CHEMSHEETS ALDEHYDES KETONES www chemsheets co uk A

![OXIDATION & REDUCTION • Tollen’s reagent, contains [Ag(NH 3)2]+ • Used to test for OXIDATION & REDUCTION • Tollen’s reagent, contains [Ag(NH 3)2]+ • Used to test for](https://slidetodoc.com/presentation_image_h/cf90bf23fa2a54b9f1f7e4873fbb2a2f/image-5.jpg)





- Slides: 17

CHEMSHEETS ALDEHYDES & KETONES © www. chemsheets. co. uk A 2 1049 2 -June-2016

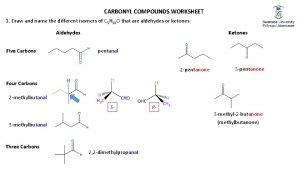
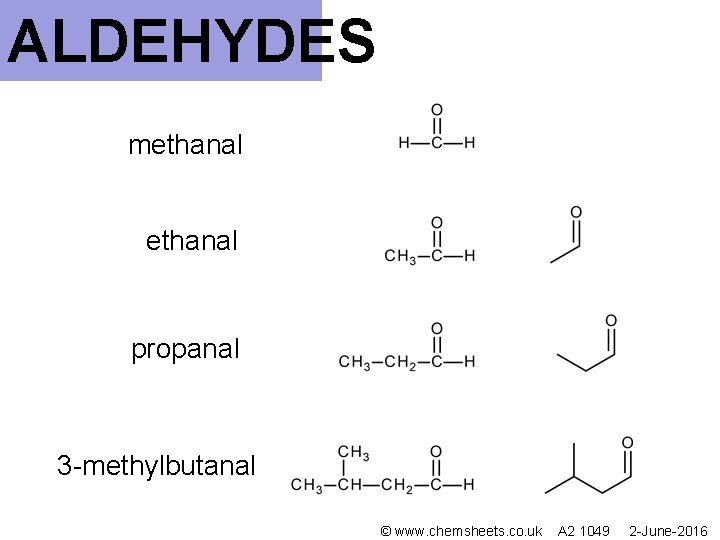
ALDEHYDES methanal propanal 3 -methylbutanal © www. chemsheets. co. uk A 2 1049 2 -June-2016

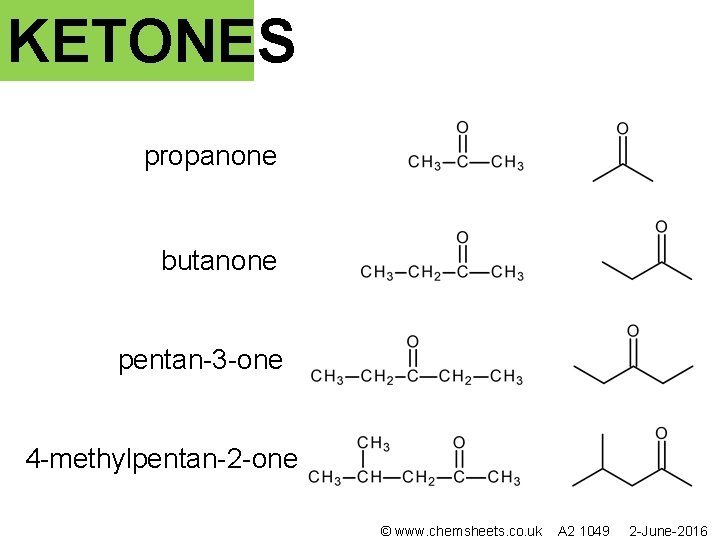
KETONES propanone butanone pentan-3 -one 4 -methylpentan-2 -one © www. chemsheets. co. uk A 2 1049 2 -June-2016

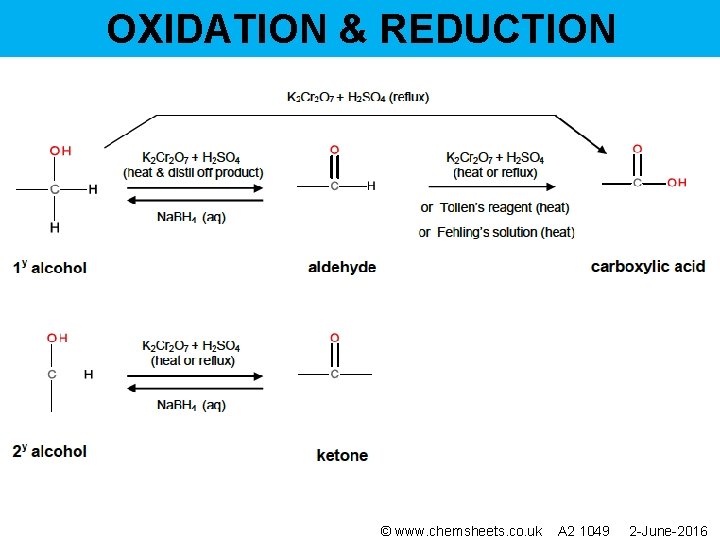
OXIDATION & REDUCTION © www. chemsheets. co. uk A 2 1049 2 -June-2016
![OXIDATION REDUCTION Tollens reagent contains AgNH 32 Used to test for OXIDATION & REDUCTION • Tollen’s reagent, contains [Ag(NH 3)2]+ • Used to test for](https://slidetodoc.com/presentation_image_h/cf90bf23fa2a54b9f1f7e4873fbb2a2f/image-5.jpg)
OXIDATION & REDUCTION • Tollen’s reagent, contains [Ag(NH 3)2]+ • Used to test for aldehydes – gives silver mirror • Reduced from Ag(+1) to Ag(0)

OXIDATION & REDUCTION • Fehling’s solution, contains Cu(+2) • Used to test for aldehydes – gives brick-red precipitate of Cu 2 O • Reduced from Cu(+2) to Cu(+1)

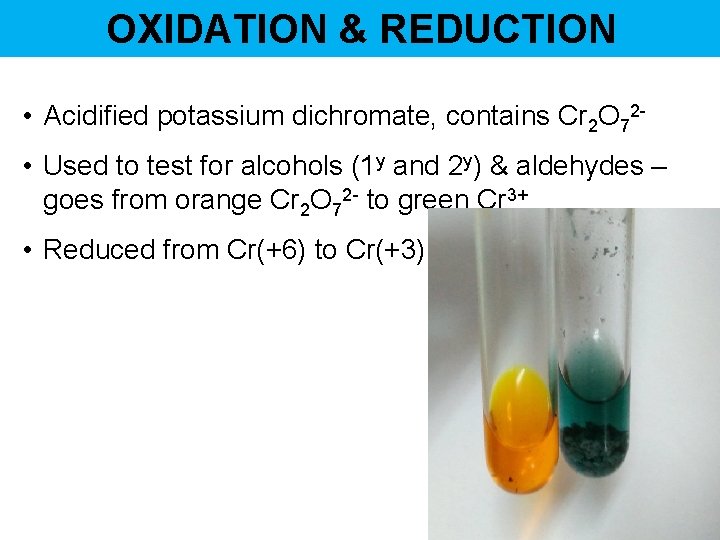
OXIDATION & REDUCTION • Acidified potassium dichromate, contains Cr 2 O 72 • Used to test for alcohols (1 y and 2 y) & aldehydes – goes from orange Cr 2 O 72 - to green Cr 3+ • Reduced from Cr(+6) to Cr(+3)

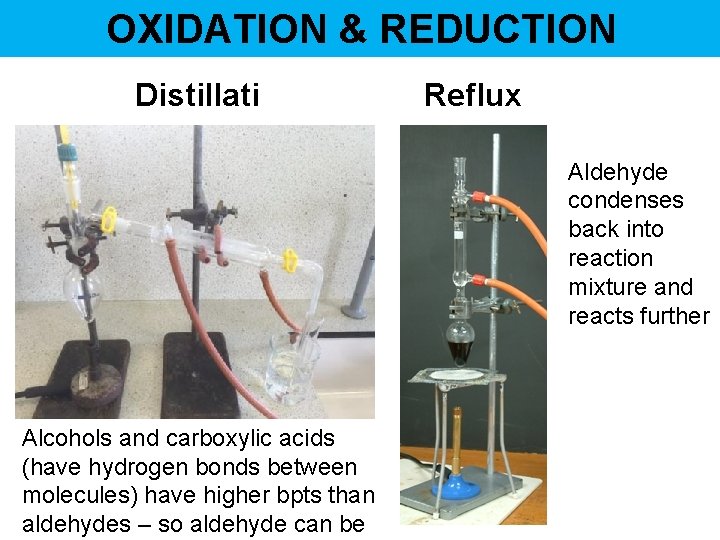
OXIDATION & REDUCTION Distillati on Reflux Aldehyde condenses back into reaction mixture and reacts further Alcohols and carboxylic acids (have hydrogen bonds between molecules) have higher bpts than aldehydes – so aldehyde can be

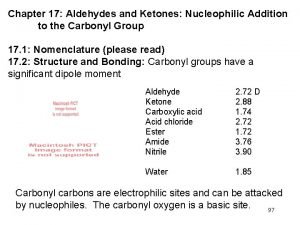
NUCLEOPHILIC ADDITION + – • Addition reactions: atoms add across C=O to the C and O • C is + so attacked by nucleophiles (electron pair donors) • The three atoms attached to the C are planar © www. chemsheets. co. uk A 2 1049 2 -June-2016

NUCLEOPHILIC ADDITION Reduction with Na. BH 4 © www. chemsheets. co. uk A 2 1026 25 -May-2016

NUCLEOPHILIC ADDITION e. g. ethanal + Na. BH 4 © www. chemsheets. co. uk A 2 1026 25 -May-2016

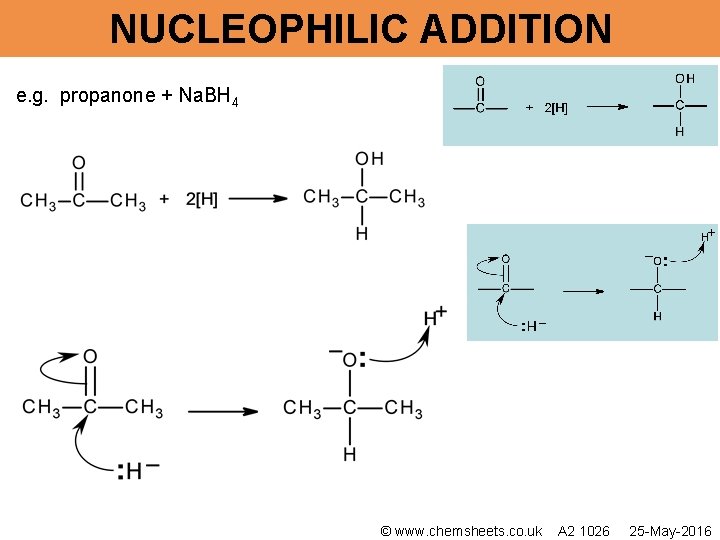
NUCLEOPHILIC ADDITION e. g. propanone + Na. BH 4 © www. chemsheets. co. uk A 2 1026 25 -May-2016

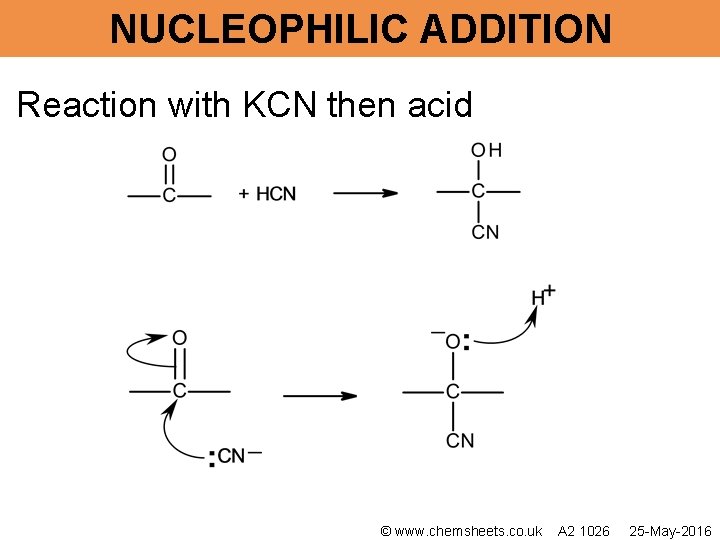
NUCLEOPHILIC ADDITION Reaction with KCN then acid © www. chemsheets. co. uk A 2 1026 25 -May-2016

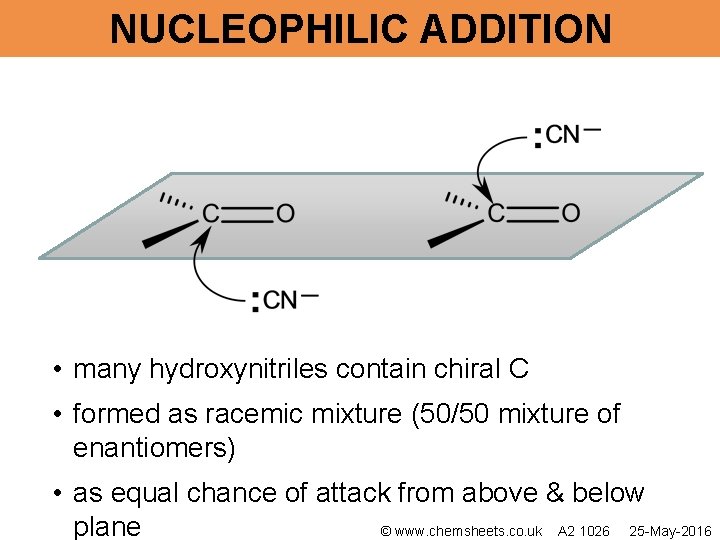
NUCLEOPHILIC ADDITION • many hydroxynitriles contain chiral C • formed as racemic mixture (50/50 mixture of enantiomers) • as equal chance of attack from above & below plane © www. chemsheets. co. uk A 2 1026 25 -May-2016

© www. chemsheets. co. uk A 2 1026 25 -May-2016

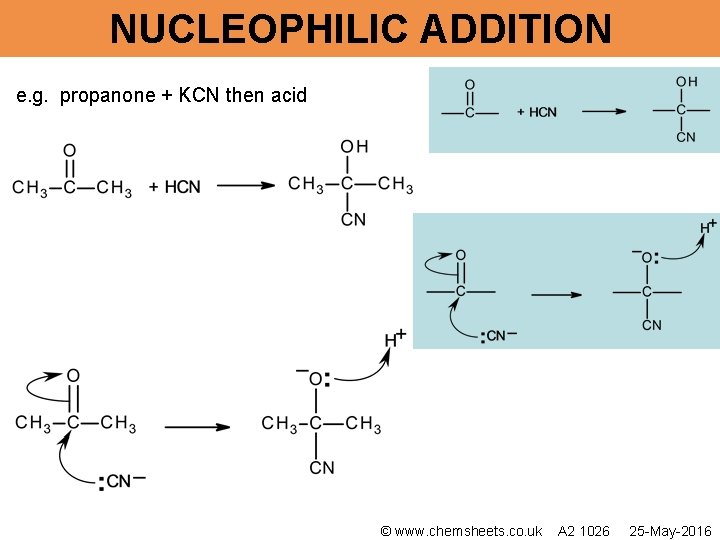
NUCLEOPHILIC ADDITION e. g. propanone + KCN then acid © www. chemsheets. co. uk A 2 1026 25 -May-2016

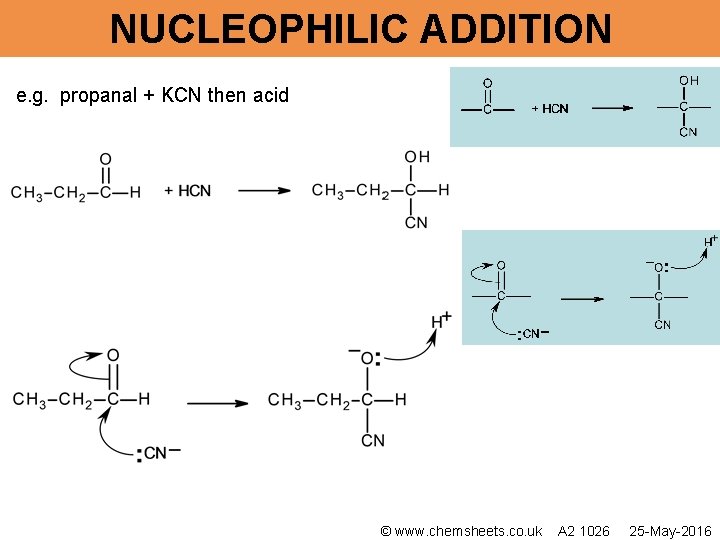
NUCLEOPHILIC ADDITION e. g. propanal + KCN then acid © www. chemsheets. co. uk A 2 1026 25 -May-2016
 Propanal + kcn
Propanal + kcn Carbonyl compounds
Carbonyl compounds Preparation of ketones from acyl chlorides
Preparation of ketones from acyl chlorides Chemical properties of aldehydes
Chemical properties of aldehydes Aldehydes and ketones structure
Aldehydes and ketones structure Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Ketone molecule
Ketone molecule Ketone reactivity
Ketone reactivity Polyhydroxy aldehyde
Polyhydroxy aldehyde Hydration of aldehydes and ketones
Hydration of aldehydes and ketones Carbonyl group aldehyde and ketone
Carbonyl group aldehyde and ketone Aldehyde protecting group
Aldehyde protecting group Aldehydes to carboxylic acids
Aldehydes to carboxylic acids Aldehyde ending
Aldehyde ending Haworth projection to fischer
Haworth projection to fischer Ch3li reaction with ketone
Ch3li reaction with ketone Alkynes to aldehydes
Alkynes to aldehydes Aldehydes scary
Aldehydes scary