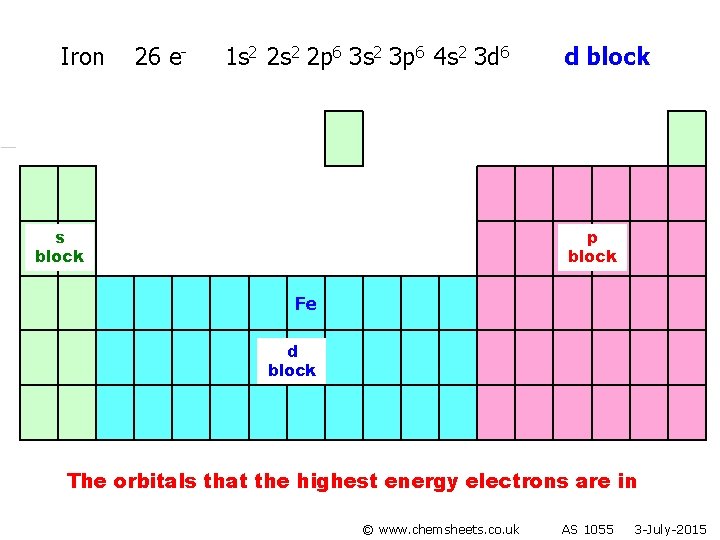
Iron 26 e- 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 s block d block p block Fe d block The orbitals that the highest energy electrons are in © www. chemsheets. co. uk AS 1055 3 -July-2015

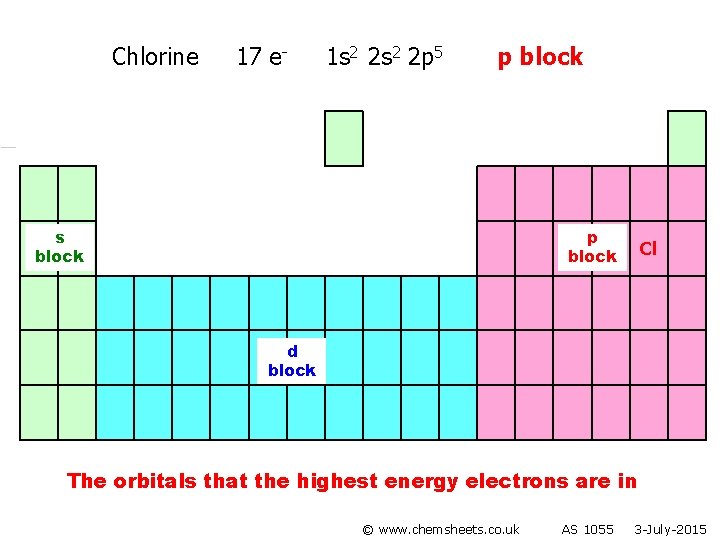
Chlorine 17 e- 1 s 2 2 p 5 p block s block p block Cl d block The orbitals that the highest energy electrons are in © www. chemsheets. co. uk AS 1055 3 -July-2015

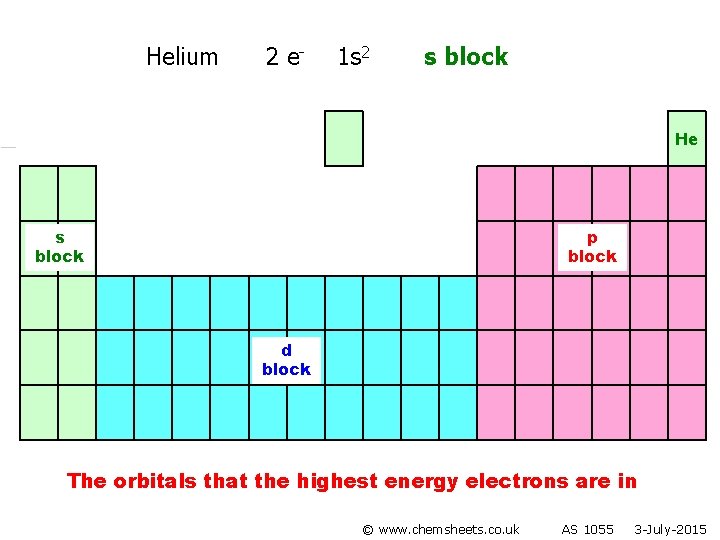
Helium 2 e- 1 s 2 s block He s block p block d block The orbitals that the highest energy electrons are in © www. chemsheets. co. uk AS 1055 3 -July-2015

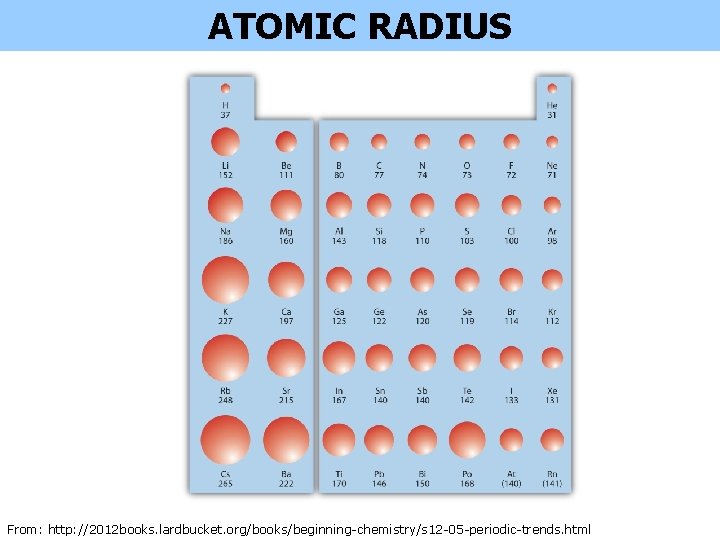
ATOMIC RADIUS From: http: //2012 books. lardbucket. org/books/beginning-chemistry/s 12 -05 -periodic-trends. html

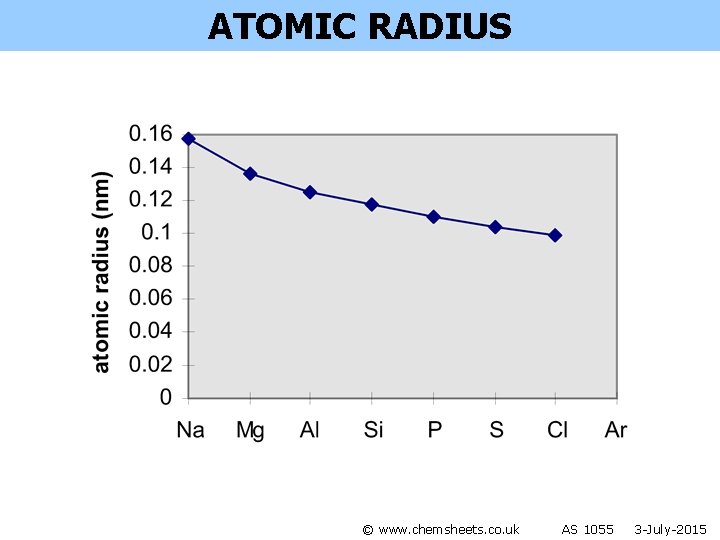
ATOMIC RADIUS © www. chemsheets. co. uk AS 1055 3 -July-2015

ATOMIC RADIUS Across a period: • outer electrons are in same shell • more protons in nucleus • same amount of shielding • so stronger attraction between nucleus and outer shell electrons • so outer shell electrons pulled closer to nucleus Na Mg Al Si P S © www. chemsheets. co. uk Cl AS 1055 3 -July-2015

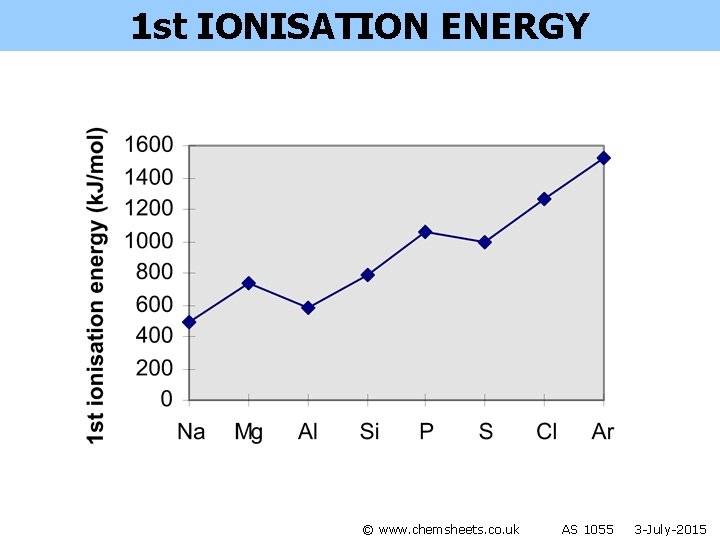
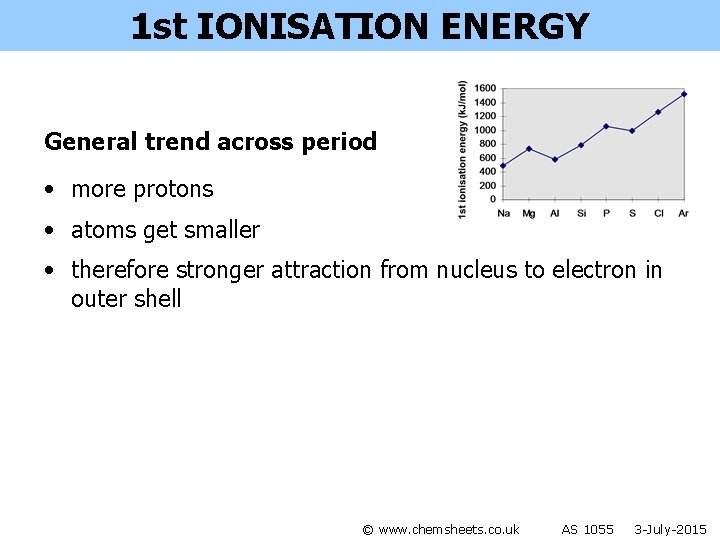
1 st IONISATION ENERGY © www. chemsheets. co. uk AS 1055 3 -July-2015

1 st IONISATION ENERGY General trend across period • more protons • atoms get smaller • therefore stronger attraction from nucleus to electron in outer shell © www. chemsheets. co. uk AS 1055 3 -July-2015

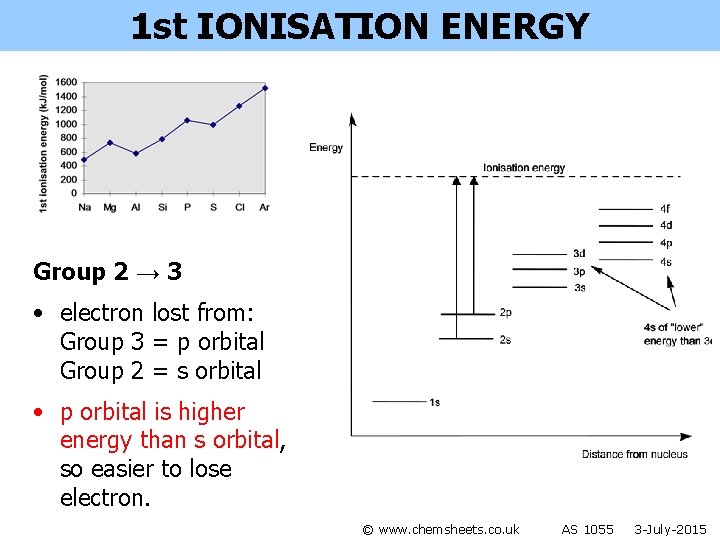
1 st IONISATION ENERGY Group 2 → 3 • electron lost from: Group 3 = p orbital Group 2 = s orbital • p orbital is higher energy than s orbital, so easier to lose electron. © www. chemsheets. co. uk AS 1055 3 -July-2015

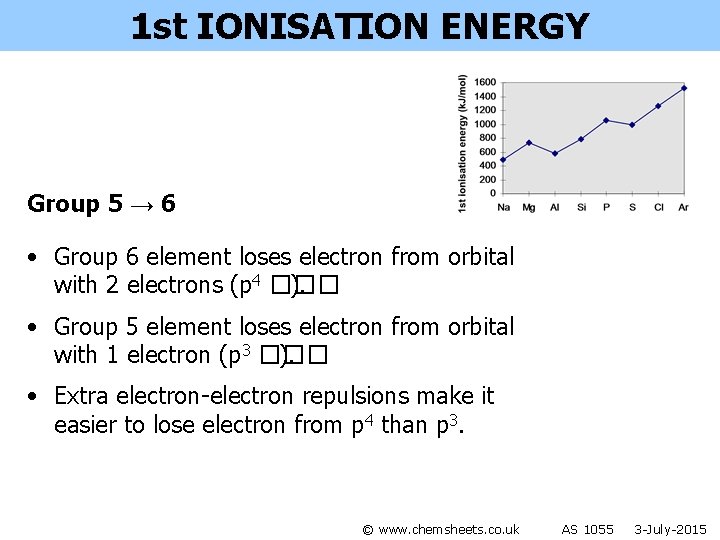
1 st IONISATION ENERGY Group 5 → 6 • Group 6 element loses electron from orbital with 2 electrons (p 4 ��� ). • Group 5 element loses electron from orbital with 1 electron (p 3 ��� ). • Extra electron-electron repulsions make it easier to lose electron from p 4 than p 3. © www. chemsheets. co. uk AS 1055 3 -July-2015

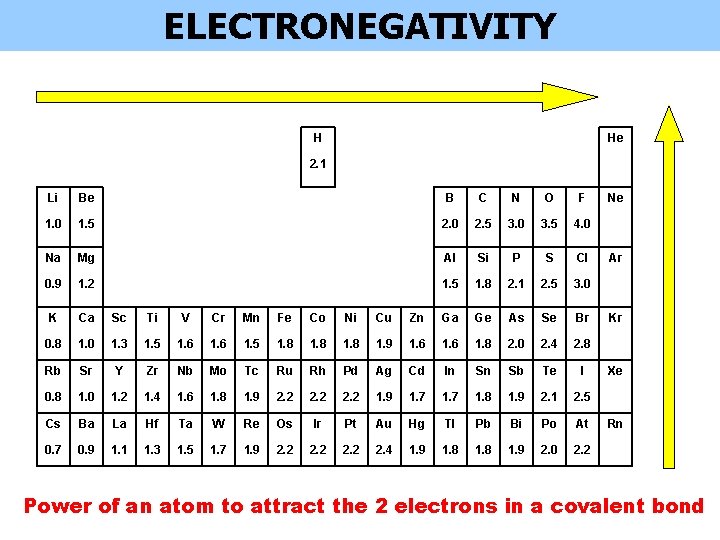
ELECTRONEGATIVITY H He 2. 1 Li Be B C N O F 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 Na Mg Al Si P S Cl 0. 9 1. 2 1. 5 1. 8 2. 1 2. 5 3. 0 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 0. 8 1. 0 1. 3 1. 5 1. 6 1. 5 1. 8 1. 9 1. 6 1. 8 2. 0 2. 4 2. 8 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I 0. 8 1. 0 1. 2 1. 4 1. 6 1. 8 1. 9 2. 2 1. 9 1. 7 1. 8 1. 9 2. 1 2. 5 Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At 0. 7 0. 9 1. 1 1. 3 1. 5 1. 7 1. 9 2. 2 2. 4 1. 9 1. 8 1. 9 2. 0 2. 2 Ne Ar Kr Xe Rn Power of an atom to attract the 2 electrons in a covalent bond

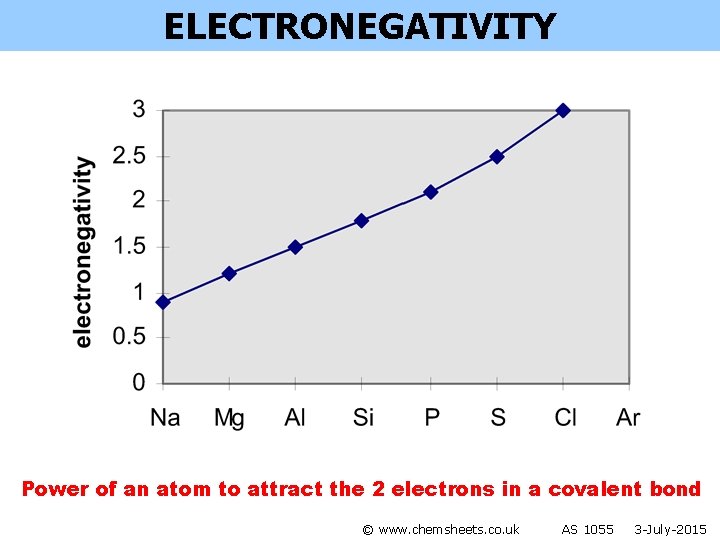
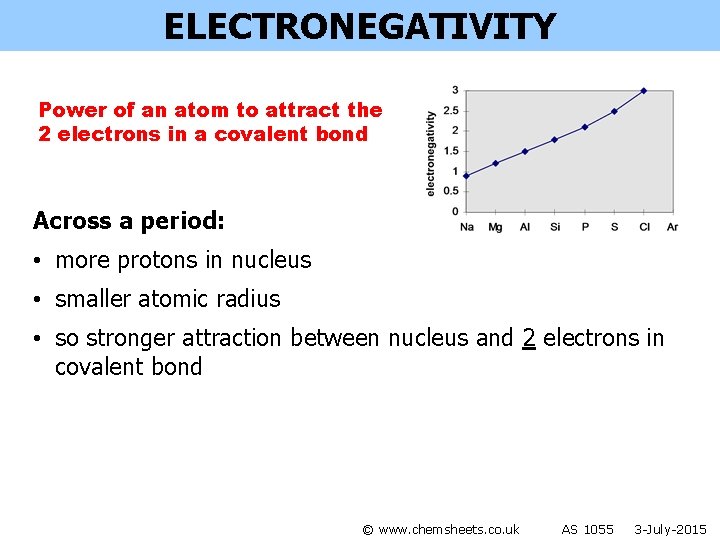
ELECTRONEGATIVITY Power of an atom to attract the 2 electrons in a covalent bond © www. chemsheets. co. uk AS 1055 3 -July-2015

ELECTRONEGATIVITY Power of an atom to attract the 2 electrons in a covalent bond Across a period: • more protons in nucleus • smaller atomic radius • so stronger attraction between nucleus and 2 electrons in covalent bond © www. chemsheets. co. uk AS 1055 3 -July-2015

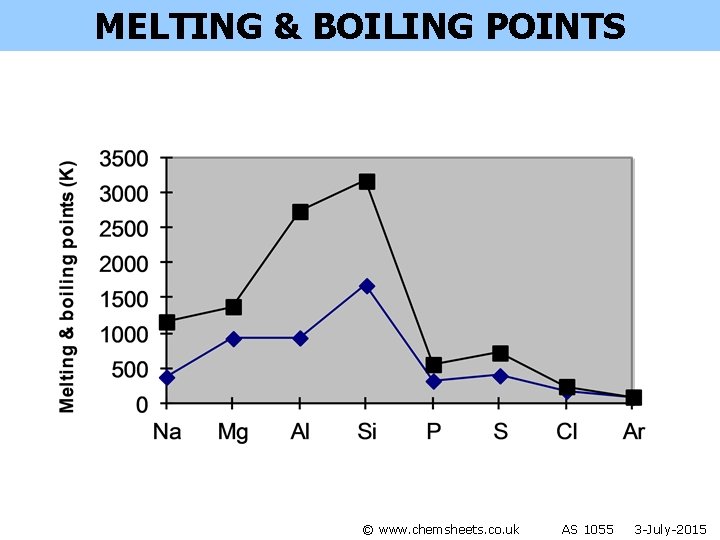
MELTING & BOILING POINTS © www. chemsheets. co. uk AS 1055 3 -July-2015

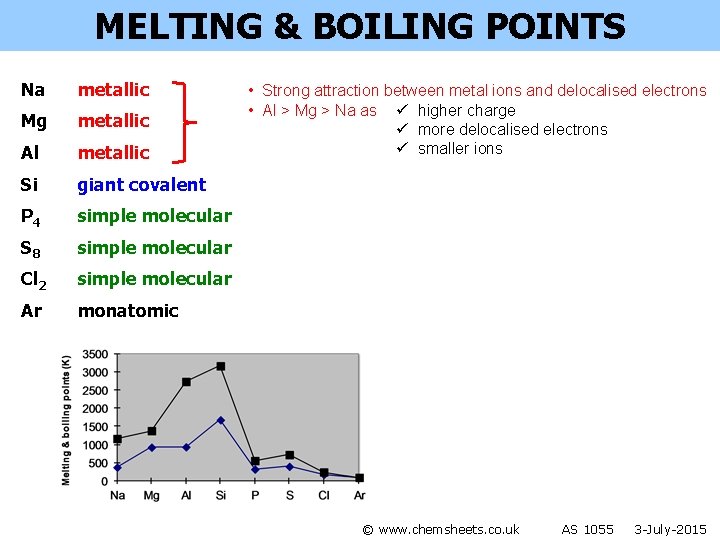
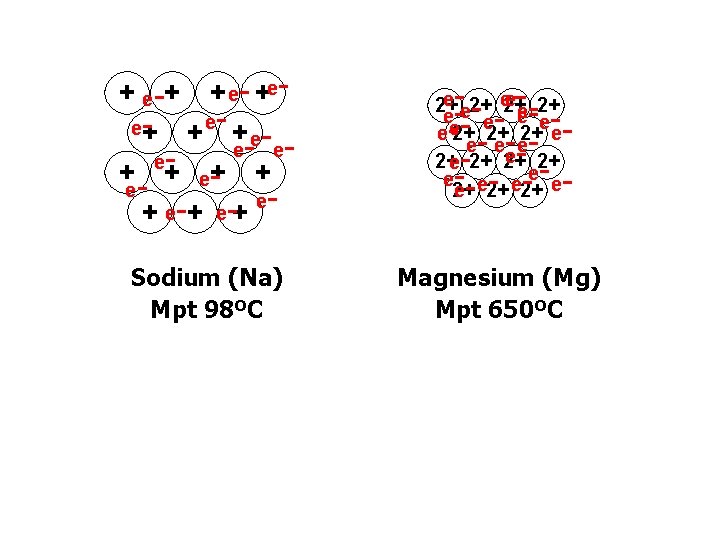
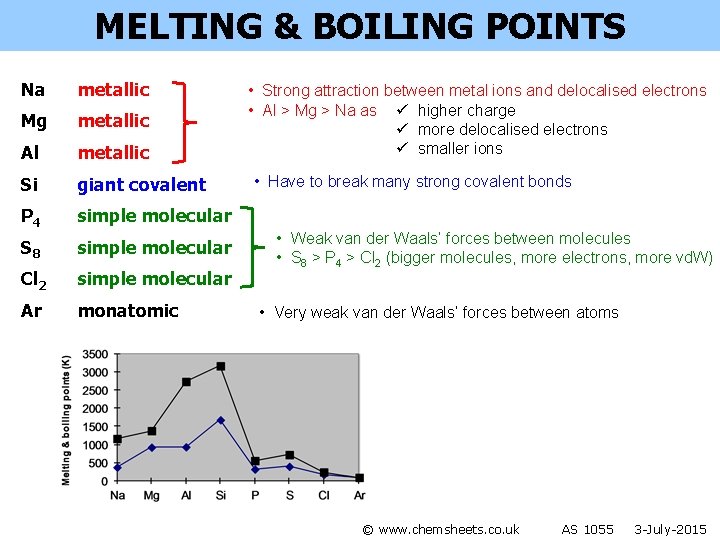
MELTING & BOILING POINTS Na metallic Mg metallic Al metallic Si giant covalent P 4 simple molecular S 8 simple molecular Cl 2 simple molecular Ar monatomic • Strong attraction between metal ions and delocalised electrons • Al > Mg > Na as ü higher charge ü more delocalised electrons ü smaller ions © www. chemsheets. co. uk AS 1055 3 -July-2015

+ e-+ + e- +ee+ - + ee- ee+ + e+ e-+ e- e 2+ 2+ e e e e 2+ 2+ 2+ e e ee e 2+ 2+ Sodium (Na) Mpt 98ºC Magnesium (Mg) Mpt 650ºC - - ---- - - -

MELTING & BOILING POINTS Na metallic Mg metallic Al metallic Si giant covalent P 4 simple molecular S 8 simple molecular Cl 2 simple molecular Ar monatomic • Strong attraction between metal ions and delocalised electrons • Al > Mg > Na as ü higher charge ü more delocalised electrons ü smaller ions • Have to break many strong covalent bonds • Weak van der Waals’ forces between molecules • S 8 > P 4 > Cl 2 (bigger molecules, more electrons, more vd. W) • Very weak van der Waals’ forces between atoms © www. chemsheets. co. uk AS 1055 3 -July-2015
 Chemsheets periodicity
Chemsheets periodicity Din 1055
Din 1055 Gaudium et spess art 3a
Gaudium et spess art 3a Canone 1055
Canone 1055 Orbital diagram for k
Orbital diagram for k Electronegativity periodic trend
Electronegativity periodic trend Caries risk assessment form texas medicaid
Caries risk assessment form texas medicaid Ap chemistry chapter 7
Ap chemistry chapter 7 Lymphatic filariasis
Lymphatic filariasis Monophagic adalah
Monophagic adalah Bright futures screening guidelines
Bright futures screening guidelines Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Electron configurations and periodicity
Electron configurations and periodicity Oxygen periodic trends
Oxygen periodic trends What is periodicity
What is periodicity Chemsheets polymers
Chemsheets polymers Chemsheets shapes of molecules
Chemsheets shapes of molecules Chemsheets reactions of alkenes 2 answers
Chemsheets reactions of alkenes 2 answers Chemsheets as 1036
Chemsheets as 1036 Reactions of alcohols 2 chemsheets answers
Reactions of alcohols 2 chemsheets answers Chemsheets shapes of molecules
Chemsheets shapes of molecules