CHEMSHEETS GROUP 2 SULFATES HYDROXIDES www chemsheets co







- Slides: 20

CHEMSHEETS GROUP 2 – SULFATES & HYDROXIDES © www. chemsheets. co. uk AS 1060 3 -July-15

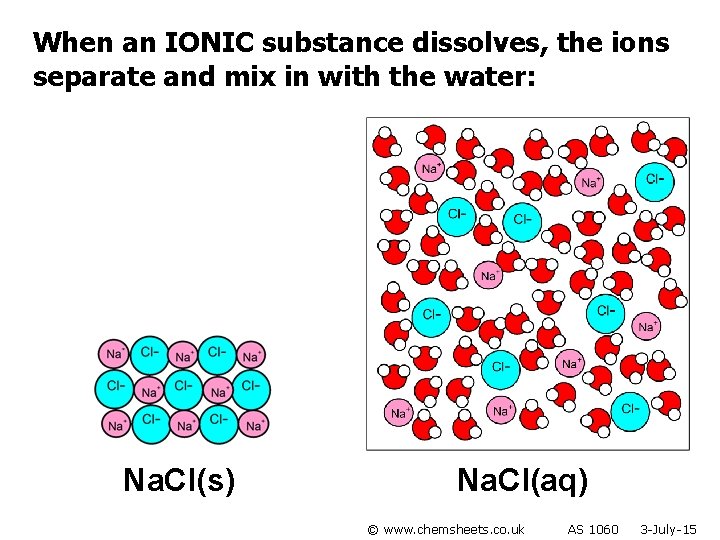
When an IONIC substance dissolves, the ions separate and mix in with the water: Na. Cl(s) Na. Cl(aq) © www. chemsheets. co. uk AS 1060 3 -July-15

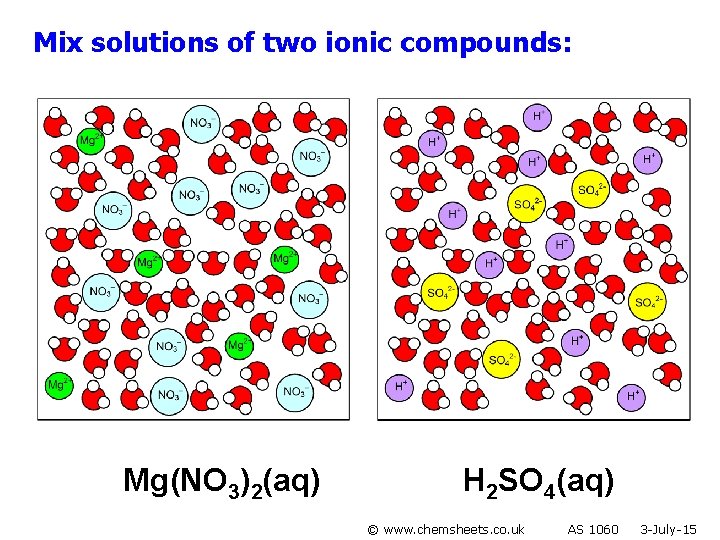
Mix solutions of two ionic compounds: Mg(NO 3)2(aq) H 2 SO 4(aq) © www. chemsheets. co. uk AS 1060 3 -July-15

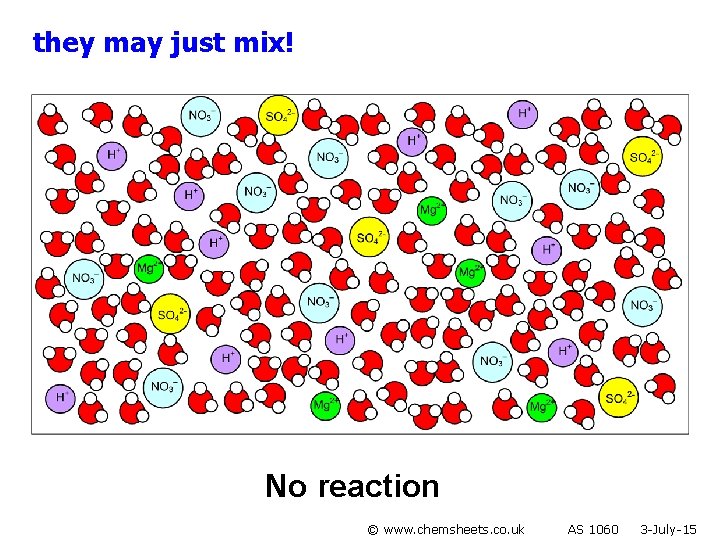
they may just mix! No reaction © www. chemsheets. co. uk AS 1060 3 -July-15

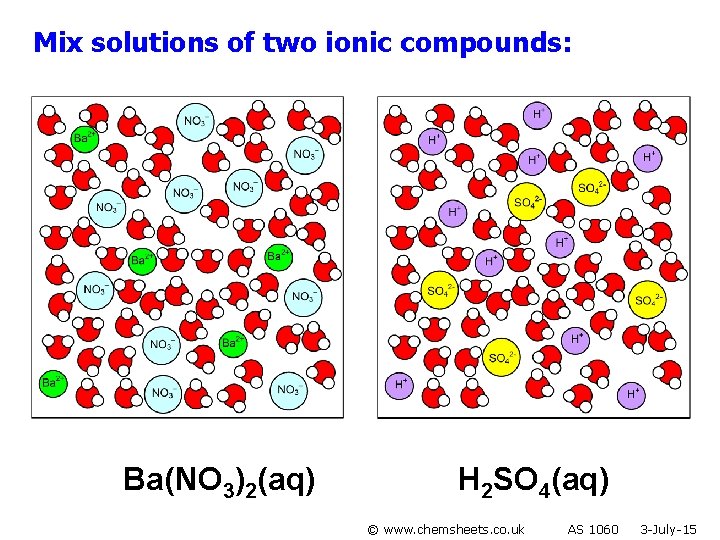
Mix solutions of two ionic compounds: Ba(NO 3)2(aq) H 2 SO 4(aq) © www. chemsheets. co. uk AS 1060 3 -July-15

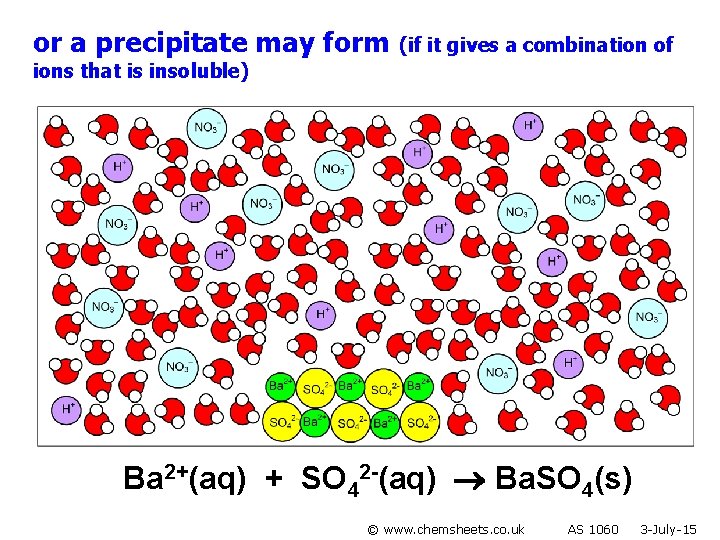
or a precipitate may form (if it gives a combination of ions that is insoluble) Ba 2+(aq) + SO 42 -(aq) Ba. SO 4(s) © www. chemsheets. co. uk AS 1060 3 -July-15

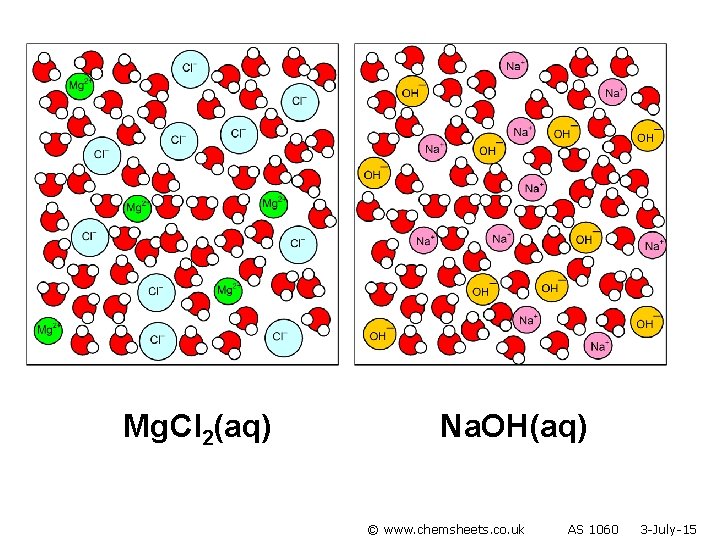
Mg. Cl 2(aq) Na. OH(aq) © www. chemsheets. co. uk AS 1060 3 -July-15

Mg 2+(aq) + 2 OH–(aq) Mg(OH)2 (s) © www. chemsheets. co. uk AS 1060 3 -July-15

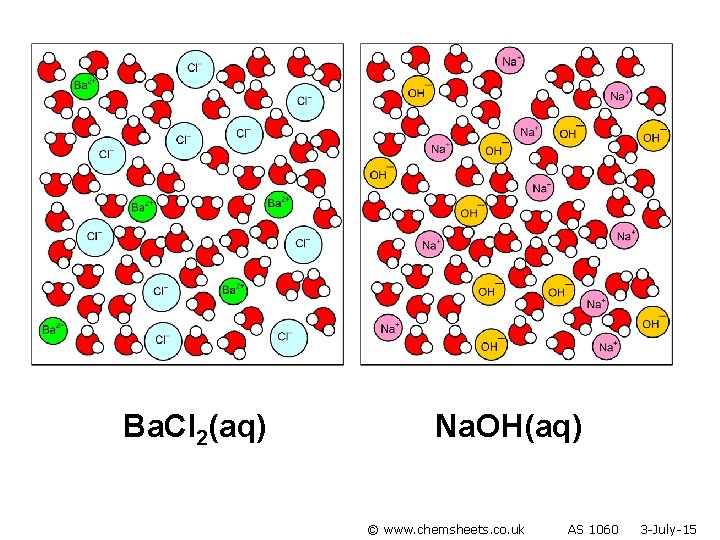
Ba. Cl 2(aq) Na. OH(aq) © www. chemsheets. co. uk AS 1060 3 -July-15

No reaction © www. chemsheets. co. uk AS 1060 3 -July-15

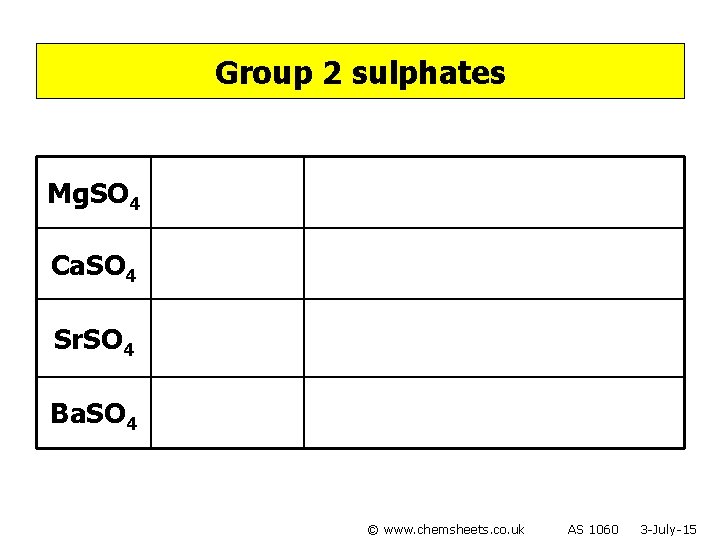
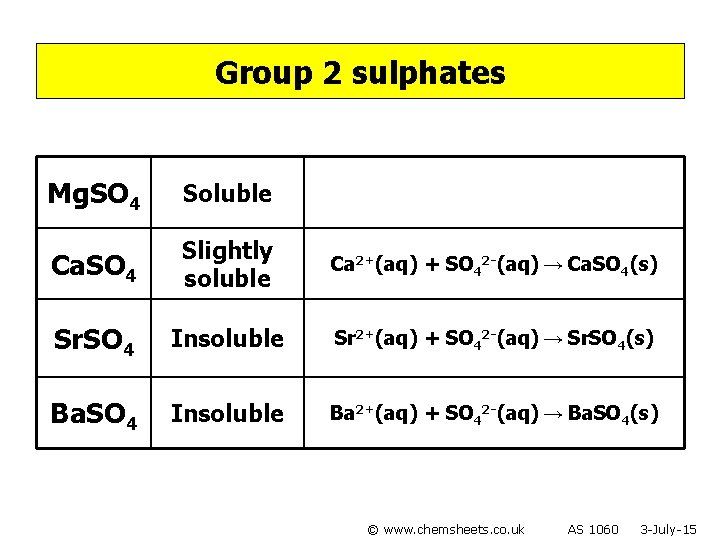
Group 2 sulphates Mg. SO 4 Ca. SO 4 Sr. SO 4 Ba. SO 4 © www. chemsheets. co. uk AS 1060 3 -July-15

Group 2 sulphates Mg. SO 4 Soluble Ca. SO 4 Slightly soluble Sr. SO 4 Insoluble Ba. SO 4 Insoluble © www. chemsheets. co. uk AS 1060 3 -July-15

Group 2 sulphates Mg. SO 4 Soluble Ca. SO 4 Slightly soluble Ca 2+(aq) + SO 42 -(aq) → Ca. SO 4(s) Sr. SO 4 Insoluble Sr 2+(aq) + SO 42 -(aq) → Sr. SO 4(s) Ba. SO 4 Insoluble Ba 2+(aq) + SO 42 -(aq) → Ba. SO 4(s) © www. chemsheets. co. uk AS 1060 3 -July-15

Group 2 hydroxides Mg(OH)2 Ca(OH)2 Sr(OH)2 Ba(OH)2 © www. chemsheets. co. uk AS 1060 3 -July-15

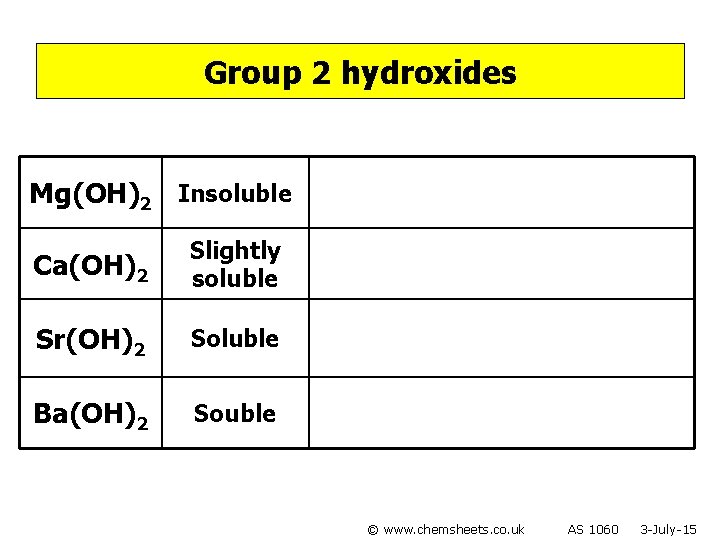
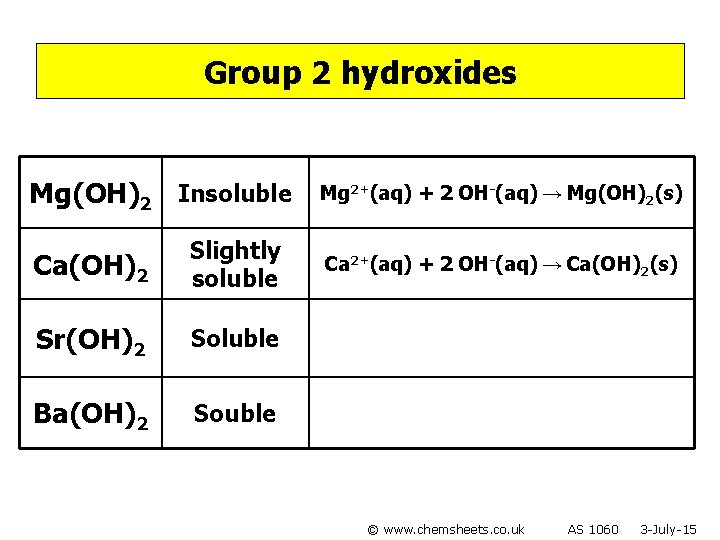
Group 2 hydroxides Mg(OH)2 Insoluble Ca(OH)2 Slightly soluble Sr(OH)2 Soluble Ba(OH)2 Souble © www. chemsheets. co. uk AS 1060 3 -July-15

Group 2 hydroxides Mg(OH)2 Insoluble Ca(OH)2 Slightly soluble Sr(OH)2 Soluble Ba(OH)2 Souble Mg 2+(aq) + 2 OH-(aq) → Mg(OH)2(s) Ca 2+(aq) + 2 OH-(aq) → Ca(OH)2(s) © www. chemsheets. co. uk AS 1060 3 -July-15

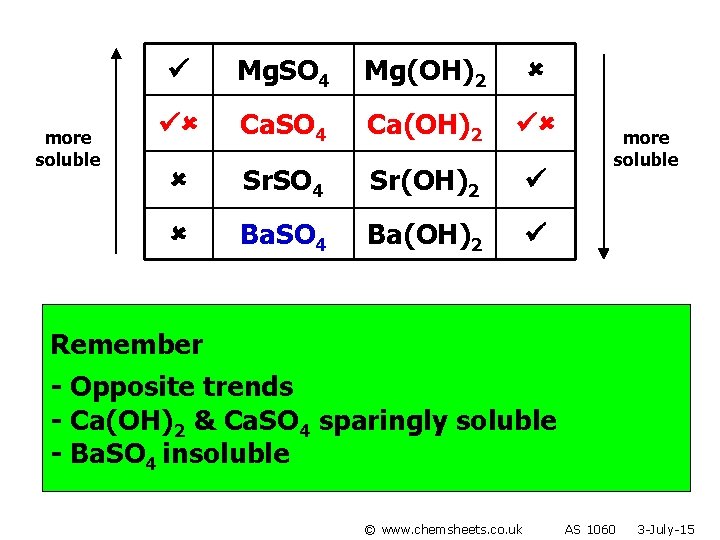
more soluble Mg. SO 4 Mg(OH)2 Ca. SO 4 Ca(OH)2 Sr. SO 4 Sr(OH)2 Ba. SO 4 Ba(OH)2 more soluble Remember - Opposite trends - Ca(OH)2 & Ca. SO 4 sparingly soluble - Ba. SO 4 insoluble © www. chemsheets. co. uk AS 1060 3 -July-15

Barium meal Ba. SO 4 © www. chemsheets. co. uk AS 1061 3 -July-15

Milk of magnesia Mg(OH)2 © www. chemsheets. co. uk AS 1061 3 -July-15

Slaked lime © www. chemsheets. co. uk Ca(OH)2 AS 1061 3 -July-15