Charles Law Boyles Law V k T PV
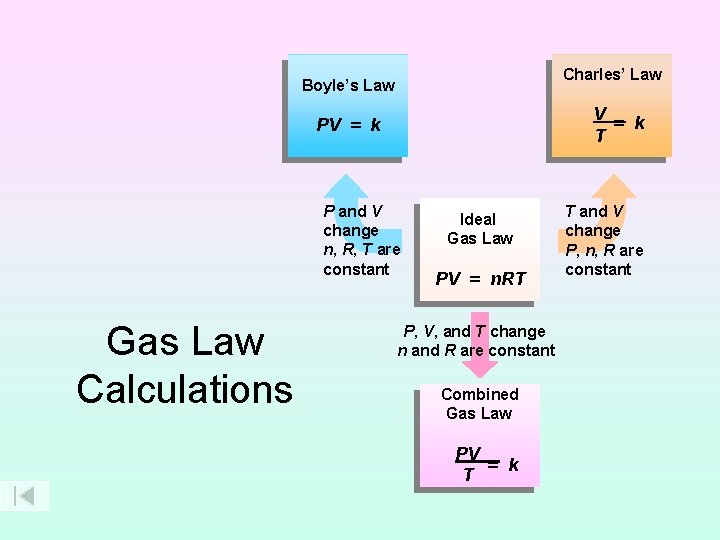
Charles’ Law Boyle’s Law V = k T PV = k P and V change n, R, T are constant Gas Law Calculations Ideal Gas Law PV = n. RT P, V, and T change n and R are constant Combined Gas Law PV = k T T and V change P, n, R are constant
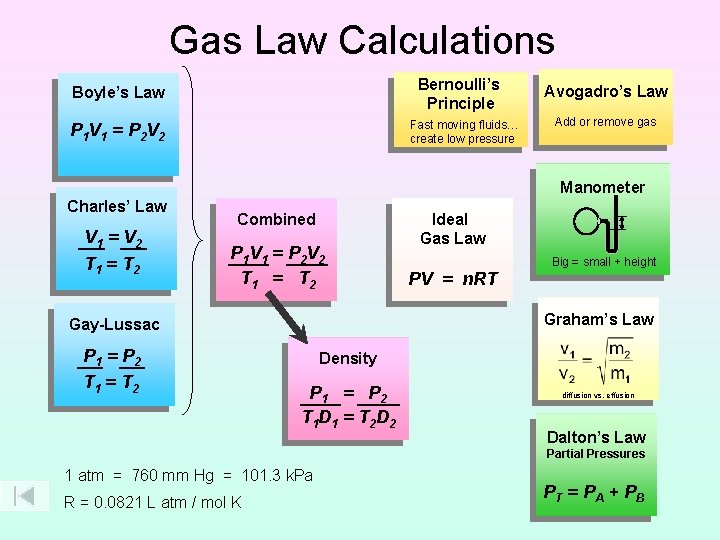
Gas Law Calculations Bernoulli’s Principle Boyle’s Law Fast moving fluids… create low pressure P 1 V 1 = P 2 V 2 Avogadro’s Law Add or remove gas Manometer Charles’ Law V 1 = V 2 T 1 = T 2 Combined P 1 V 1 = P 2 V 2 T 1 = T 2 PV = n. RT Big = small + height Graham’s Law Gay-Lussac P 1 = P 2 T 1 = T 2 Ideal Gas Law Density P 1 = P 2 T 1 D 1 = T 2 D 2 diffusion vs. effusion Dalton’s Law Partial Pressures 1 atm = 760 mm Hg = 101. 3 k. Pa R = 0. 0821 L atm / mol K PT = P A + P B
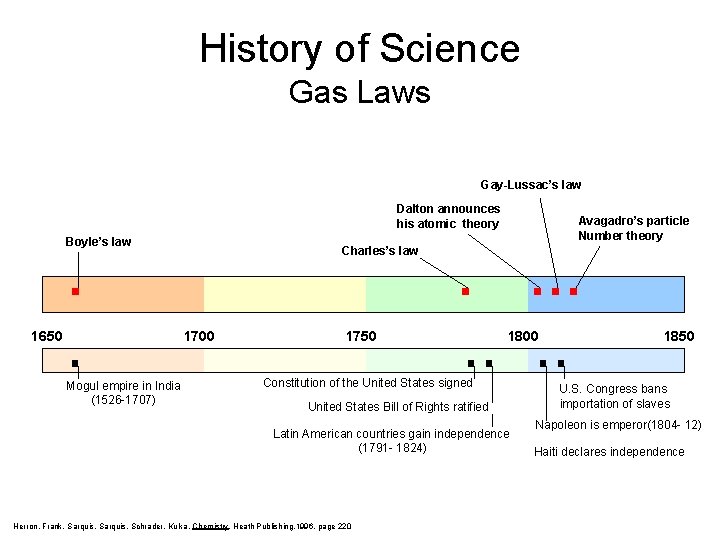
History of Science Gas Laws Gay-Lussac’s law Dalton announces his atomic theory Boyle’s law 1650 Charles’s law 1700 Mogul empire in India (1526 -1707) Avagadro’s particle Number theory 1750 1800 Constitution of the United States signed United States Bill of Rights ratified Latin American countries gain independence (1791 - 1824) Herron, Frank, Sarquis, Schrader, Kulka, Chemistry, Heath Publishing, 1996, page 220 1850 U. S. Congress bans importation of slaves Napoleon is emperor(1804 - 12) Haiti declares independence

Scientists • Evangelista Torricelli (1608 -1647) – Published first scientific explanation of a vacuum. – Invented mercury barometer. • Robert Boyle (1627 - 1691) – Volume inversely related to pressure (temperature remains constant) • Jacques Charles (1746 -1823) – Volume directly related to temperature (pressure remains constant) • Joseph Gay-Lussac (1778 -1850) – Pressure directly related to temperature (volume remains constant)
Eggsplosion Gas Demonstrations Gas: Demonstrations Effect of Temperature on Volume of a Gas VIDEO Air Pressure Crushes a Popcan VIDEO Air Pressure Inside a Balloon (Needle through a balloon) VIDEO Effect of Pressure on Volume (Shaving Creme in a Belljar) VIDEO http: //www. unit 5. org/chemistry/Gas. Laws. html
- Slides: 5