GLUCONEOGENESIS Dr Ngweina Francis Magitta MD Ph D














- Slides: 24

GLUCONEOGENESIS Dr. Ng’weina Francis Magitta, MD, Ph. D University of Dar es Salaam 2015

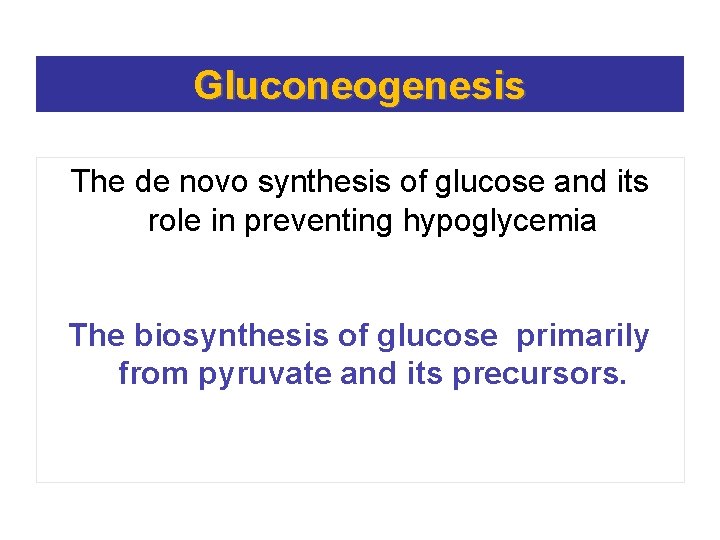
Gluconeogenesis The de novo synthesis of glucose and its role in preventing hypoglycemia The biosynthesis of glucose primarily from pyruvate and its precursors.

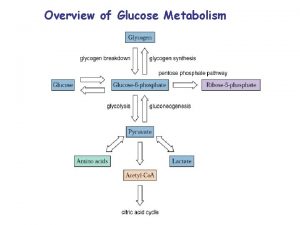
OVERVIEW • Is not a reversal of glycolysis. • Glucose is made from non-carbohydrate precursors of carbon skeleton. • Takes place in liver, minor in kidney, brain, skeletal and heart muscle, to maintain the glucose level in the blood. • Glucose is the primary fuel of brain, and the only fuel for red blood cells.

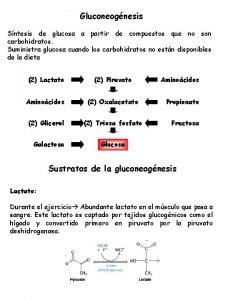
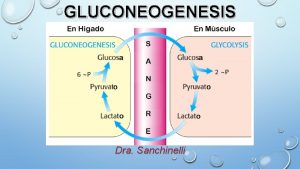
Precursors for Gluconeogenesis • Gluconeogenesis begins with various substrates converted into pyruvate and this proceed though what is essentially the reverse of glycolysis (except for a few committed steps). • 3 and 4 -carbon substrates can enter the gluconeogenesis pathway. Lactate from anaerobic exercise in skeletal muscle is easily converted to pyruvate; this happens as part of the Cori cycle.

Precursors. . . • Oxaloacetate (an intermediate in the TCA cycle can be used for gluconeogenesis. Amino acids, after their amino group has been removed, feed into parts of the TCA, and can thus generate glucose in this pathway. • Fatty acids cannot be turned into glucose, as they are broken down into the two carbon acetyl Co. A. (However glycerol which is a part of all triglycerides can be used in gluconeogenesis).

WHY DO WE PRODUCE GLUCOSE? a) Need to maintain glucose levels within a narrow range in blood. b) Some tissue-- brain, erythrocytes, and muscles in exertion use glucose at a rapid rate and sometimes require glucose in addition to dietary glucose. c) The brain uses mostly glucose and erythrocytes can use only glucose as a source of energy.

GLUCUNEOGENESIS VS GLYCOLYSIS The gluconeogenesis pathway is similar to the reverse of glycolysis but differs at critical sites. Control of these opposing pathways is RECIPROCAL so that physiological conditions favoring one disfavor the other and vice versa. General principles of metabolic control: a) Pathways are not simple reversals of each other b) Opposing pathways are under reciprocal control

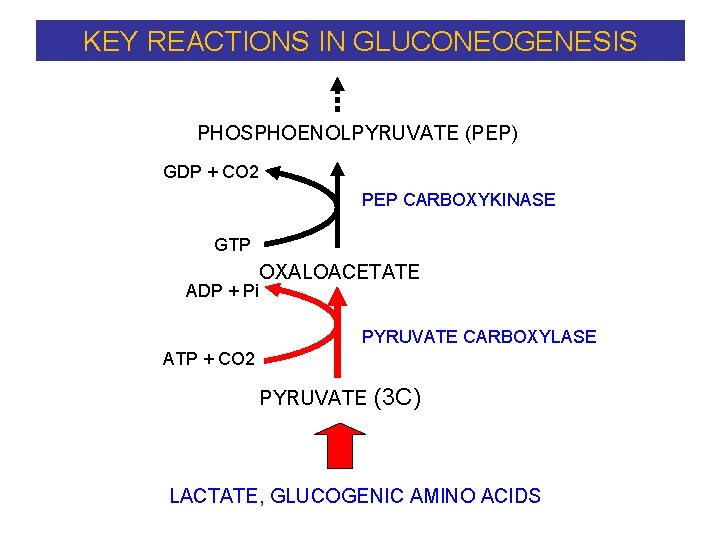
KEY REACTIONS IN GLUCONEOGENESIS PHOSPHOENOLPYRUVATE (PEP) GDP + CO 2 PEP CARBOXYKINASE GTP ADP + Pi OXALOACETATE PYRUVATE CARBOXYLASE ATP + CO 2 PYRUVATE (3 C) LACTATE, GLUCOGENIC AMINO ACIDS

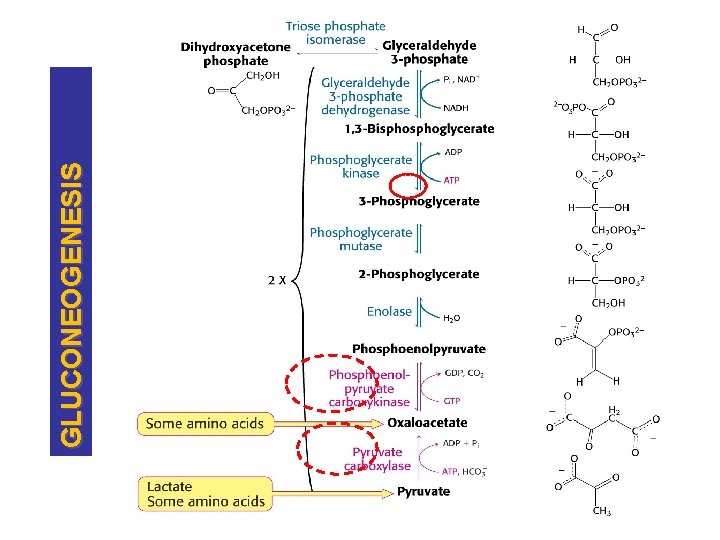
STEPS, CONT. GLUCOSE Pi GLUCOSE 6 PHOSPHATASE GLUCOSE 6 -PHOSPHATE PHOSPHOGLUCOMUTASE FRUCTOSE 6 -PHOSPHATE Pi F 1, 6 -BISPHOSPHATASE FRUCTOSE 1, 6 -BISPHOSPHATE

GLUCONEOGENESIS

GLUCONEOGENESIS

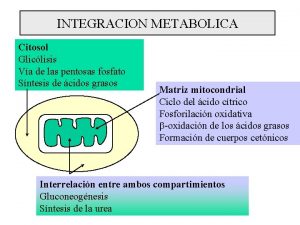
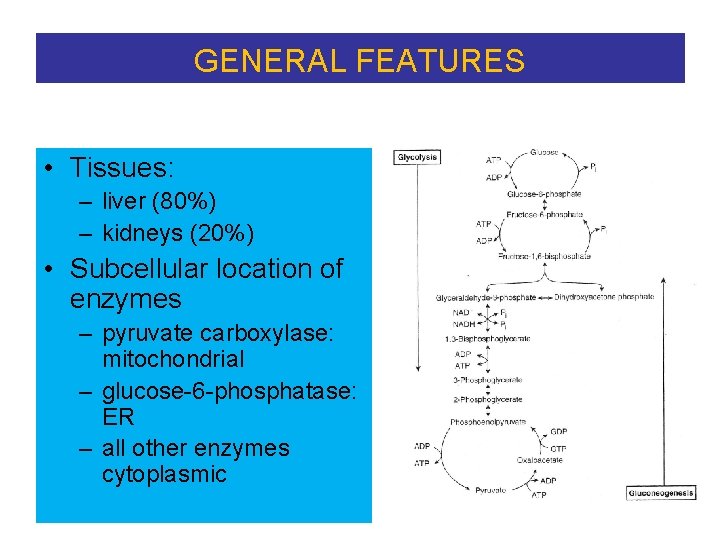
GENERAL FEATURES • Tissues: – liver (80%) – kidneys (20%) • Subcellular location of enzymes – pyruvate carboxylase: mitochondrial – glucose-6 -phosphatase: ER – all other enzymes cytoplasmic

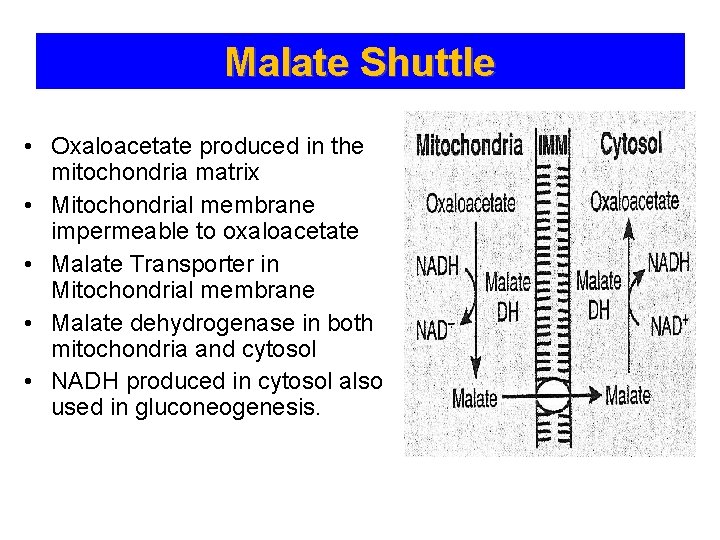
Malate Shuttle • Oxaloacetate produced in the mitochondria matrix • Mitochondrial membrane impermeable to oxaloacetate • Malate Transporter in Mitochondrial membrane • Malate dehydrogenase in both mitochondria and cytosol • NADH produced in cytosol also used in gluconeogenesis.

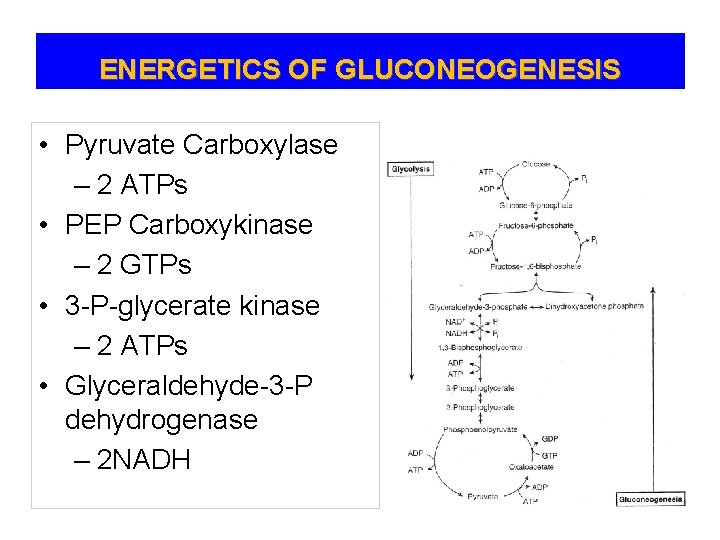
ENERGETICS OF GLUCONEOGENESIS • Pyruvate Carboxylase – 2 ATPs • PEP Carboxykinase – 2 GTPs • 3 -P-glycerate kinase – 2 ATPs • Glyceraldehyde-3 -P dehydrogenase – 2 NADH

BIOENERGETICS § Cost: The production of glucose via Gluconeogenesis is energitically expensive. § Input: 2 pyruvate + 4 ATP + 2 GTP + 2 NADH § Output: Glucose + 4 ADP + 2 GDP + 2 NAD+ + 6 Pi

PRECURSORS FOR GLUCONEOGENESIS • Glycerol – derived from adipocyte lipolysis – hepatic glycerol kinase

PRECURSORS FOR GLUCONEOGENESIS • Lactate – RBC – muscle – the Cori Cycle

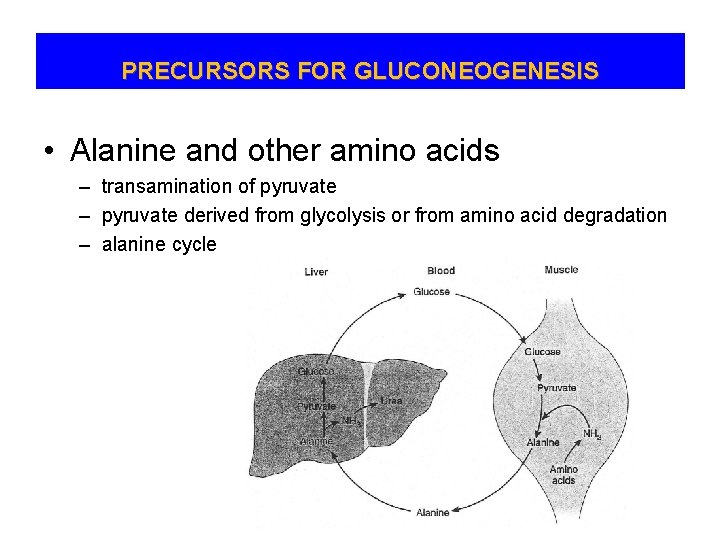
PRECURSORS FOR GLUCONEOGENESIS • Alanine and other amino acids – transamination of pyruvate – pyruvate derived from glycolysis or from amino acid degradation – alanine cycle

REGULATION OF GLUCONEOGENESIS AND GLYCOLYSIS • Gluconeogenesis and Glycolysis are regulated by similar effector molecues but in the opposite direction – avoid futile cycles • PK vs PC&PEPCK • PFK-1 vs FDP’tase • GK vs G 6 P’tase

REGULATION, CONT. • Regulation of enzyme quantity • Fasting: glucagon, cortisol – induces gluconeogenic enzymes – represses glycolytic enzymes – liver making glucose • Feeding: insulin – induces glycolytic enzymes – represses gluconeogenic enzymes – liver using glucose

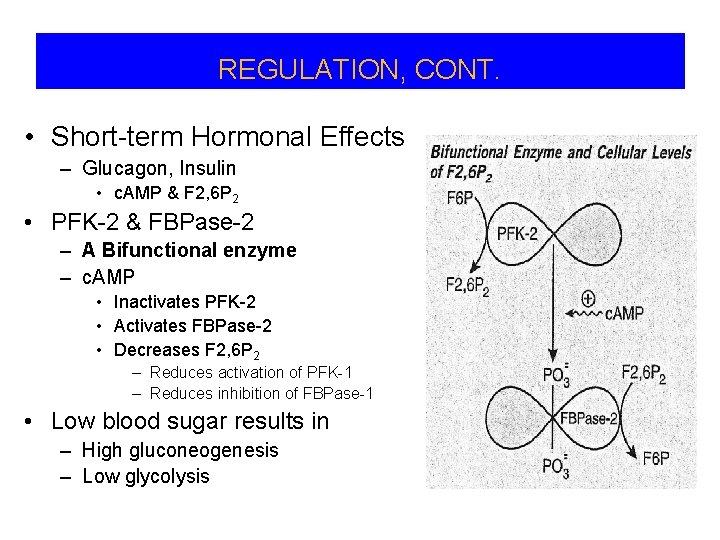
REGULATION, CONT. • Short-term Hormonal Effects – Glucagon, Insulin • c. AMP & F 2, 6 P 2 • PFK-2 & FBPase-2 – A Bifunctional enzyme – c. AMP • Inactivates PFK-2 • Activates FBPase-2 • Decreases F 2, 6 P 2 – Reduces activation of PFK-1 – Reduces inhibition of FBPase-1 • Low blood sugar results in – High gluconeogenesis – Low glycolysis

REGULATION, CONT. • Allosteric Effects • Pyruvate kinase vs Pyruvate carboxylase – PK - Inhibited by ATP and alanine – PC - Activated by acetyl Co. A – Fasting results in gluconeogenesis • PFK-1 vs FBPase-1 – FBPase-1 inhibited by AMP & F 2, 6 P 2 – PFK-1 activated by AMP and & F 2, 6 P 2 – Feeding results in glycolysis

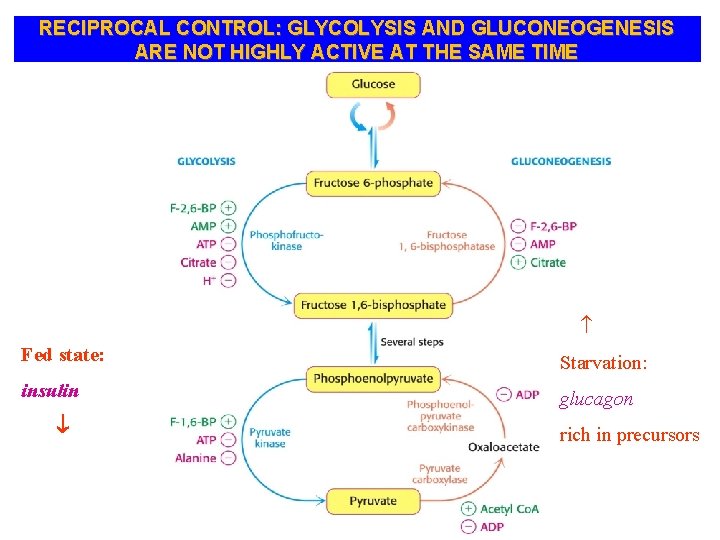
RECIPROCAL CONTROL: GLYCOLYSIS AND GLUCONEOGENESIS ARE NOT HIGHLY ACTIVE AT THE SAME TIME Fed state: Starvation: insulin glucagon rich in precursors

THE END. . .
 Precursor of gluconeogenesis
Precursor of gluconeogenesis Gluconeogenesis of amino acids
Gluconeogenesis of amino acids Gluconeogenesis nedir
Gluconeogenesis nedir Gluconeogenesis definition
Gluconeogenesis definition Glycogenolysis and gluconeogenesis
Glycogenolysis and gluconeogenesis Gluconeogenesis purpose
Gluconeogenesis purpose Gluco neo genesis
Gluco neo genesis Energetics of gluconeogenesis
Energetics of gluconeogenesis Regulacion de gluconeogenesis
Regulacion de gluconeogenesis Glycogenolysis and gluconeogenesis
Glycogenolysis and gluconeogenesis Glucolisis en el musculo
Glucolisis en el musculo Glycogen storage disease table
Glycogen storage disease table Odd chain fatty acids gluconeogenesis
Odd chain fatty acids gluconeogenesis Hexokinase phosphofructokinase and pyruvate kinase
Hexokinase phosphofructokinase and pyruvate kinase Pyruvate carboxylase gluconeogenesis
Pyruvate carboxylase gluconeogenesis Krebs cycle and gluconeogenesis
Krebs cycle and gluconeogenesis Noncarbohydrate precursors
Noncarbohydrate precursors Sustratos gluconeogenicos
Sustratos gluconeogenicos Glucose 6 phosphatase
Glucose 6 phosphatase Net reaction of gluconeogenesis
Net reaction of gluconeogenesis Net reaction of gluconeogenesis
Net reaction of gluconeogenesis Gluconeogenesis irreversible steps
Gluconeogenesis irreversible steps Precursores de gluconeogenesis
Precursores de gluconeogenesis Krebs cycle and gluconeogenesis
Krebs cycle and gluconeogenesis Gluconeogenesis regulacion
Gluconeogenesis regulacion