Boyles Law Boyles Law The relationship among pressure

Boyle’s Law
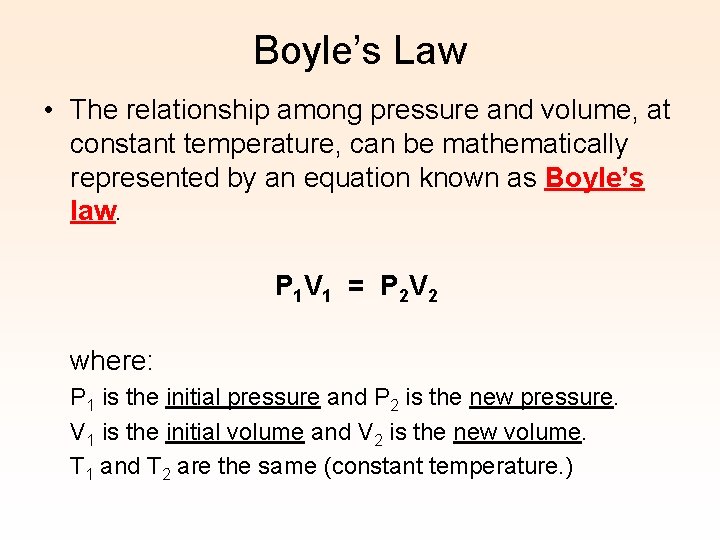
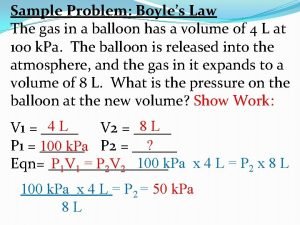
Boyle’s Law • The relationship among pressure and volume, at constant temperature, can be mathematically represented by an equation known as Boyle’s law. P 1 V 1 = P 2 V 2 where: P 1 is the initial pressure and P 2 is the new pressure. V 1 is the initial volume and V 2 is the new volume. T 1 and T 2 are the same (constant temperature. )
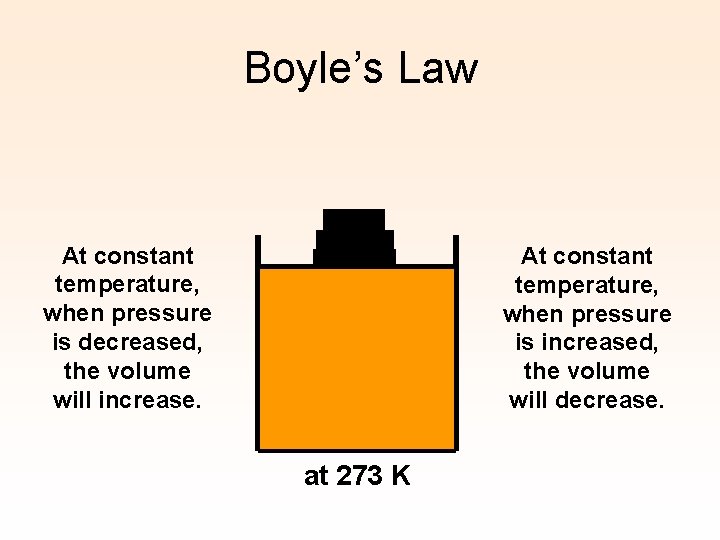
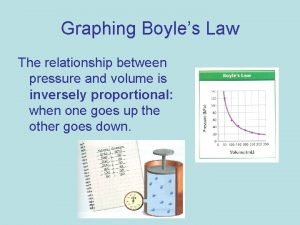
Boyle’s Law At constant temperature, when pressure is decreased, the volume will increase. At constant temperature, when pressure is increased, the volume will decrease. at 273 K
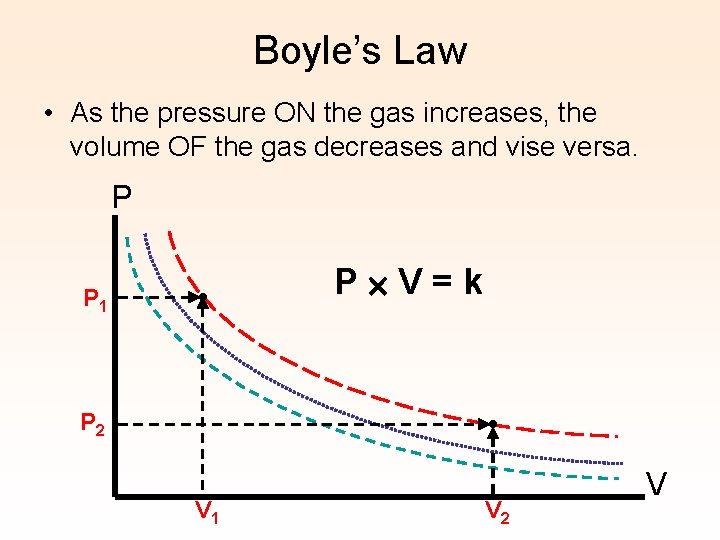
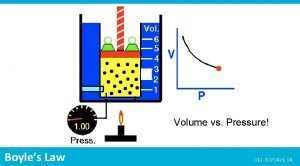
Boyle’s Law • As the pressure ON the gas increases, the volume OF the gas decreases and vise versa. P P 1 • P 2 P V=k • V 1 V 2 V
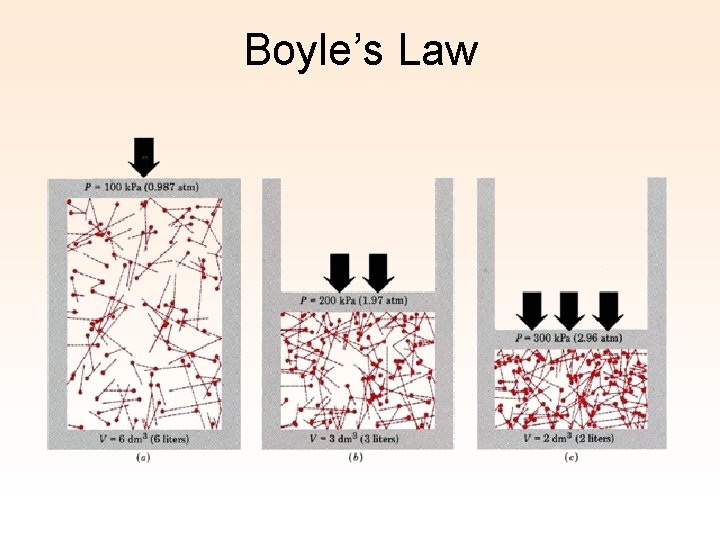
Boyle’s Law
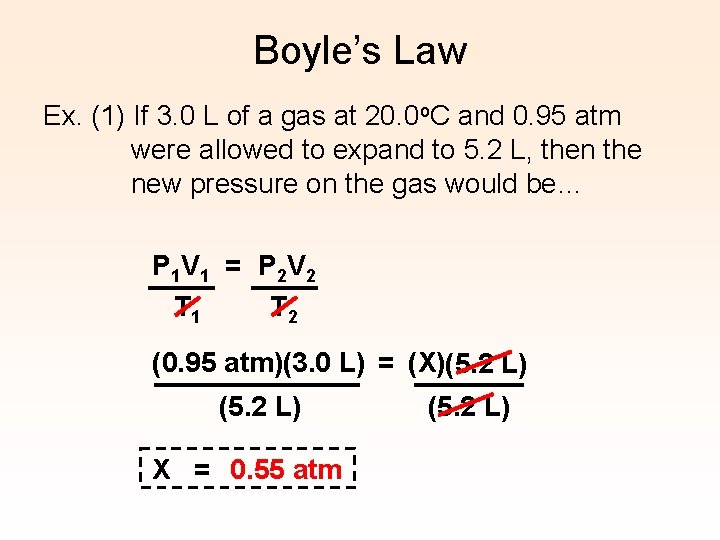
Boyle’s Law Ex. (1) If 3. 0 L of a gas at 20. 0 o. C and 0. 95 atm were allowed to expand to 5. 2 L, then the new pressure on the gas would be… P 1 V 1 = P 2 V 2 T 1 T 2 (0. 95 atm)(3. 0 L) = (X)(5. 2 L) X = 0. 55 atm (5. 2 L)
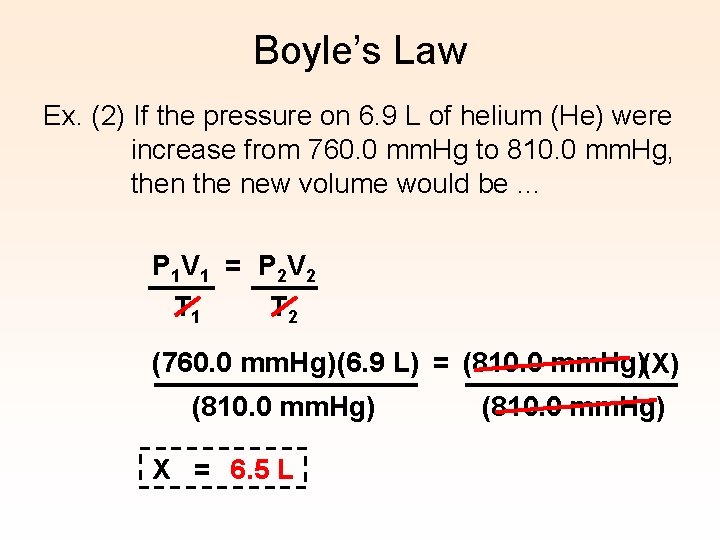
Boyle’s Law Ex. (2) If the pressure on 6. 9 L of helium (He) were increase from 760. 0 mm. Hg to 810. 0 mm. Hg, then the new volume would be … P 1 V 1 = P 2 V 2 T 1 T 2 (760. 0 mm. Hg)(6. 9 L) = (810. 0 mm. Hg)(X) (810. 0 mm. Hg) X = 6. 5 L (810. 0 mm. Hg)
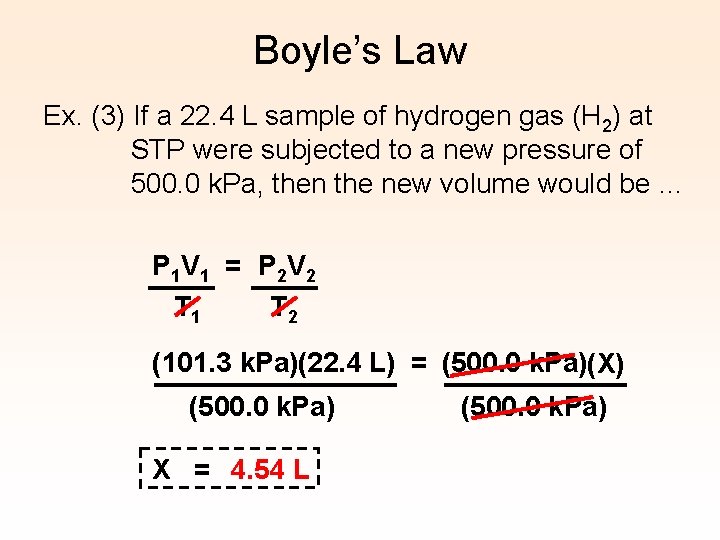
Boyle’s Law Ex. (3) If a 22. 4 L sample of hydrogen gas (H 2) at STP were subjected to a new pressure of 500. 0 k. Pa, then the new volume would be … P 1 V 1 = P 2 V 2 T 1 T 2 (101. 3 k. Pa)(22. 4 L) = (500. 0 k. Pa)(X) (500. 0 k. Pa) X = 4. 54 L (500. 0 k. Pa)
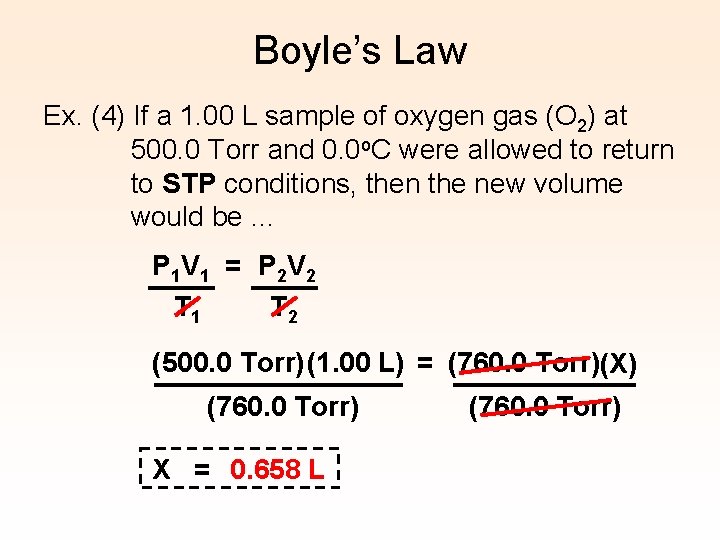
Boyle’s Law Ex. (4) If a 1. 00 L sample of oxygen gas (O 2) at 500. 0 Torr and 0. 0 o. C were allowed to return to STP conditions, then the new volume would be … P 1 V 1 = P 2 V 2 T 1 T 2 (500. 0 Torr) (1. 00 L) = (760. 0 Torr)(X) (760. 0 Torr) X = 0. 658 L (760. 0 Torr)
- Slides: 9