CHEMSHEETS ELECTRON ARRANGEMENT www chemsheets co uk AS







- Slides: 17

CHEMSHEETS ELECTRON ARRANGEMENT © www. chemsheets. co. uk AS 1009 3 -Jun-2015

Shells, sub-shells & orbitals • Electrons are arranged in electrons shells (energy levels). • The shells have sub-shells (sub-levels). • Each shell/sub-shell is made up of electron orbitals which can each hold 2 electrons. © www. chemsheets. co. uk AS 1009 3 -Jun-2015

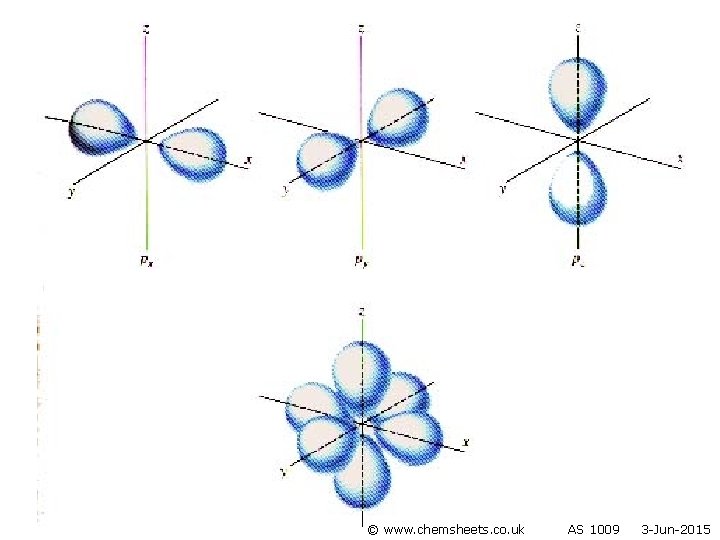
Orbitals • Each sub-level consists of electron orbitals (region of space in which the electron spends most of its time). • Each orbital can hold 2 electrons with opposite spins (one electron spins clockwise and one anticlockwise). • Orbitals are regions of space that electrons are most likely to be in. © www. chemsheets. co. uk AS 1009 3 -Jun-2015

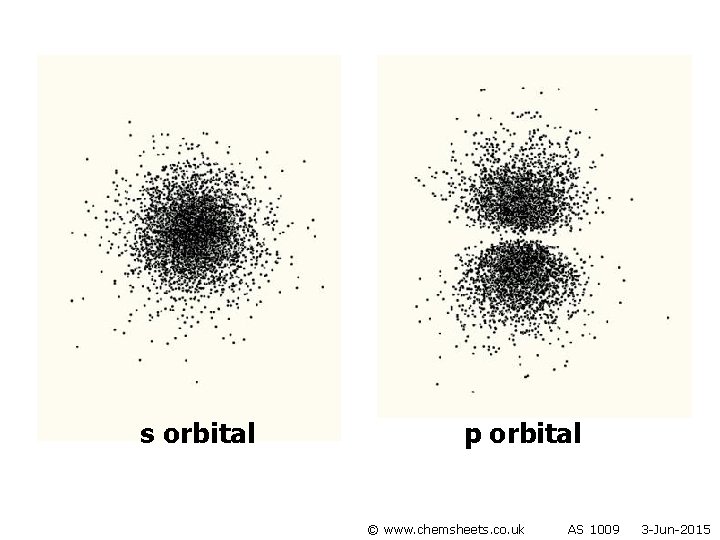
s orbital p orbital © www. chemsheets. co. uk AS 1009 3 -Jun-2015

© www. chemsheets. co. uk AS 006 19 -Feb-12

© www. chemsheets. co. uk AS 1009 3 -Jun-2015

The Orbitron http: //winter. group. shef. ac. uk/orbitron/AOs/1 s/index. html © www. chemsheets. co. uk AS 006 19 -Feb-12

© www. chemsheets. co. uk AS 006 19 -Feb-12

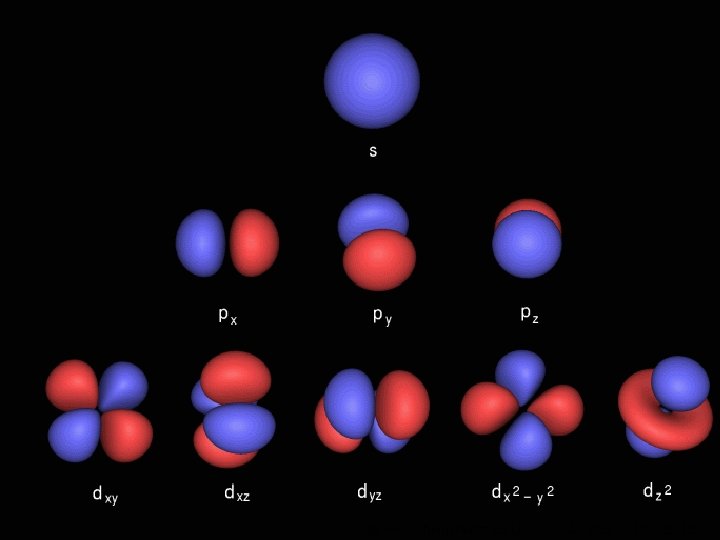
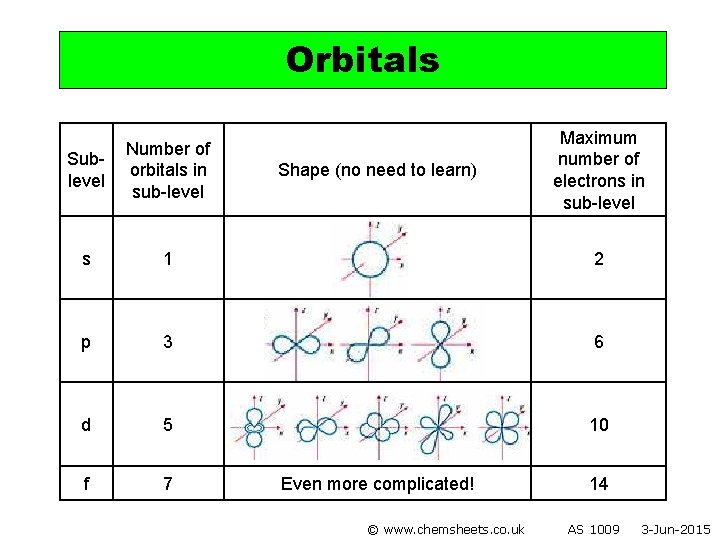
Orbitals Maximum number of electrons in sub-level Sublevel Number of orbitals in sub-level s 1 2 p 3 6 d 5 10 f 7 Shape (no need to learn) Even more complicated! © www. chemsheets. co. uk 14 AS 1009 3 -Jun-2015

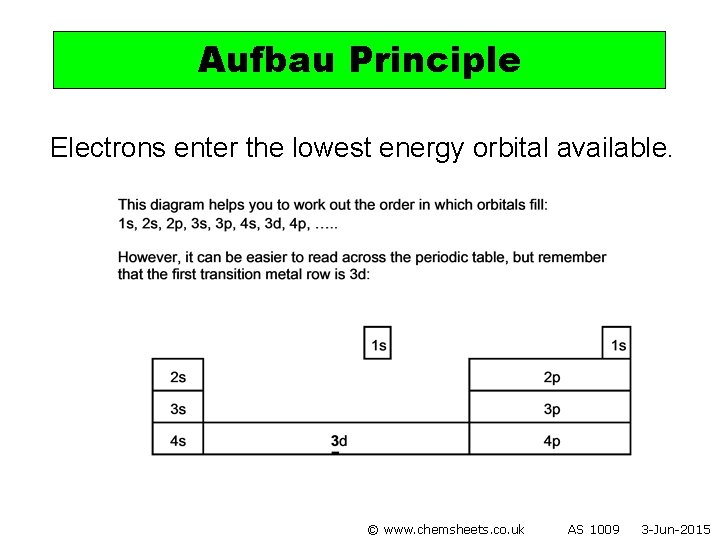
Aufbau Principle Electrons enter the lowest energy orbital available. © www. chemsheets. co. uk AS 1009 3 -Jun-2015

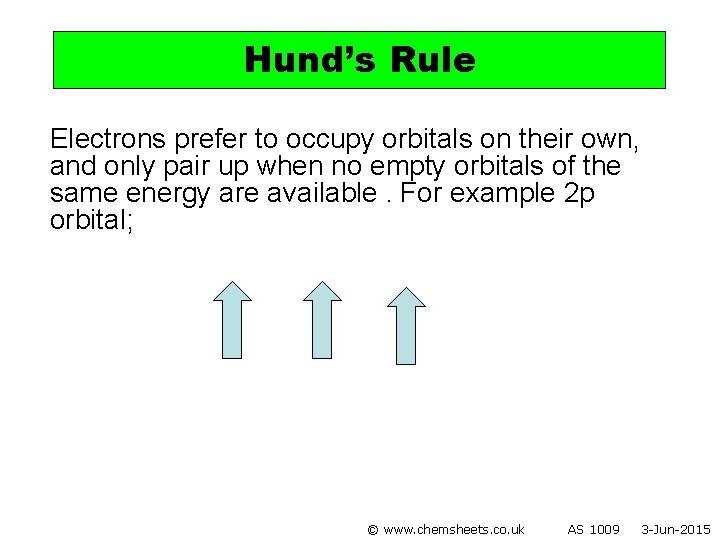
Hund’s Rule Electrons prefer to occupy orbitals on their own, and only pair up when no empty orbitals of the same energy are available. For example 2 p orbital; © www. chemsheets. co. uk AS 1009 3 -Jun-2015

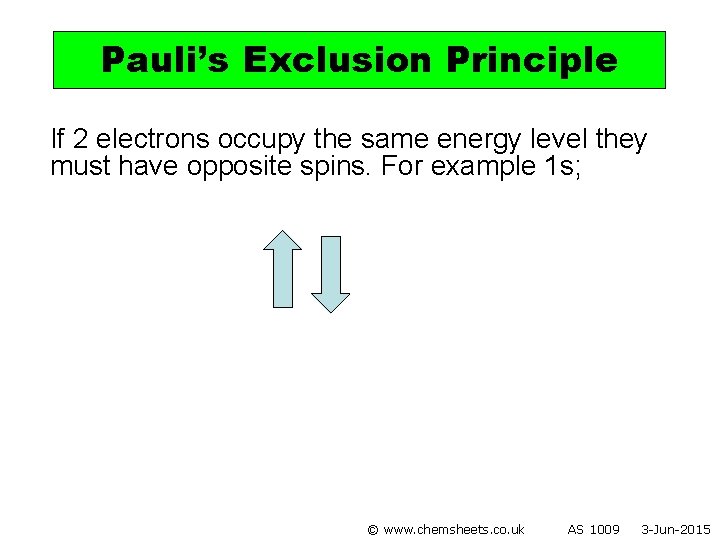
Pauli’s Exclusion Principle If 2 electrons occupy the same energy level they must have opposite spins. For example 1 s; © www. chemsheets. co. uk AS 1009 3 -Jun-2015

e. g. silicon 14 e- 1 s 2 2 p 6 3 s 2 3 p 2

e. g. calcium 20 e- 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2

Ions • The highest energy electrons are lost when an ion is formed. • Note that 4 s electrons are lost before 3 d (as once 4 s and 3 d are occupied, 4 s moves above 3 d). © www. chemsheets. co. uk AS 1009 3 -Jun-2015

e. g. Ca 2+ 18 e- 1 s 2 2 p 6 3 s 2 3 p 6 © www. chemsheets. co. uk AS 1009 3 -Jun-2015

Cu & Cr • Cu and Cr do not have the expected electron structure. Cr = 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 NOT 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 4 Cu = 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10 NOT 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 9 © www. chemsheets. co. uk AS 1009 3 -Jun-2015
 Tetrachloromethane electron arrangement
Tetrachloromethane electron arrangement Orbital p
Orbital p Magnesium ion electron configuration
Magnesium ion electron configuration Chemsheets group 7 halogens answers
Chemsheets group 7 halogens answers Chemsheets as 1052
Chemsheets as 1052 Steric number
Steric number Chemsheets shapes of molecules
Chemsheets shapes of molecules Chemsheets reactions of halogenoalkanes 2
Chemsheets reactions of halogenoalkanes 2 Www.chemsheets
Www.chemsheets Chemsheets a2 1102 amino acids 2 answers
Chemsheets a2 1102 amino acids 2 answers Chemsheets shapes of molecules
Chemsheets shapes of molecules Aliphatic amines
Aliphatic amines Addition polymers 1 chemsheets
Addition polymers 1 chemsheets Chemsheets as 1079
Chemsheets as 1079 Butanone and acidified kcn
Butanone and acidified kcn Chemsheets
Chemsheets Chemsheets
Chemsheets Chemsheets periodicity
Chemsheets periodicity