Do Now 1 What can aliphatic amines act









- Slides: 19

Do Now 1. What can aliphatic amines act as? 2. Do you think aliphatic and aromatic amines behave the same or differently? Explain why.

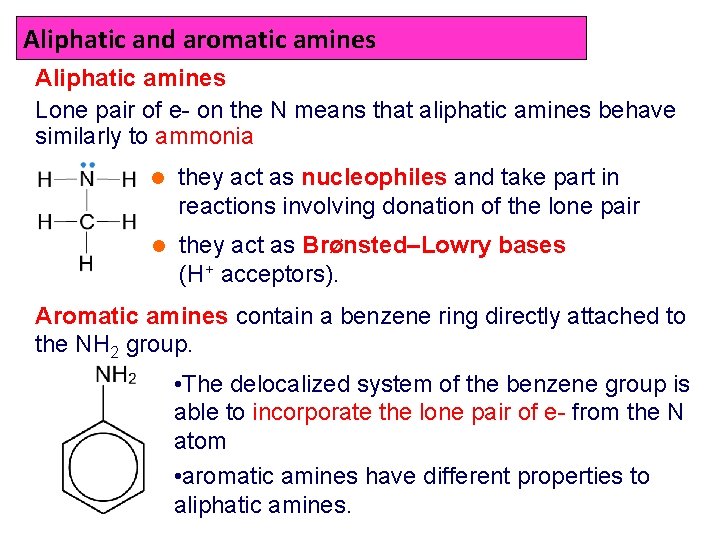
Aliphatic and aromatic amines Aliphatic amines Lone pair of e- on the N means that aliphatic amines behave similarly to ammonia l they act as nucleophiles and take part in reactions involving donation of the lone pair l they act as Brønsted–Lowry bases (H+ acceptors). Aromatic amines contain a benzene ring directly attached to the NH 2 group. • The delocalized system of the benzene group is able to incorporate the lone pair of e- from the N atom • aromatic amines have different properties to aliphatic amines.

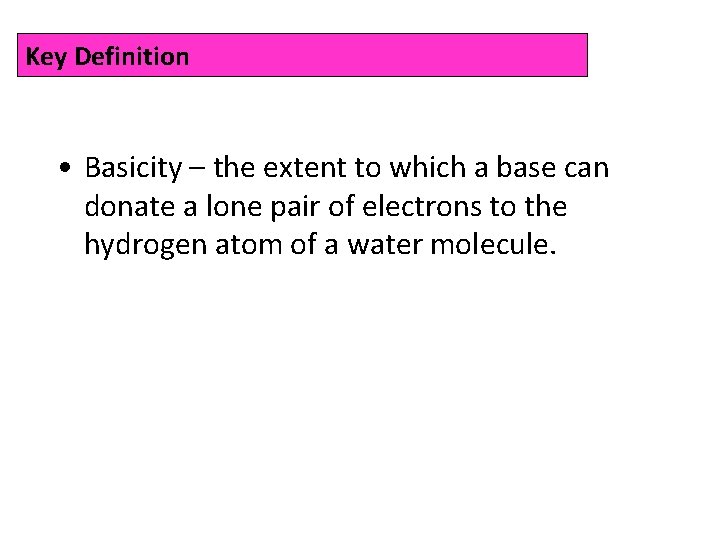
Key Definition • Basicity – the extent to which a base can donate a lone pair of electrons to the hydrogen atom of a water molecule.

Reactions with water • Can amines dissolve in water? • If so, which ones? • Why can they/can’t they?

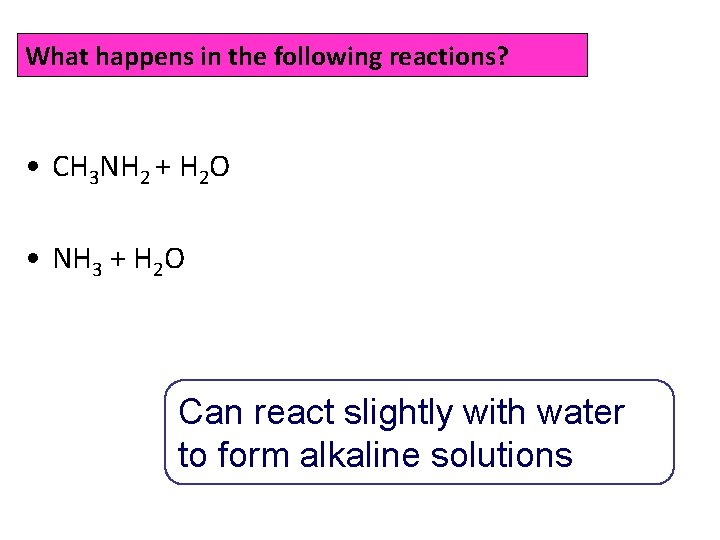
What happens in the following reactions? • CH 3 NH 2 + H 2 O • NH 3 + H 2 O Can react slightly with water to form alkaline solutions

Think back to electrophilic addition…. What does the term “inductive effect” mean? How do we show it?

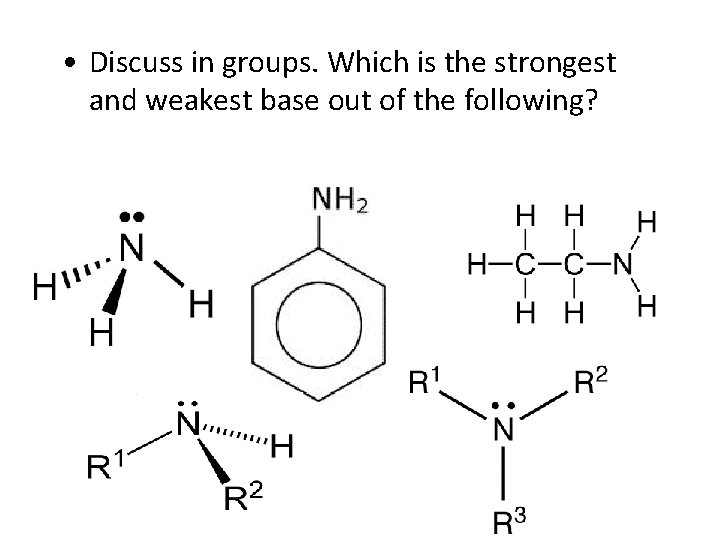
• Discuss in groups. Which is the strongest and weakest base out of the following?

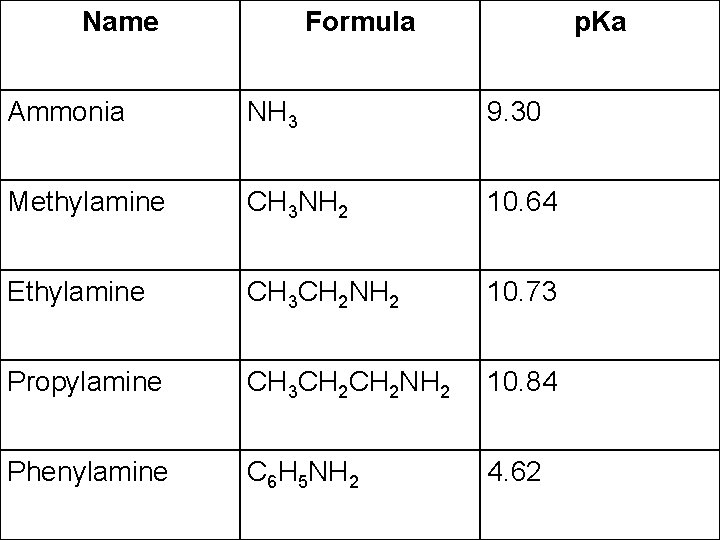
Name Formula p. Ka Ammonia NH 3 9. 30 Methylamine CH 3 NH 2 10. 64 Ethylamine CH 3 CH 2 NH 2 10. 73 Propylamine CH 3 CH 2 NH 2 10. 84 Phenylamine C 6 H 5 NH 2 4. 62

Base strength Two of the factors which influence the strength of a base are: • the ease with which the lone pair picks up a hydrogen ion, • the stability of the ions being formed.

• Alkyl groups have a tendency to "push" electrons away from themselves. • That means that there will be a small amount of extra negative charge built up on the nitrogen atom. • That extra negativity around the nitrogen makes the lone pair even more attractive towards hydrogen ions.

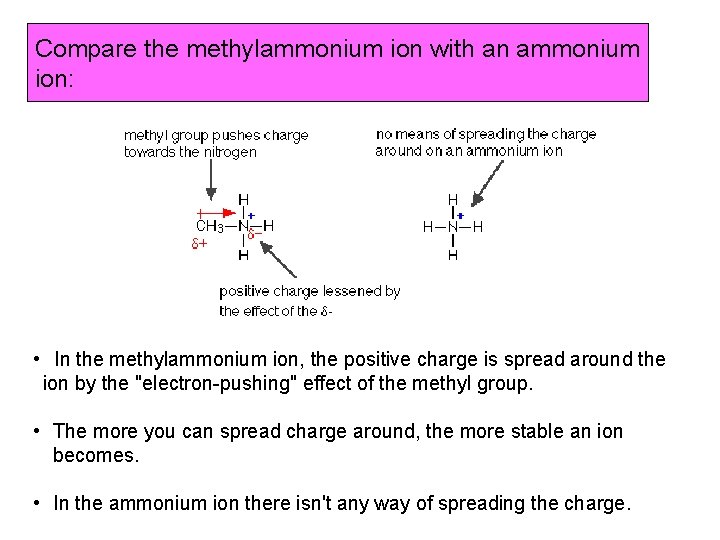
Compare the methylammonium ion with an ammonium ion: • In the methylammonium ion, the positive charge is spread around the ion by the "electron-pushing" effect of the methyl group. • The more you can spread charge around, the more stable an ion becomes. • In the ammonium ion there isn't any way of spreading the charge.

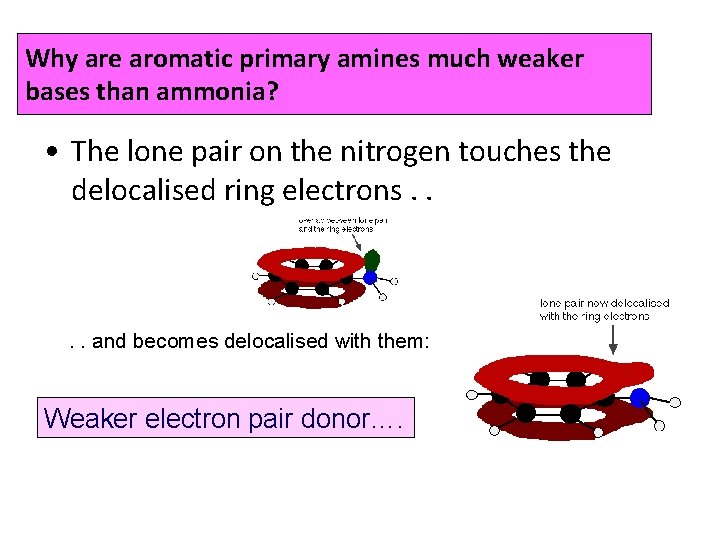
Why are aromatic primary amines much weaker bases than ammonia? • The lone pair on the nitrogen touches the delocalised ring electrons. . and becomes delocalised with them: Weaker electron pair donor….

Complete Task 1 of Amines Chemsheets


Questions 1. Write out the equation for the reaction between propylamine and water 2. Explain why this reaction can occur 3. Explain why it is reversible 4. What do you think will happen when an amine reacts with a strong acid?

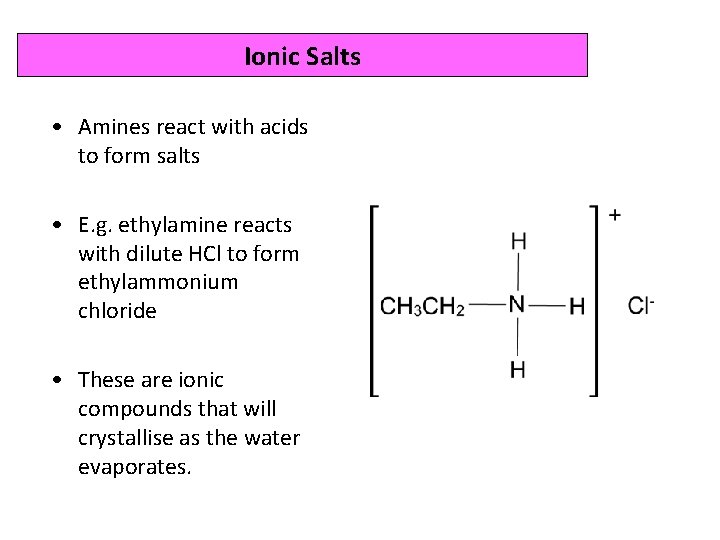
Reactions of amines as bases Amines accept protons (H+) from acids to form salts: ethylamine ethylammonium chloride

Ionic Salts • Amines react with acids to form salts • E. g. ethylamine reacts with dilute HCl to form ethylammonium chloride • These are ionic compounds that will crystallise as the water evaporates.

Questions 1. What will form when propylamine reacts with hydrochloric acid? 2. What will form when ethylamine reacts with nitric acid? 3. What will form when methylamine reacts with sulfuric acid?

Exam Questions