Energetics 6 1 What is Energetics 6 2





![6. 3 Standard Enthalpy Changes of Reactions ø What is Hf [N 2(g)] ? 6. 3 Standard Enthalpy Changes of Reactions ø What is Hf [N 2(g)] ?](https://slidetodoc.com/presentation_image_h2/762fe56c980075475b8d50622212fc61/image-16.jpg)


![6. 5 Enthalpy Change of Formation of CO(g) ø Hf CO(g)] + H 2 6. 5 Enthalpy Change of Formation of CO(g) ø Hf CO(g)] + H 2](https://slidetodoc.com/presentation_image_h2/762fe56c980075475b8d50622212fc61/image-27.jpg)


- Slides: 30

Energetics 6. 1 What is Energetics? 6. 2 Ideal Enthalpy Changes Related to Breaking and Formation of Bonds 6. 3 Standard Enthalpy Changes

6. 4 Experimental Determination of Enthalpy Changes by Calorimetry 6. 5 Hess’s Law 6. 6 Calculations involving Enthalpy Changes of Reactions

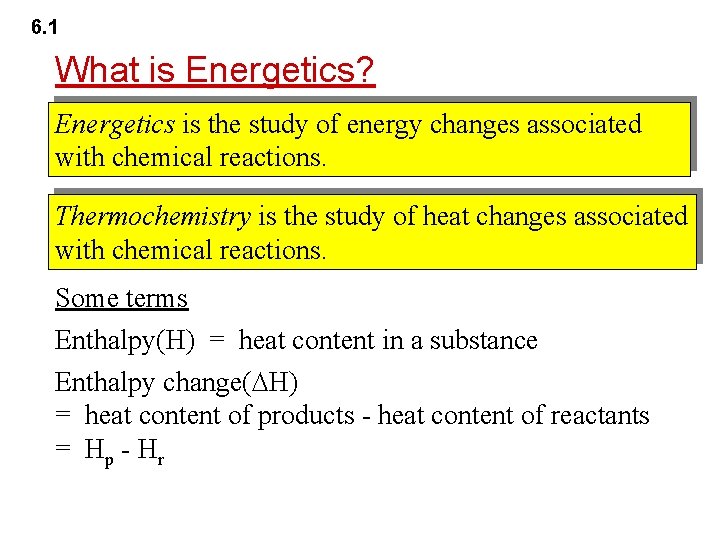
6. 1 What is Energetics? Energetics is the study of energy changes associated with chemical reactions. Thermochemistry is the study of heat changes associated with chemical reactions. Some terms Enthalpy(H) = heat content in a substance Enthalpy change( H) = heat content of products - heat content of reactants = H p - Hr

6. 1 Internal Energy and Enthalpy e. g. Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) Heat change at constant pressure Enthalpy change = Change in internal Work done on the + energy surroundings (Heat change at constant volume)

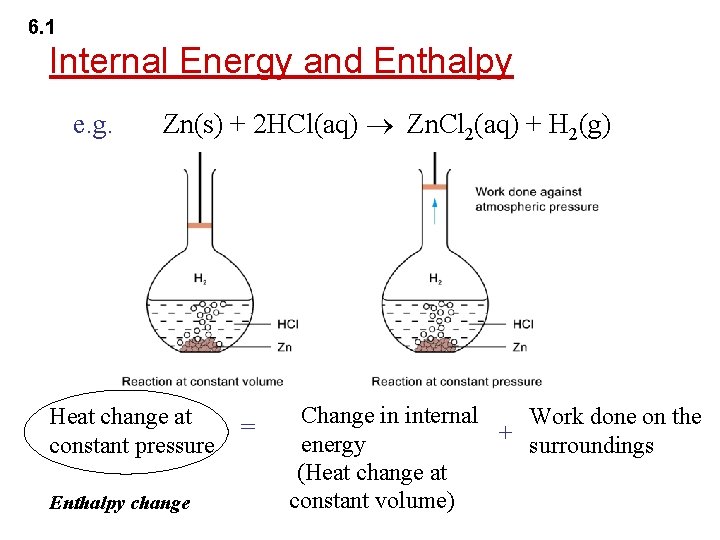
6. 1 Exothermic and Endothermic Reactions An exothermic reaction is a reaction that releases heat energy to the surroundings. ( H = -ve)

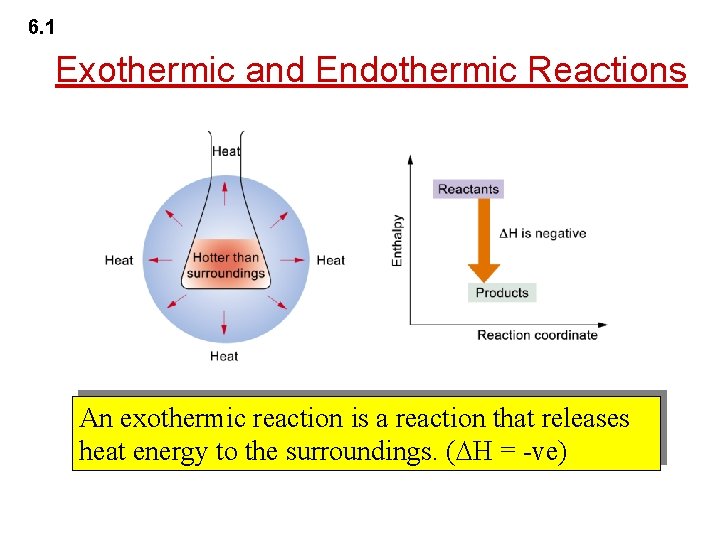
6. 1 Exothermic and Endothermic Reactions An endothermic reaction is a reaction that absorbs heat energy from the surroundings. ( H = +ve)

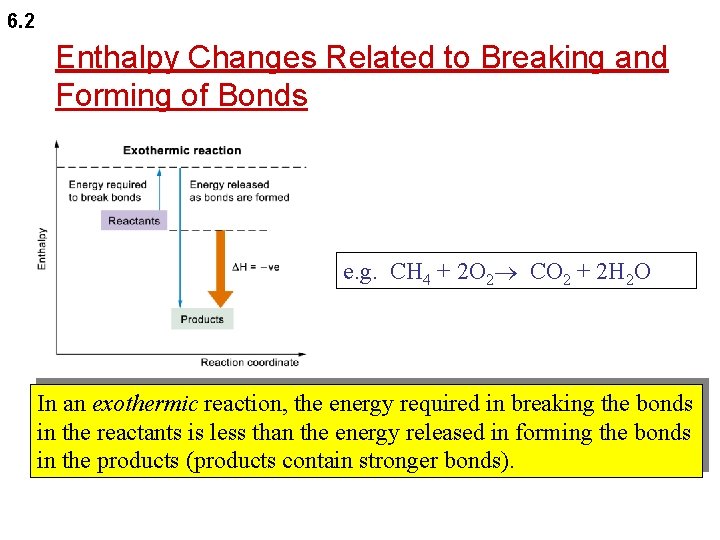
6. 2 Enthalpy Changes Related to Breaking and Forming of Bonds e. g. CH 4 + 2 O 2 CO 2 + 2 H 2 O In an exothermic reaction, the energy required in breaking the bonds in the reactants is less than the energy released in forming the bonds in the products (products contain stronger bonds).

6. 2 Enthalpy Changes Related to Breaking and Forming of Bonds In an endothermic reaction, the energy required in breaking the bonds in the reactants is more than the energy released in forming the bonds in the products (reactants contain stronger bonds).

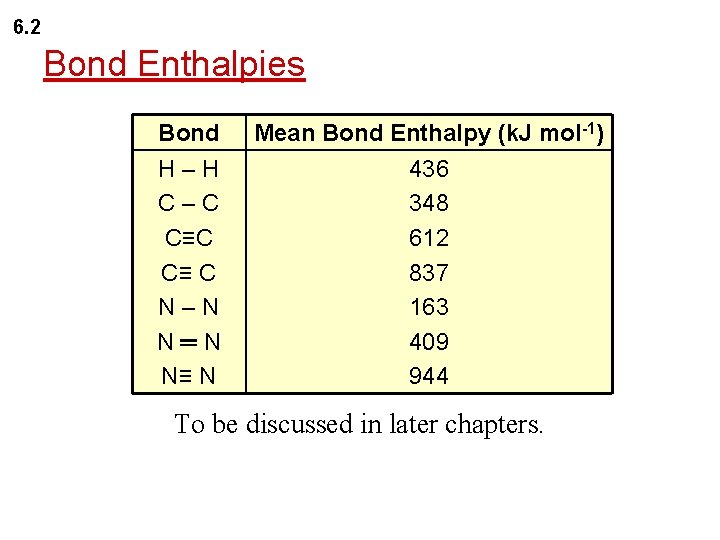
6. 2 Bond Enthalpies Bond H–H C–C C≡ C N–N N═N N≡ N Mean Bond Enthalpy (k. J mol-1) 436 348 612 837 163 409 944 To be discussed in later chapters.

Standard Enthalpy Changes CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) H = -802 k. J mol-1 CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) H = -890 k. J mol-1 As enthalpy changes depend on temperature and pressure, chemists find it convenient to report enthalpy changes based on an internationally agreed set standard conditions: 1. elements or compounds in their normal physical states; 2. a pressure of 1 atm (101325 Nm-2); and 3. a temperature of 250 C (298 K) ø Enthalpy change under standard conditions denoted by symbol: H

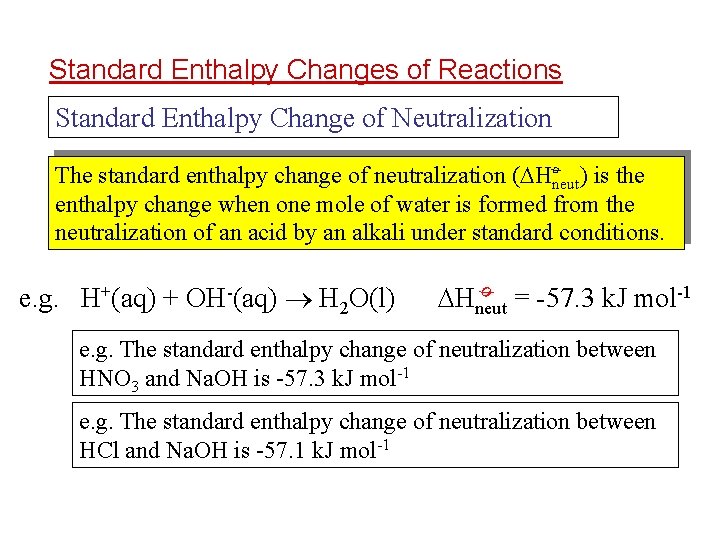
Standard Enthalpy Changes of Reactions Standard Enthalpy Change of Neutralization ø The standard enthalpy change of neutralization ( Hneut) is the enthalpy change when one mole of water is formed from the neutralization of an acid by an alkali under standard conditions. Hneut = -57. 3 k. J mol-1 ø e. g. H+(aq) + OH-(aq) H 2 O(l) e. g. The standard enthalpy change of neutralization between HNO 3 and Na. OH is -57. 3 k. J mol-1 e. g. The standard enthalpy change of neutralization between HCl and Na. OH is -57. 1 k. J mol-1

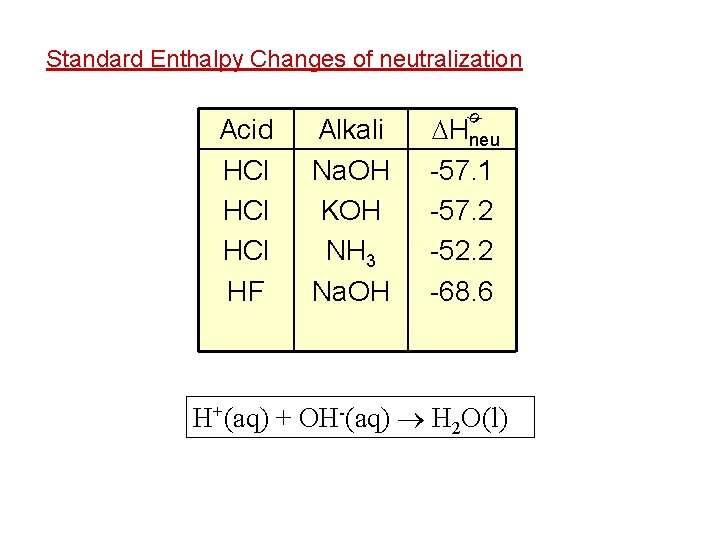
Standard Enthalpy Changes of neutralization Alkali Na. OH KOH NH 3 Na. OH Hneu -57. 1 -57. 2 -52. 2 -68. 6 ø Acid HCl HCl HF H+(aq) + OH-(aq) H 2 O(l)

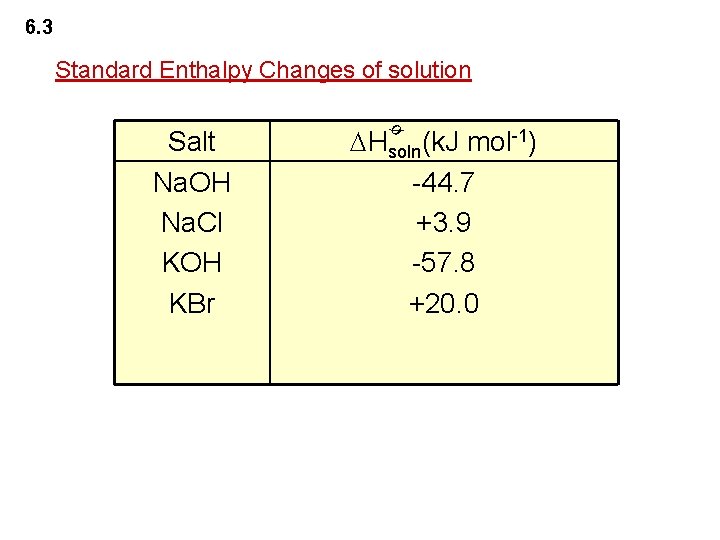
6. 3 Standard Enthalpy Changes of Reactions Standard Enthalpy Change of Solution ø The standard enthalpy change of solution ( Hsoln) is the enthalpy change when one mole of a solute is dissolved to form an infinitely dilute solution under standard conditions. ø e. g. Na. Cl(s) + water Na+(aq)+Cl-(aq) Hsoln=+3. 9 k. J mol-1 ø e. g. Li. Cl(s) + water Li+(aq) + Cl-(aq) Hsoln=-37. 2 k. J mol-1 Note that enthalpy changes of solution associate with physical changes.

6. 3 Standard Enthalpy Changes of solution Hsoln(k. J mol-1) -44. 7 +3. 9 -57. 8 +20. 0 ø Salt Na. OH Na. Cl KOH KBr

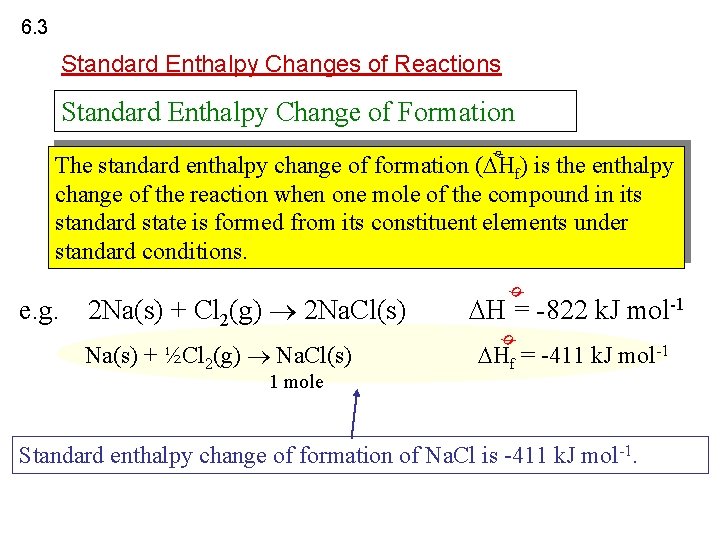
6. 3 Standard Enthalpy Changes of Reactions Standard Enthalpy Change of Formation ø The standard enthalpy change of formation ( Hf) is the enthalpy change of the reaction when one mole of the compound in its standard state is formed from its constituent elements under standard conditions. 2 Na(s) + Cl 2(g) 2 Na. Cl(s) 1 mole Hf = -411 k. J mol-1 ø Na(s) + ½Cl 2(g) Na. Cl(s) H = -822 k. J mol-1 ø e. g. Standard enthalpy change of formation of Na. Cl is -411 k. J mol-1.
![6 3 Standard Enthalpy Changes of Reactions ø What is Hf N 2g 6. 3 Standard Enthalpy Changes of Reactions ø What is Hf [N 2(g)] ?](https://slidetodoc.com/presentation_image_h2/762fe56c980075475b8d50622212fc61/image-16.jpg)
6. 3 Standard Enthalpy Changes of Reactions ø What is Hf [N 2(g)] ? N 2(g) ø Hf [N 2(g)] = 0 The enthalpy change of formation of an element is always zero.

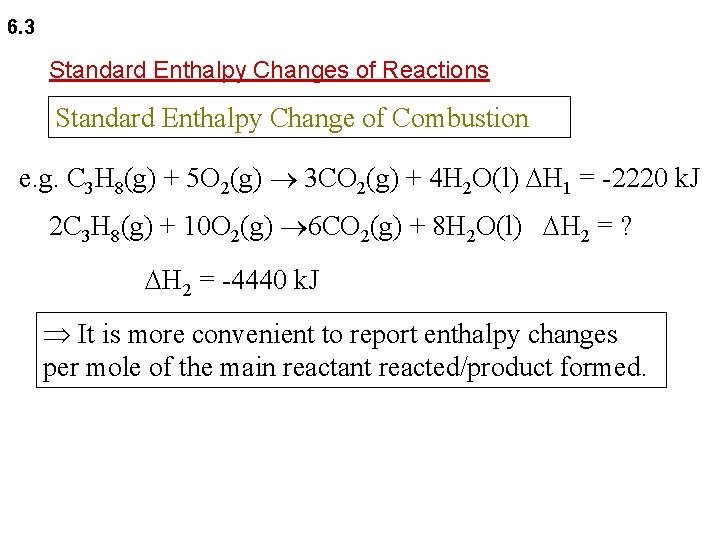
6. 3 Standard Enthalpy Changes of Reactions Standard Enthalpy Change of Combustion e. g. C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) H 1 = -2220 k. J 2 C 3 H 8(g) + 10 O 2(g) 6 CO 2(g) + 8 H 2 O(l) H 2 = ? H 2 = -4440 k. J It is more convenient to report enthalpy changes per mole of the main reactant reacted/product formed.

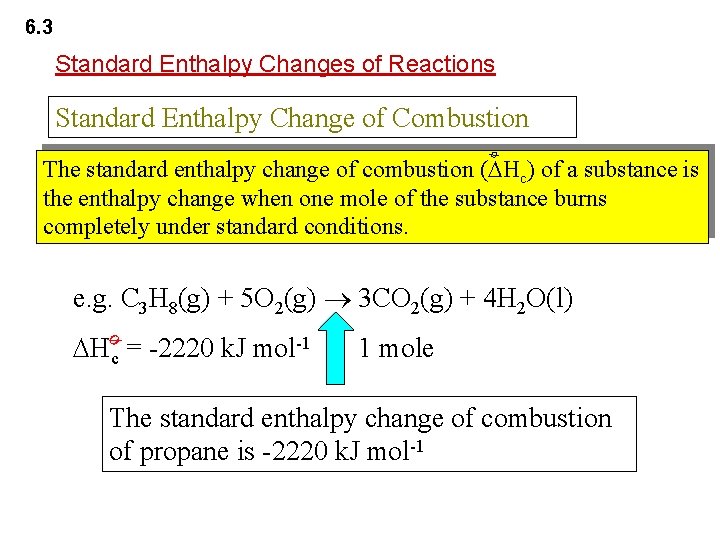
6. 3 Standard Enthalpy Changes of Reactions Standard Enthalpy Change of Combustion ø The standard enthalpy change of combustion ( Hc) of a substance is the enthalpy change when one mole of the substance burns completely under standard conditions. e. g. C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) ø Hc = -2220 k. J mol-1 1 mole The standard enthalpy change of combustion of propane is -2220 k. J mol-1

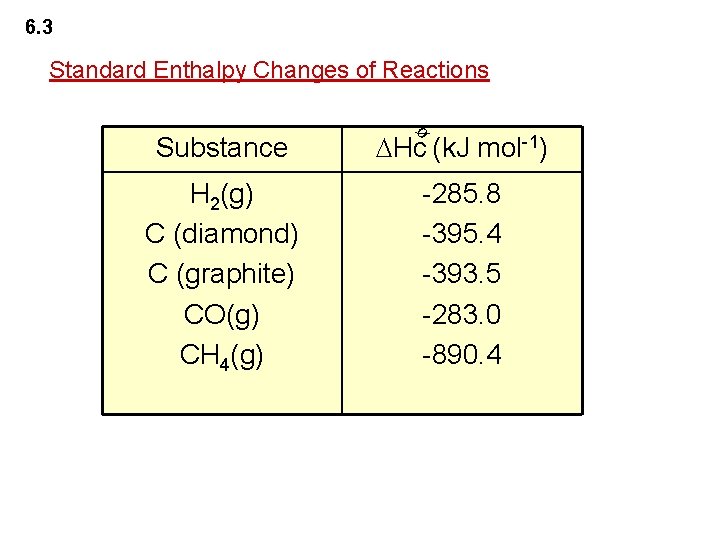
6. 3 Standard Enthalpy Changes of Reactions Hc (k. J mol-1) H 2(g) C (diamond) C (graphite) CO(g) CH 4(g) -285. 8 -395. 4 -393. 5 -283. 0 -890. 4 ø Substance

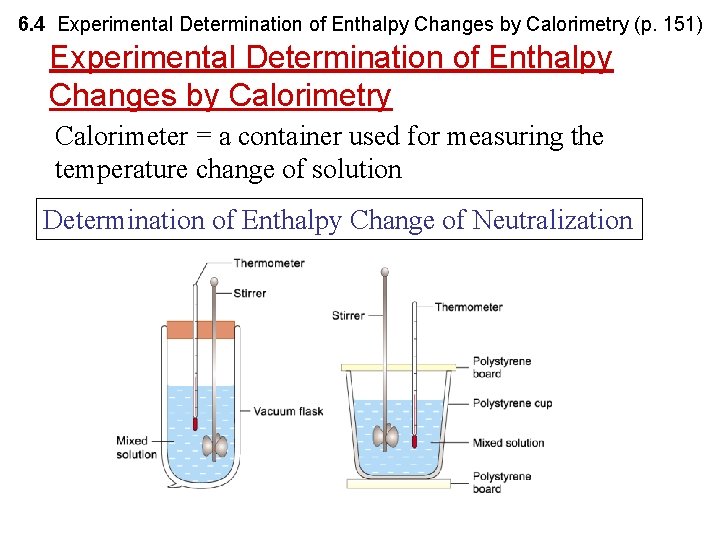
6. 4 Experimental Determination of Enthalpy Changes by Calorimetry (p. 151) Experimental Determination of Enthalpy Changes by Calorimetry Calorimeter = a container used for measuring the temperature change of solution Determination of Enthalpy Change of Neutralization

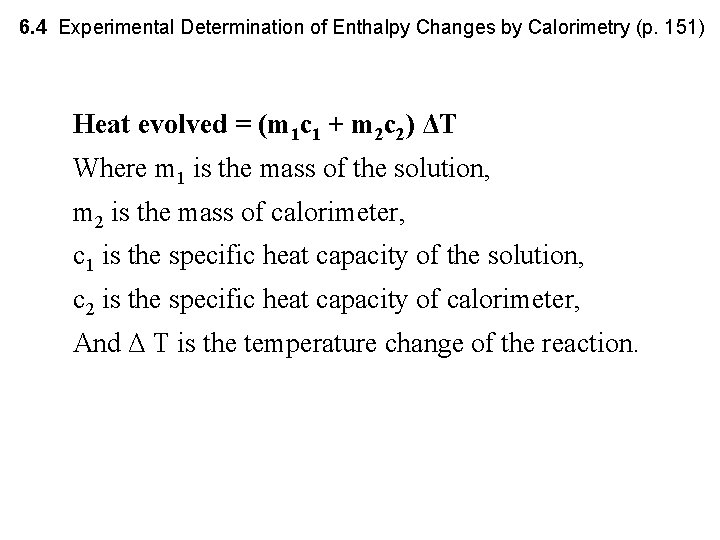
6. 4 Experimental Determination of Enthalpy Changes by Calorimetry (p. 151) Heat evolved = (m 1 c 1 + m 2 c 2) ΔT Where m 1 is the mass of the solution, m 2 is the mass of calorimeter, c 1 is the specific heat capacity of the solution, c 2 is the specific heat capacity of calorimeter, And Δ T is the temperature change of the reaction.

6. 4 Experimental Determination of Enthalpy Changes by Calorimetry (p. 153) Determination of Enthalpy Change of Combustion

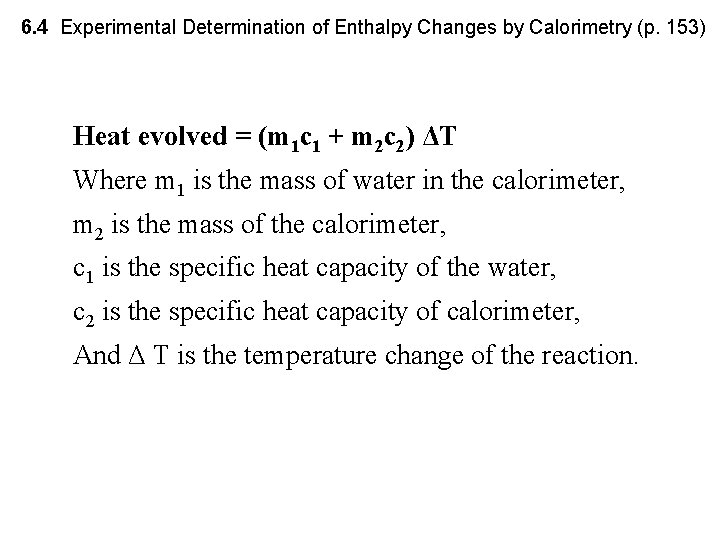
6. 4 Experimental Determination of Enthalpy Changes by Calorimetry (p. 153) Heat evolved = (m 1 c 1 + m 2 c 2) ΔT Where m 1 is the mass of water in the calorimeter, m 2 is the mass of the calorimeter, c 1 is the specific heat capacity of the water, c 2 is the specific heat capacity of calorimeter, And Δ T is the temperature change of the reaction.

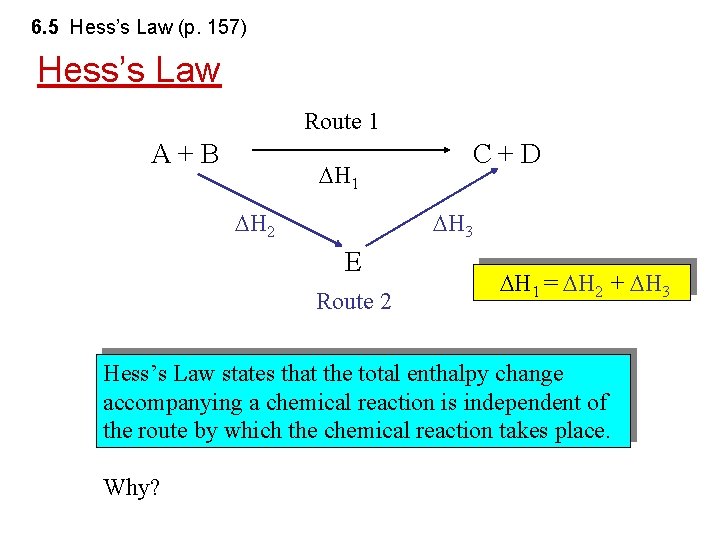
6. 5 Hess’s Law (p. 157) Hess’s Law Route 1 A+B H 1 H 2 C+D H 3 E Route 2 H 1 = H 2 + H 3 Hess’s Law states that the total enthalpy change accompanying a chemical reaction is independent of the route by which the chemical reaction takes place. Why?

6. 5 Hess’s Law (p. 158) Importance of Hess’s Law The enthalpy change of some chemical reactions cannot be determined directly because: • the reactions cannot be performed in the laboratory • the reaction rates are too slow • the reactions may involve the formation of side products But the enthalpy change of such reactions can be determined indirectly by applying Hess’s Law.

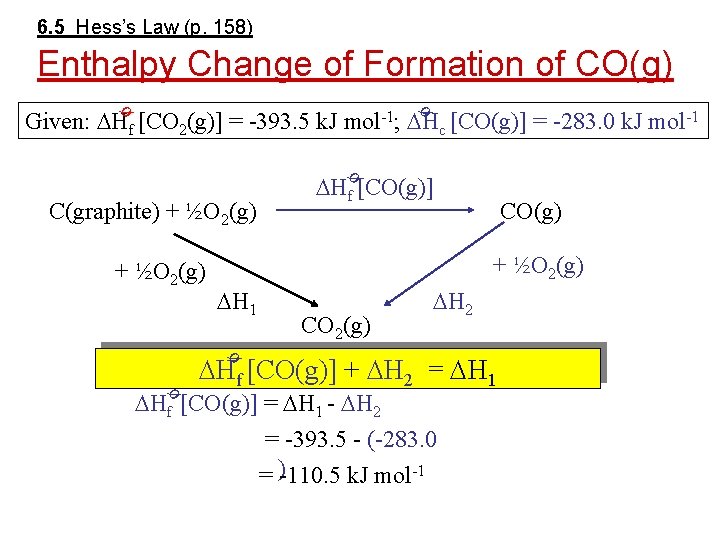
6. 5 Hess’s Law (p. 158) Enthalpy Change of Formation of CO(g) ø ø Given: Hf [CO 2(g)] = -393. 5 k. J mol-1; Hc [CO(g)] = -283. 0 k. J mol-1 ø Hf [CO(g)] C(graphite) + ½O 2(g) CO(g) + ½O 2(g) H 1 CO 2(g) H 2 ø Hf [CO(g)] + H 2 = H 1 ø Hf [CO(g)] = H 1 - H 2 = -393. 5 - (-283. 0 = )-110. 5 k. J mol-1
![6 5 Enthalpy Change of Formation of COg ø Hf COg H 2 6. 5 Enthalpy Change of Formation of CO(g) ø Hf CO(g)] + H 2](https://slidetodoc.com/presentation_image_h2/762fe56c980075475b8d50622212fc61/image-27.jpg)
6. 5 Enthalpy Change of Formation of CO(g) ø Hf CO(g)] + H 2 = H 1

6. 5 ø Enthalpy Change of Hydration of Mg. SO 4(s) ΔH Mg. SO 4(s) + 7 H 2 O(l) Mg. SO 4· 7 H 2 O(s) aq ΔH 1 aq ΔH 2 Mg 2+(aq) + SO 42 -(aq) + 7 H 2 O(l) ø ΔH = enthalpy of hydration of Mg. SO 4(s) ΔH 1 = molar enthalpy change of solution of anhydrous magnesium sulphate(VI) ΔH 2 = molar enthalpy change of solution of magnesium sulphate(VI)-7 -water ø ΔH = ΔH 1 - ΔH 2

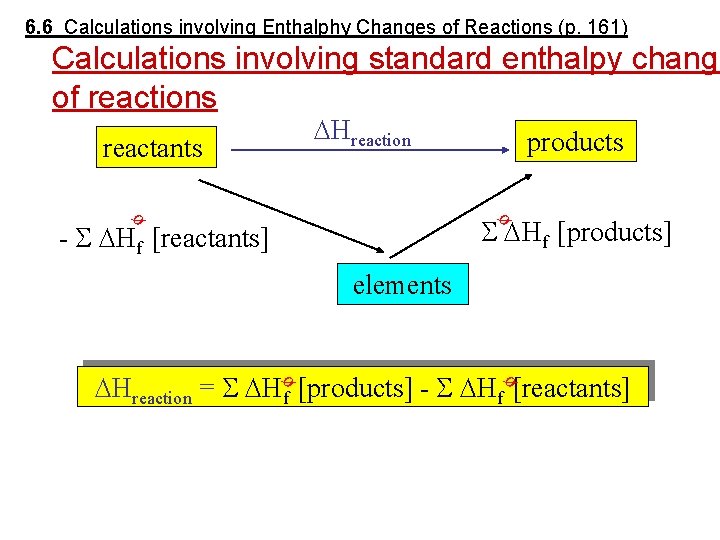
6. 6 Calculations involving Enthalphy Changes of Reactions (p. 161) Calculations involving standard enthalpy change of reactions Hreaction reactants products Hf [products] ø ø - Hf [reactants] elements ø ø Hreaction = Hf [products] - Hf [reactants]

The END