10 5 Hesss Law Hesss law states that



![Worked Example 13. 6 Ethylene glycol [CH 2(OH)] is a common automobile antifreeze. It Worked Example 13. 6 Ethylene glycol [CH 2(OH)] is a common automobile antifreeze. It](https://slidetodoc.com/presentation_image_h2/6740e03b9e677bd65d287ee3fa01d3b5/image-17.jpg)



- Slides: 26

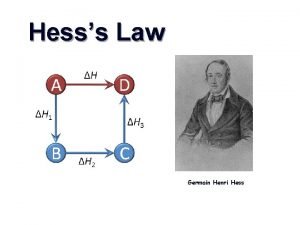
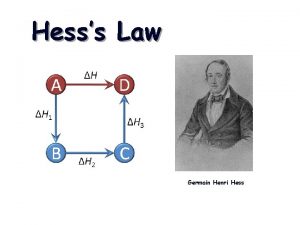
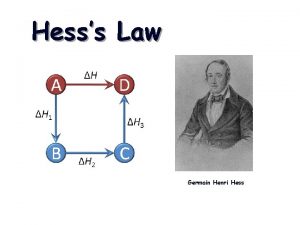
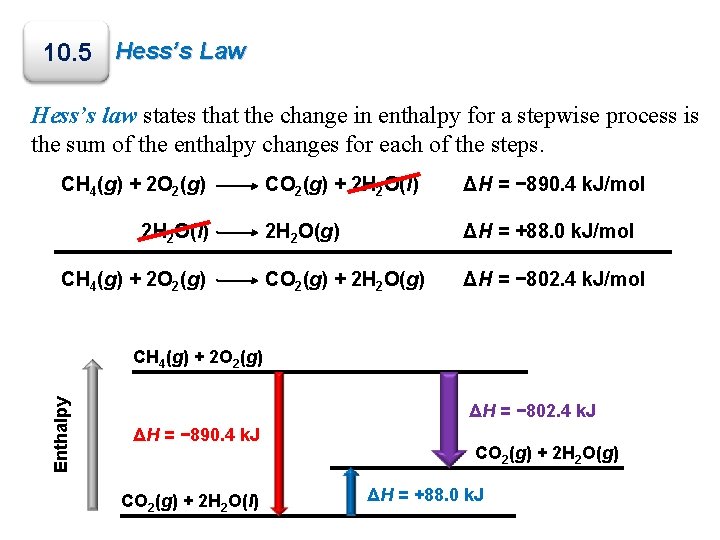
10. 5 Hess’s Law Hess’s law states that the change in enthalpy for a stepwise process is the sum of the enthalpy changes for each of the steps. CH 4(g) + 2 O 2(g) 2 H 2 O(l) CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) ΔH = − 890. 4 k. J/mol 2 H 2 O(g) ΔH = +88. 0 k. J/mol CO 2(g) + 2 H 2 O(g) ΔH = − 802. 4 k. J/mol Enthalpy CH 4(g) + 2 O 2(g) ΔH = − 802. 4 k. J ΔH = − 890. 4 k. J CO 2(g) + 2 H 2 O(l) CO 2(g) + 2 H 2 O(g) ΔH = +88. 0 k. J

Hess’s Law When applying Hess’s Law: 1) Manipulate thermochemical equations in a manner that gives the overall desired equation. 2) Remember the rules for manipulating thermochemical equations: Always specify the physical states of reactants and products because they help determine the actual enthalpy changes. When multiplying an equation by a factor (n), multiply the ΔH value by same factor. Reversing an equation changes the sign but not the magnitude of ΔH. 3) Add the ΔH for each step after proper manipulation. 4) Process is useful for calculating enthalpies that cannot be found directly.

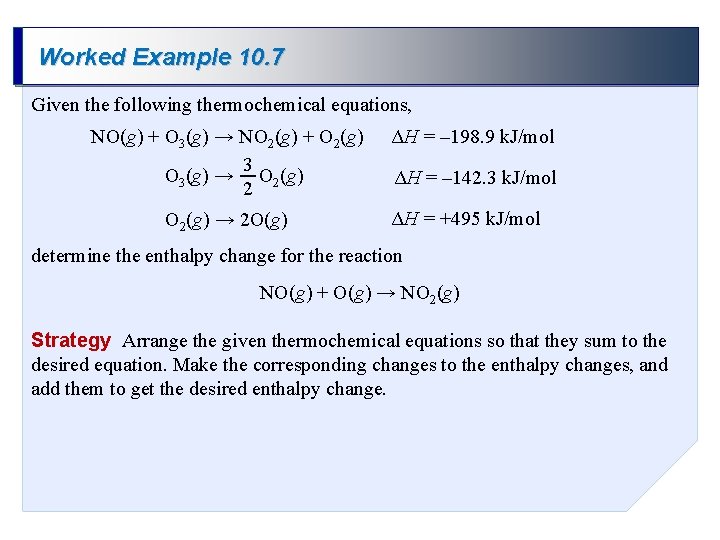
Worked Example 10. 7 Given the following thermochemical equations, NO(g) + O 3(g) → NO 2(g) + O 2(g) 3 O 3(g) → O 2(g) 2 O 2(g) → 2 O(g) ΔH = – 198. 9 k. J/mol ΔH = – 142. 3 k. J/mol ΔH = +495 k. J/mol determine the enthalpy change for the reaction NO(g) + O(g) → NO 2(g) Strategy Arrange the given thermochemical equations so that they sum to the desired equation. Make the corresponding changes to the enthalpy changes, and add them to get the desired enthalpy change.

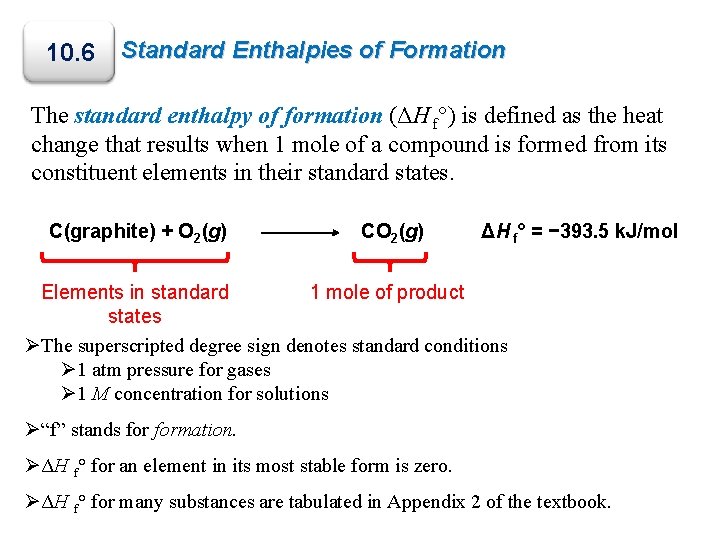
10. 6 Standard Enthalpies of Formation The standard enthalpy of formation (ΔH f°) is defined as the heat change that results when 1 mole of a compound is formed from its constituent elements in their standard states. C(graphite) + O 2(g) CO 2(g) ΔH f° = − 393. 5 k. J/mol Elements in standard 1 mole of product states ØThe superscripted degree sign denotes standard conditions Ø 1 atm pressure for gases Ø 1 M concentration for solutions Ø“f” stands formation. ØΔH f° for an element in its most stable form is zero. ØΔH f° for many substances are tabulated in Appendix 2 of the textbook.

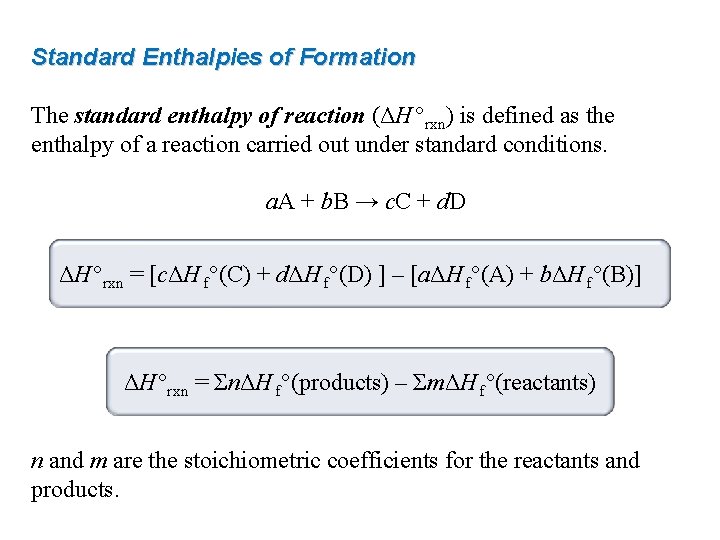
Standard Enthalpies of Formation The standard enthalpy of reaction (ΔH °rxn) is defined as the enthalpy of a reaction carried out under standard conditions. a. A + b. B → c. C + d. D ΔH °rxn = [cΔH f°(C) + dΔH f°(D) ] – [aΔH f°(A) + bΔH f°(B)] ΔH °rxn = ΣnΔH f°(products) – ΣmΔH f°(reactants) n and m are the stoichiometric coefficients for the reactants and products.

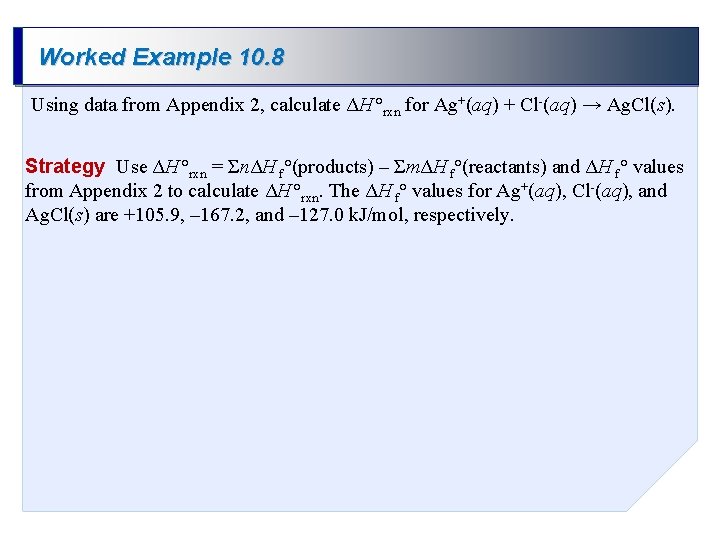
Worked Example 10. 8 Using data from Appendix 2, calculate ΔH °rxn for Ag+(aq) + Cl-(aq) → Ag. Cl(s). Strategy Use ΔH °rxn = ΣnΔH f°(products) – ΣmΔH f°(reactants) and ΔH f° values from Appendix 2 to calculate ΔH °rxn. The ΔH f° values for Ag+(aq), Cl-(aq), and Ag. Cl(s) are +105. 9, – 167. 2, and – 127. 0 k. J/mol, respectively.

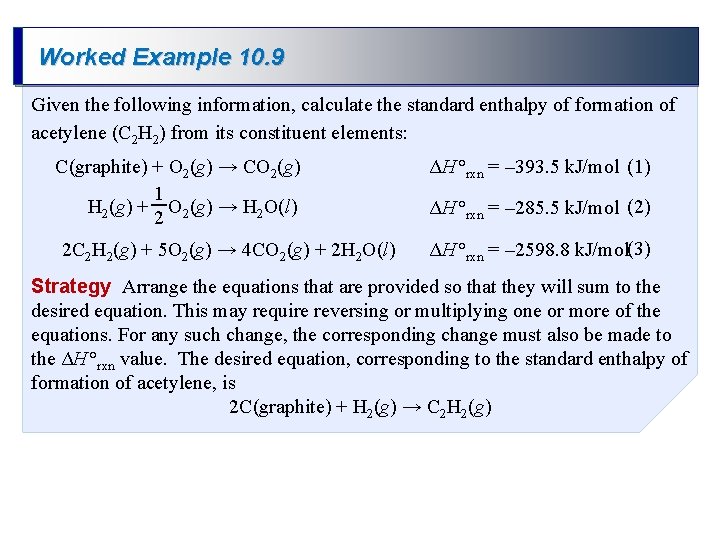
Worked Example 10. 9 Given the following information, calculate the standard enthalpy of formation of acetylene (C 2 H 2) from its constituent elements: C(graphite) + O 2(g) → CO 2(g) 1 H 2(g) + O 2(g) → H 2 O(l) 2 2 C 2 H 2(g) + 5 O 2(g) → 4 CO 2(g) + 2 H 2 O(l) ΔH °rxn = – 393. 5 k. J/mol (1) ΔH °rxn = – 285. 5 k. J/mol (2) ΔH °rxn = – 2598. 8 k. J/mol(3) Strategy Arrange the equations that are provided so that they will sum to the desired equation. This may require reversing or multiplying one or more of the equations. For any such change, the corresponding change must also be made to the ΔH °rxn value. The desired equation, corresponding to the standard enthalpy of formation of acetylene, is 2 C(graphite) + H 2(g) → C 2 H 2(g)

10. 7 Bond Enthalpy and the Stability of Covalent Molecules The bond enthalpy is the enthalpy change associated with breaking a bond in 1 mole of gaseous molecule. H 2(g) → H(g) + H(g) ΔH° = 436. 4 k. J/mol The enthalpy for a gas phase reaction is given by: ΔH° = ΣBE(reactants) – ΣBE(products) ΔH° = total energy input – total energy released bonds broken bonds formed

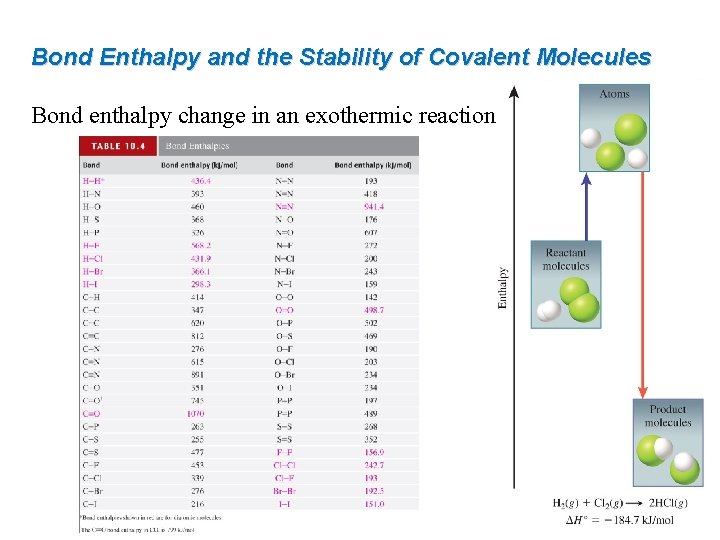
Bond Enthalpy and the Stability of Covalent Molecules Bond enthalpy change in an exothermic reaction. :

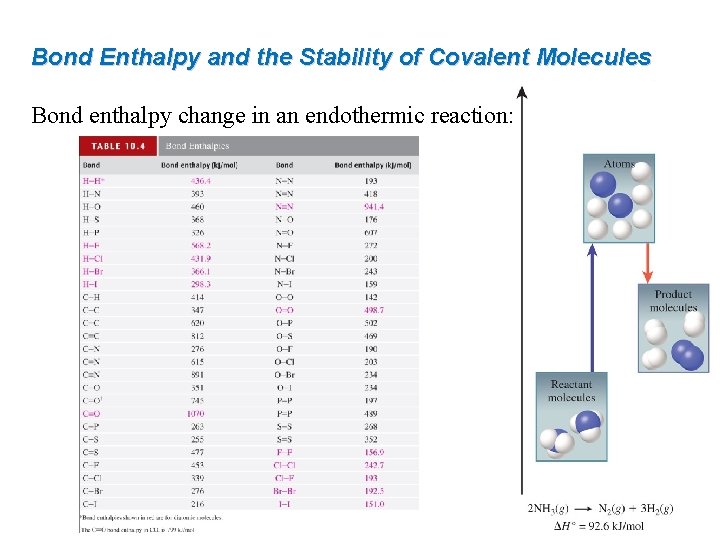
Bond Enthalpy and the Stability of Covalent Molecules Bond enthalpy change in an endothermic reaction:

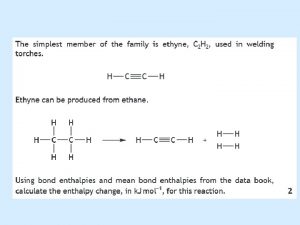
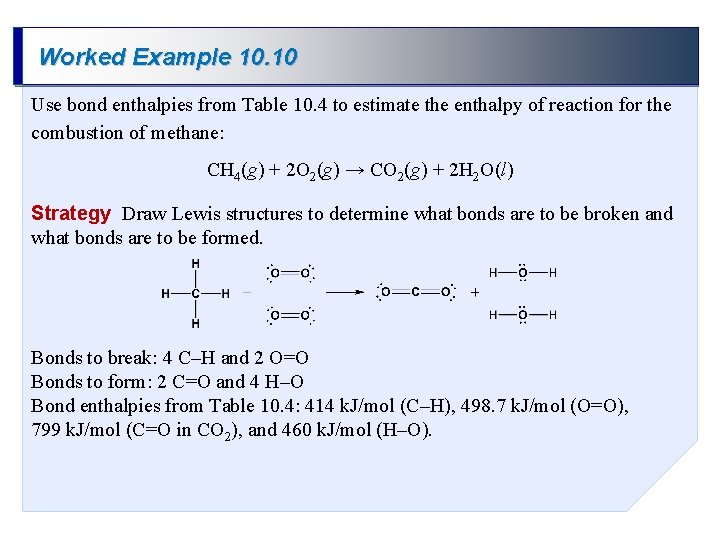
Worked Example 10. 10 Use bond enthalpies from Table 10. 4 to estimate the enthalpy of reaction for the combustion of methane: CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) Strategy Draw Lewis structures to determine what bonds are to be broken and what bonds are to be formed. Bonds to break: 4 C–H and 2 O=O Bonds to form: 2 C=O and 4 H–O Bond enthalpies from Table 10. 4: 414 k. J/mol (C–H), 498. 7 k. J/mol (O=O), 799 k. J/mol (C=O in CO 2), and 460 k. J/mol (H–O).

10. 8 Lattice Energy and Stability of Ionic Solids Lattice Energy relates to how strongly an ionic solid is held together. What holds an ionic solid together? Why would some solids have greater lattice energies than others? Should it have a negative or positive sign associated with it? Text Practice: 10. 81 a) c)

13. 5 Colligative Properties Colligative properties are properties that depend on the number of solute particles in solution. Colligative properties do not depend on the nature of the solute particles. The colligative properties are: Ø vapor-pressure lowering Ø boiling-point elevation Ø freezing-point depression Ø osmotic pressure

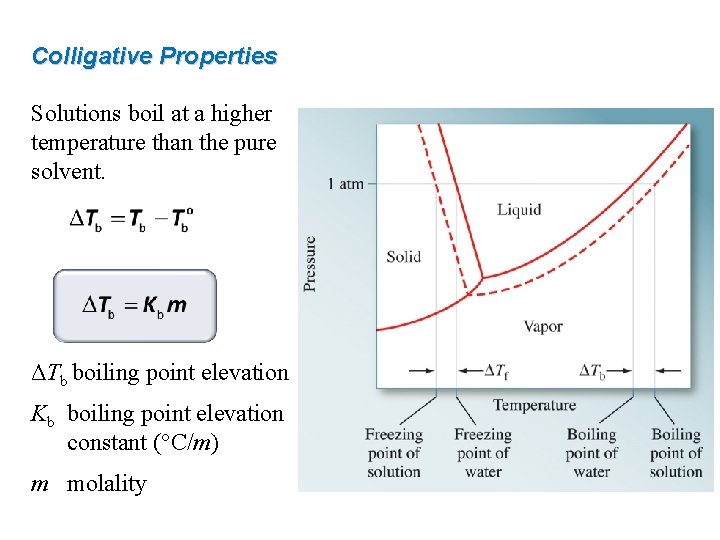
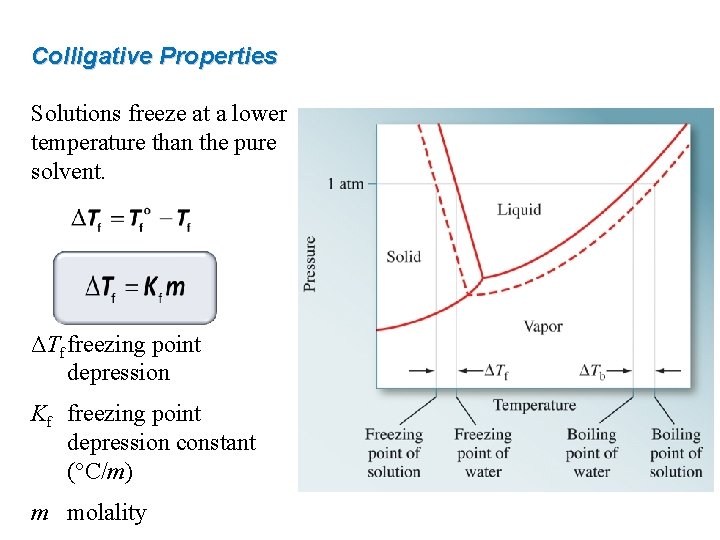
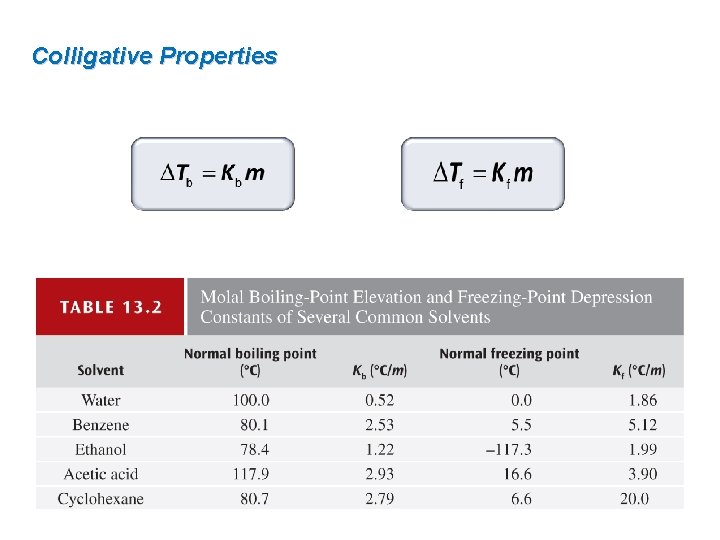
Colligative Properties Solutions boil at a higher temperature than the pure solvent. ΔTb boiling point elevation Kb boiling point elevation constant (°C/m) m molality

Colligative Properties Solutions freeze at a lower temperature than the pure solvent. ΔTf freezing point depression Kf freezing point depression constant (°C/m) m molality

Colligative Properties
![Worked Example 13 6 Ethylene glycol CH 2OH is a common automobile antifreeze It Worked Example 13. 6 Ethylene glycol [CH 2(OH)] is a common automobile antifreeze. It](https://slidetodoc.com/presentation_image_h2/6740e03b9e677bd65d287ee3fa01d3b5/image-17.jpg)
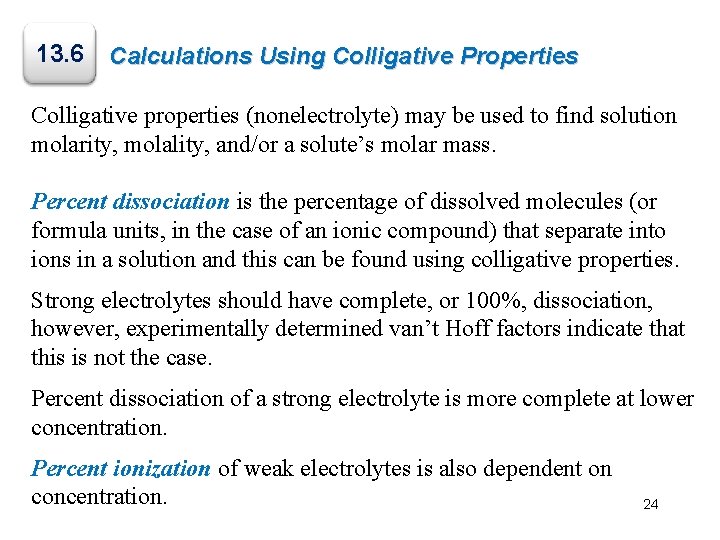
Worked Example 13. 6 Ethylene glycol [CH 2(OH)] is a common automobile antifreeze. It is water soluble and fairly nonvolatile (b. p. 197°C). Calculate (a) the freezing point and (b) the boiling point of a solution containing 685 g of ethylene glycol in 2075 g of water.

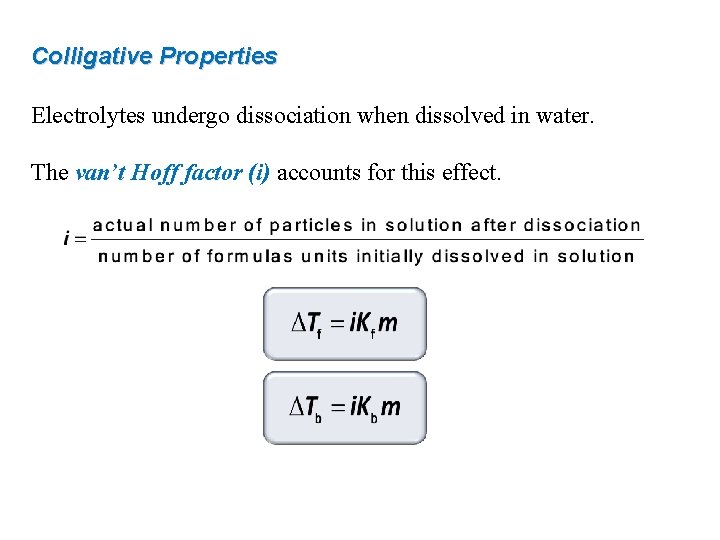
Colligative Properties Electrolytes undergo dissociation when dissolved in water. The van’t Hoff factor (i) accounts for this effect.

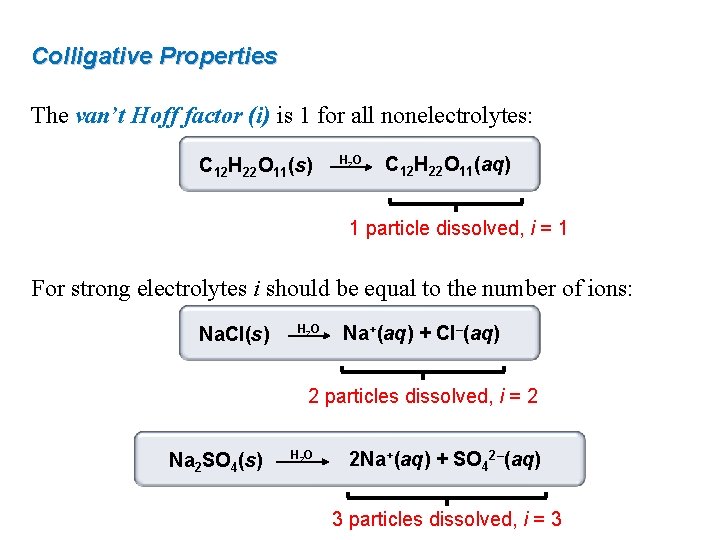
Colligative Properties The van’t Hoff factor (i) is 1 for all nonelectrolytes: C 12 H 22 O 11(s) H 2 O C 12 H 22 O 11(aq) 1 particle dissolved, i = 1 For strong electrolytes i should be equal to the number of ions: Na. Cl(s) H 2 O Na+(aq) + Cl–(aq) 2 particles dissolved, i = 2 Na 2 SO 4(s) H 2 O 2 Na+(aq) + SO 42–(aq) 3 particles dissolved, i = 3

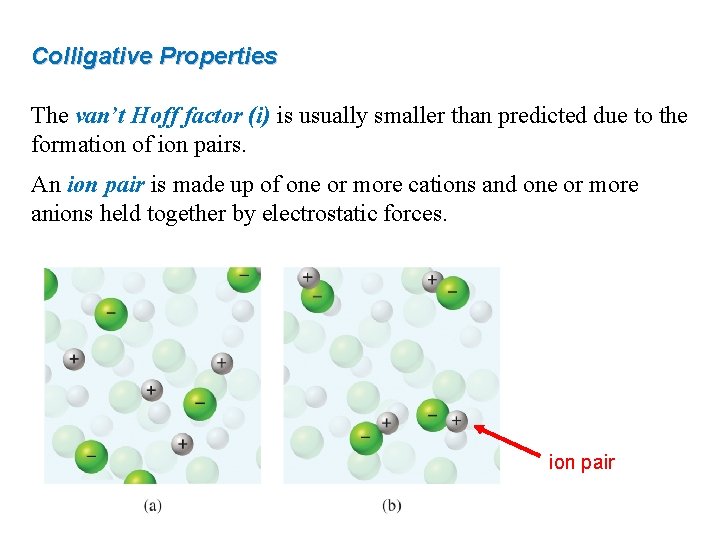
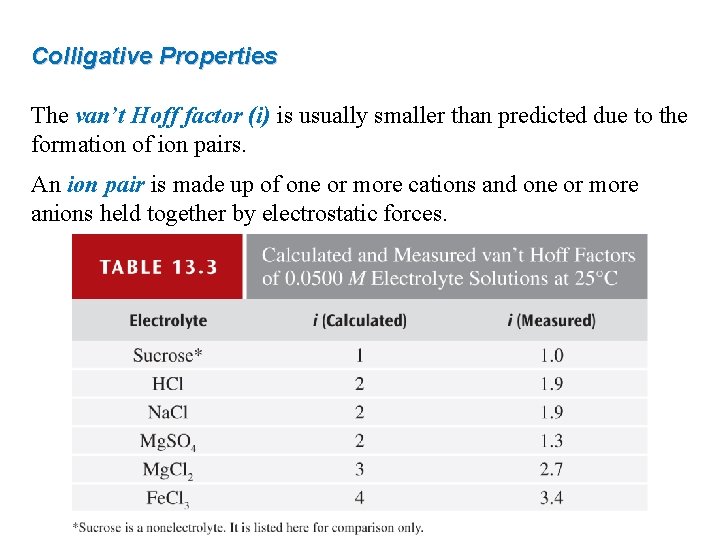
Colligative Properties The van’t Hoff factor (i) is usually smaller than predicted due to the formation of ion pairs. An ion pair is made up of one or more cations and one or more anions held together by electrostatic forces. ion pair

Colligative Properties The van’t Hoff factor (i) is usually smaller than predicted due to the formation of ion pairs. An ion pair is made up of one or more cations and one or more anions held together by electrostatic forces.

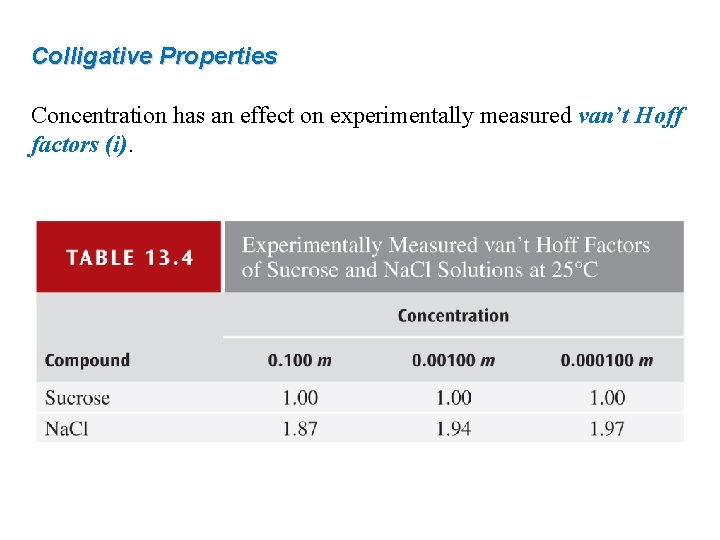
Colligative Properties Concentration has an effect on experimentally measured van’t Hoff factors (i).

Worked Example 13. 8 Quinine was the first drug widely used to treat malaria, and it remains the treatment of choice for severe cases. A solution prepared by dissolving 10. 0 g of quinine in 50. 0 m. L of ethanol has a freezing point 1. 55°C below that of pure ethanol. Determine the molar mass of quinine. (The density of ethanol is 0. 789 g/m. L. ) Assume that quinine is a nonelectrolyte.

13. 6 Calculations Using Colligative Properties Colligative properties (nonelectrolyte) may be used to find solution molarity, molality, and/or a solute’s molar mass. Percent dissociation is the percentage of dissolved molecules (or formula units, in the case of an ionic compound) that separate into ions in a solution and this can be found using colligative properties. Strong electrolytes should have complete, or 100%, dissociation, however, experimentally determined van’t Hoff factors indicate that this is not the case. Percent dissociation of a strong electrolyte is more complete at lower concentration. Percent ionization of weak electrolytes is also dependent on concentration. 24

Study Guide for Sections 10. 5 -10. 8, 13. 5 -13. 6 DAY 27, Terms to know: Sections 10. 5 -10. 8, 13. 5 -13. 6 Hess’s law, standard enthalpy of formation, bond enthalpy, colligative properties, van’t Hoff factor DAY 27, Specific outcomes and skills that may be tested on exam 4: Sections 10. 5 -10. 8, 13. 5 -13. 6 • Be able to use Hess’s law and given heats of reactions to determine the heat of reaction for another reaction • Be able to determine enthalpies of formation for elements in their standard states • Be able to use given enthalpies of formation to determine the enthalpy of a reaction • Given bond enthalpies, be able to determine the enthalpy of reaction for a given reaction • Be able to use a given heat of reaction as a relationship in a conversion question • Be able to explain WHY each of the colligative properties changes upon increasing concentration of solute • Be able to use the formulas for the colligative properties to calculate any of the variables in the equation given the rest of the variables • Be able to predict the van’t Hoff factor for a given solute and use it in the formulas for colligative properties • Be able to explain WHY the van’t Hoff factors for ionic solids at higher concentrations are often less than would normally be predicted

Extra Practice Problems for Sections 10. 5 -10. 8, 13. 5 -13. 6 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 10. 45 10. 47 10. 49 10. 55 10. 57 10. 59 10. 63 10. 65 10. 67 10. 71 10. 75 10. 77 10. 79 10. 85 10. 93 10. 95 10. 135 13. 63 13. 67 13. 69 13. 75 13. 77 13. 79
 Application of hess law
Application of hess law Hesss law
Hesss law Hess law
Hess law Hesss
Hesss Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block xoang nhĩ độ 2
Block xoang nhĩ độ 2 Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt There were 11
There were 11 Northern and southern states
Northern and southern states Tyranny
Tyranny Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Boyle's law charles law avogadro's law
Boyle's law charles law avogadro's law Charles law constant
Charles law constant Ohms law resistance
Ohms law resistance The law of increasing opportunity costs states that
The law of increasing opportunity costs states that Periodic law states
Periodic law states Ohms law states that
Ohms law states that Lambert's law
Lambert's law Mendel's law of independent assortment states that
Mendel's law of independent assortment states that