Shapes of Molecules 4 2 7 Predict the











- Slides: 26

Shapes of Molecules

• 4. 2. 7: Predict the shape and bond angles for species with four, three and two negative charge centres on the central atom using the valence shell pair repulsion theory (VESPR).

• Before you start it would be helpful to… • know the definition of a covalent bond • know what a lone pair is • know that like charges repel A lone pair is a pair of electrons in the valence shell of an atom, which are not used to form covalent bonds. We came across them when we did Lewis structures!

• Covalent molecules are not flat – even though that’s how we usually draw them. • They exist in 3 dimensions and have specific shapes. • We can see these shapes using computer simulations, or by making models using Molymod kits.


• During exams(!) we won’t have either of these methods available, so we have to use VSEPR theory to predict the shape of the molecule. • We then use certain conventions to draw the molecule.


VSEPR • This means “valence shell electron pair repulsion” • You can probably see why we just use the initials. . . • BUT it does tell us that the shape of a molecule is due to the number of electron pairs in the outer (valence!) shell.

• Its best to learn the principles behind this. • You can worry about all the fancy words later! • It is possible to consider the shape around any atom in a (covalently bonded) molecule • But at IB level they will usually only ask questions where its obvious which atom they want you to consider.


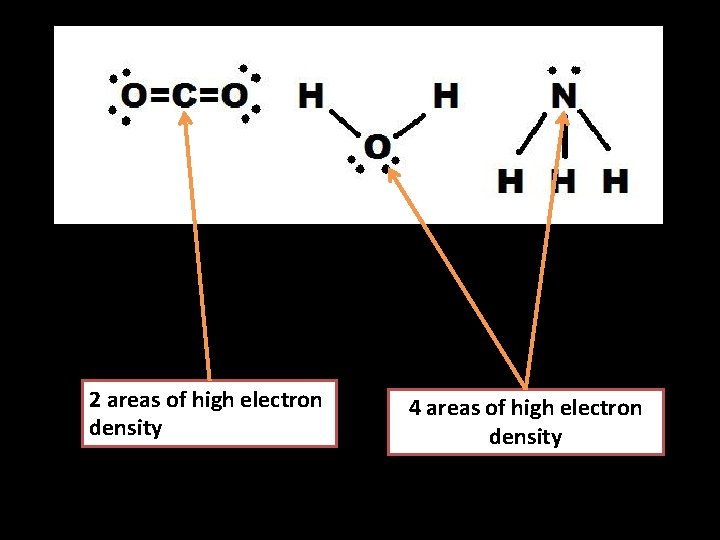
• The first step is to figure out how many areas of “high electron density” there around the atom we’re interested in. • This means IF IN DOUBT DRAW A LEWIS STRUCTURE! • Areas of high electron density could be a covalent bond, a double or triple bond or a lone pair of electrons. • Notice that double and triple bonds are only ONE area of high electron density.

2 areas of high electron density 4 areas of high electron density

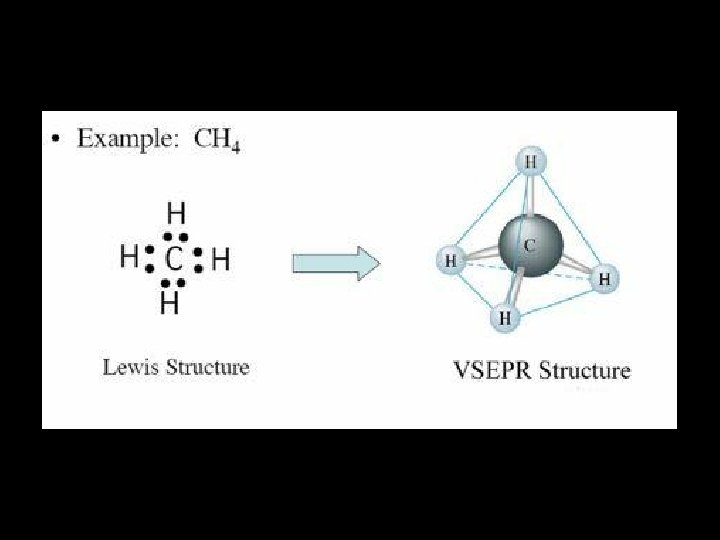
• Because like charges repel, • Areas of high electron density try to get as far apart as possible. • Because valence shells usually have 8 electrons, • The commonest case is 4 areas of HED • This gives a tetrahedron


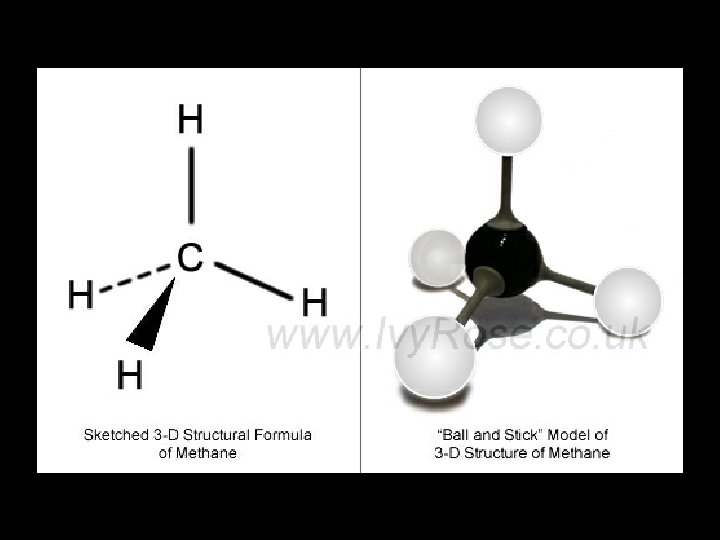
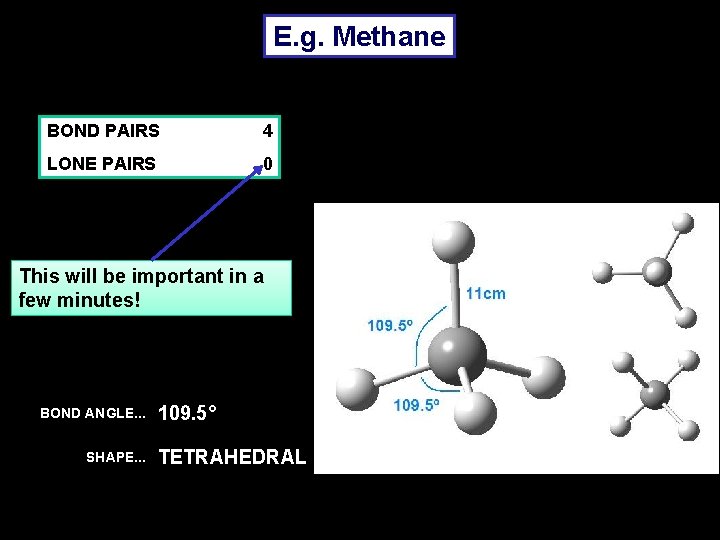
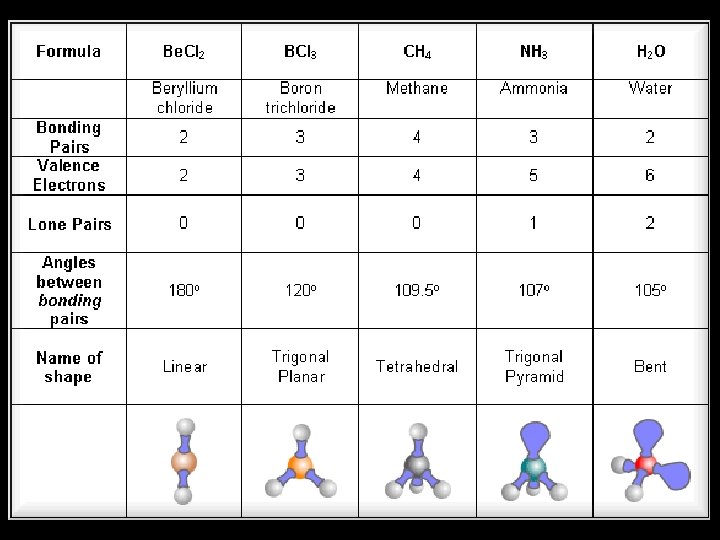
E. g. Methane BOND PAIRS 4 LONE PAIRS 0 This will be important in a few minutes! BOND ANGLE. . . SHAPE. . . 109. 5° TETRAHEDRAL

• The bond angle of 109. 5 degrees is fixed as a property of a tetrahedron. • Where a central molecule is surrounded by 4 bonding electron pairs, the bond angle is ALWAYS 109. 5 degrees. • However, sometimes one of the areas of HED will be a lone pair. • Lone pairs are closer to the central atom and repel the bonding electrons a bit more strongly.

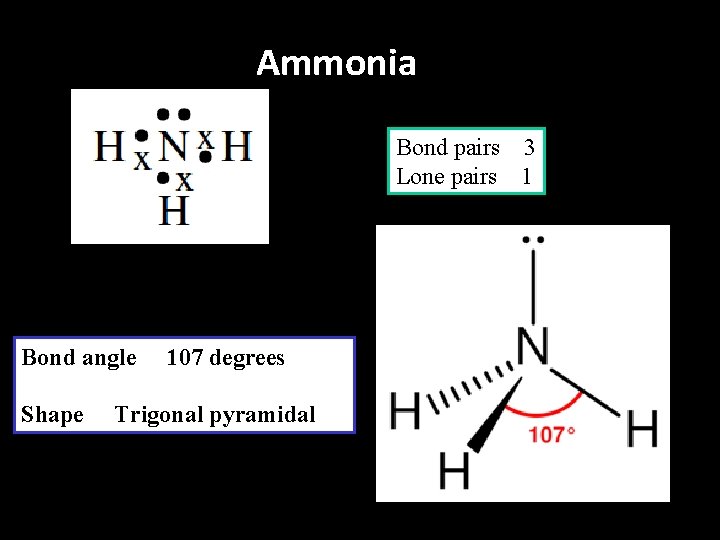
Ammonia Bond pairs 3 Lone pairs 1 Bond angle Shape 107 degrees Trigonal pyramidal

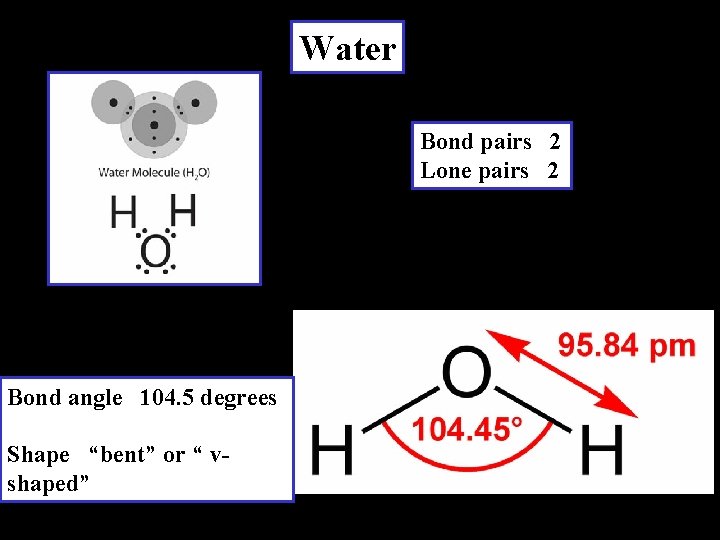
• If there are 2 lone pairs of electrons, the bonds are forced even closer together.

Water Bond pairs 2 Lone pairs 2 Bond angle 104. 5 degrees Shape “bent” or “ vshaped”

• Its important to keep in mind that the shapes we have just discussed are all just variations on a tetrahedron. • Its just that lone pair electrons make part of the tetrahedron “invisible”! • If you know the science involved, you can work out the bond angles. • Don’t stress too much about learning the names of the shapes. • You will need to do it, but it shouldn’t be too hard.

• Exactly the same science is involved for shapes which aren’t based on a tetrahedron.

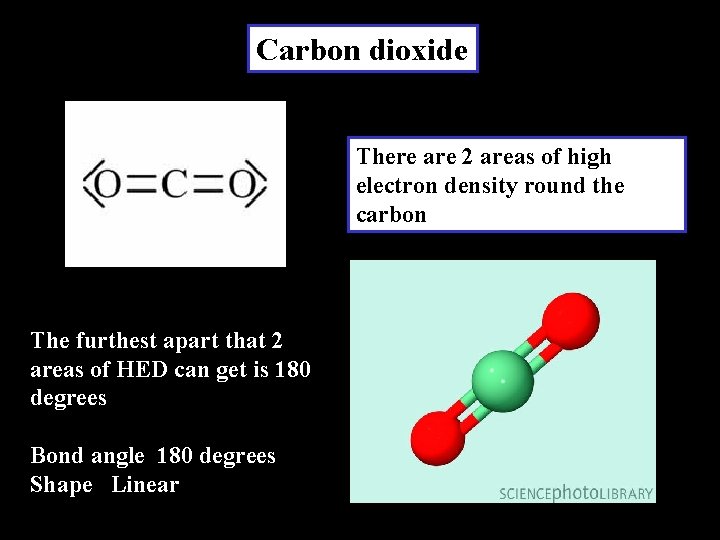
Carbon dioxide There are 2 areas of high electron density round the carbon The furthest apart that 2 areas of HED can get is 180 degrees Bond angle 180 degrees Shape Linear

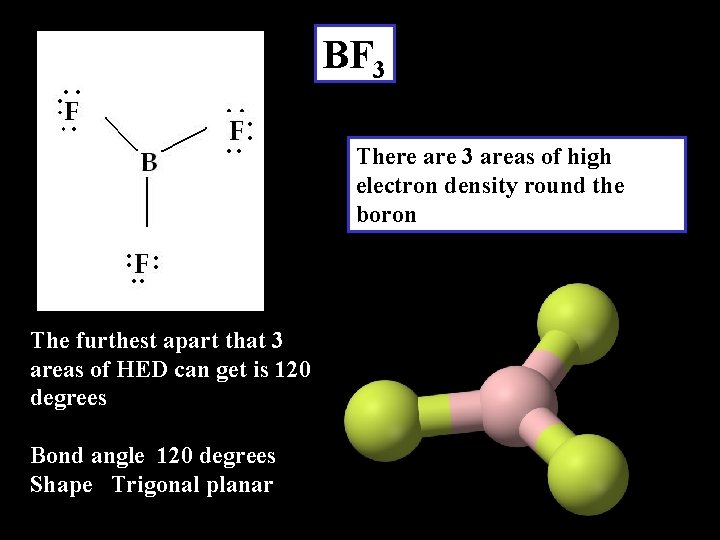
BF 3 There are 3 areas of high electron density round the boron The furthest apart that 3 areas of HED can get is 120 degrees Bond angle 120 degrees Shape Trigonal planar

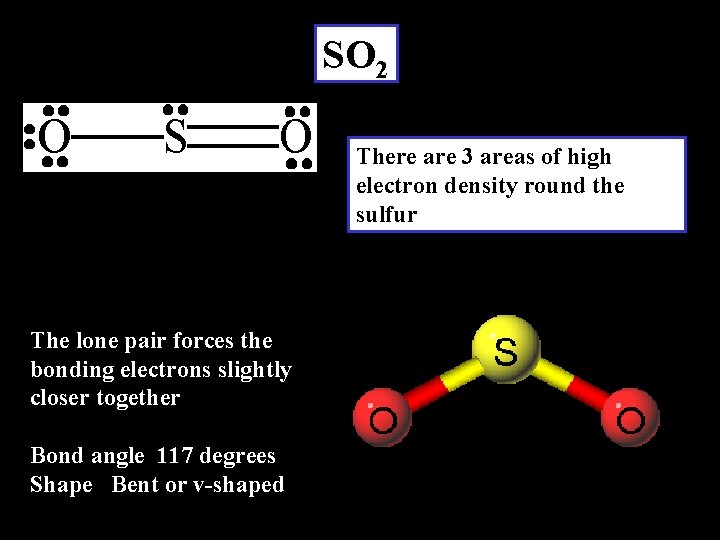
SO 2 There are 3 areas of high electron density round the sulfur The lone pair forces the bonding electrons slightly closer together Bond angle 117 degrees Shape Bent or v-shaped

• These are the only shapes that SL students need to know! • HL need a few more! • Notice that in some cases the actual bond angles given are only approximate! • The “real” angle changes depending on the environment.
