Periodic Law PERIODIC LAW states that many of






- Slides: 14

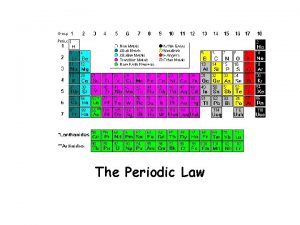
Periodic Law • PERIODIC LAW states that many of the physical and chemical properties of the elements tend to recur in a systematic manner with increasing atomic number. • Properties of elements are periodic functions of their atomic numbers.

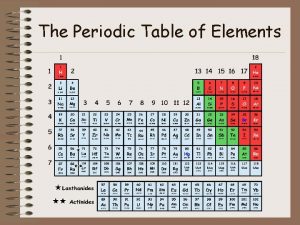
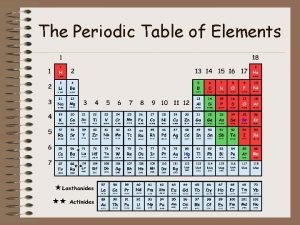
Trends on the Periodic Table • Period - a row of elements on the periodic table. – Remember that sentences are written in rows and end with a period. • Group - a column of elements on the periodic table. – Remember that group is spelled group and groups go up and down.

Element Stability • Elements will tend to lose or gain electrons to become stable!! • Elements are stable when their outermost energy level is full(of electrons)! • Although some energy levels can hold more than 8 electrons, 8 is enough for them to think they’re full. • Eight is the “magic #” except for H and He. • Their magic # is 2!

What happens when Elements gain or lose electrons to become stable? • When atoms gain or lose electrons to become stable, they become “charged”(ions)!! How many electrons will an atom gain or lose to become stable? • The number of electrons gained or lost depends on: 1. How many valence electrons the atom has! 2. What is easiest!! • It is easiest to gain or lose the fewest!

Ex. The alkali metals have 1 valence electron. Is it easier for them to lose 1 or gain 7? Lose 1 Ex. The alkaline earth metals have 2 valence electrons. Is it easier for them to lose 2 or gain 6? Lose 2 Ex. The Boron family has 3 valence electrons. Is it easier for them to lose 3 or gain 5? Lose 3 Ex. The Carbon family has 4 valence electrons. Is it easier for them to lose 4 or gain 4? Neither is easier!!

Ex. The Nitrogen Family has 5 valence electrons. Is it easier for them to lose 5 or gain 3? Gain 3 Ex. The Oxygen Family has 6 valence electrons. Is it easier for them to lose 6 or gain 2? Gain 2 Ex. The Halogens have 7 valence electrons. Is it easier for them to lose 7 or gain 1? Gain 1 Ex. The Nobel gases have 8 valence electrons(He has 2 but is full) They will not gain or lose electrons!!

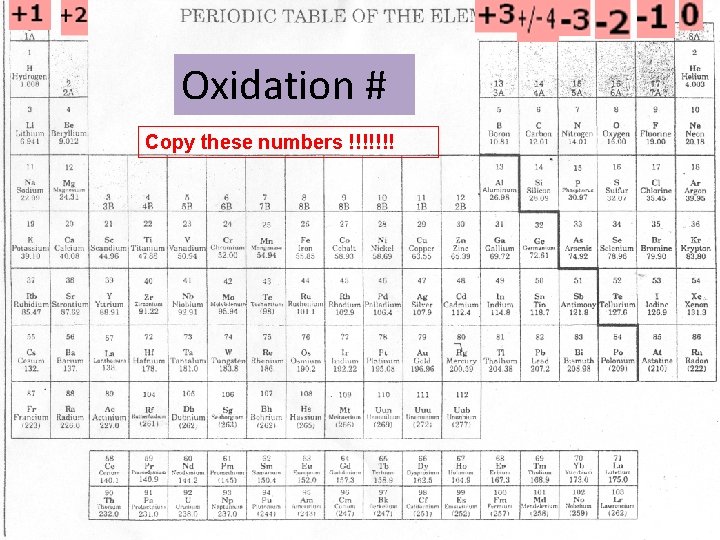
Oxidation Number • The Oxidation number is the “charge” of an atom once it has gained or lost electrons to become stable!

• Alkali metals lose 1 electron – Oxidation # is +1 • Alkaline Earth Metals lose 2 electrons – Oxidation # is +2 • Boron Family loses 3 electrons – Oxidation # is +3 • Carbon Family – Oxidation # is +/- 4 • Nitrogen Family gains 3 electrons – Oxidation # is -3 • Oxygen Family gains 2 electrons – Oxidation # is -2 • Halogens gain 1 electron – Oxidation # is -1 • Nobel Gases are stable – Oxidation # is 0

Oxidation # Copy these numbers !!!!!!!

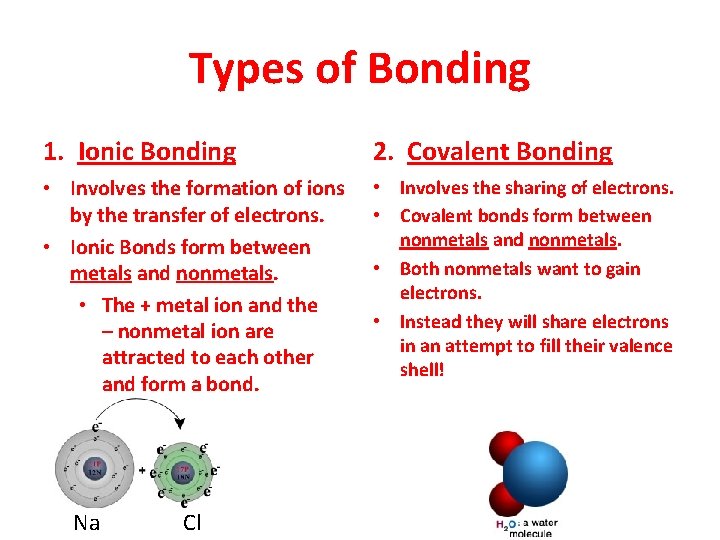
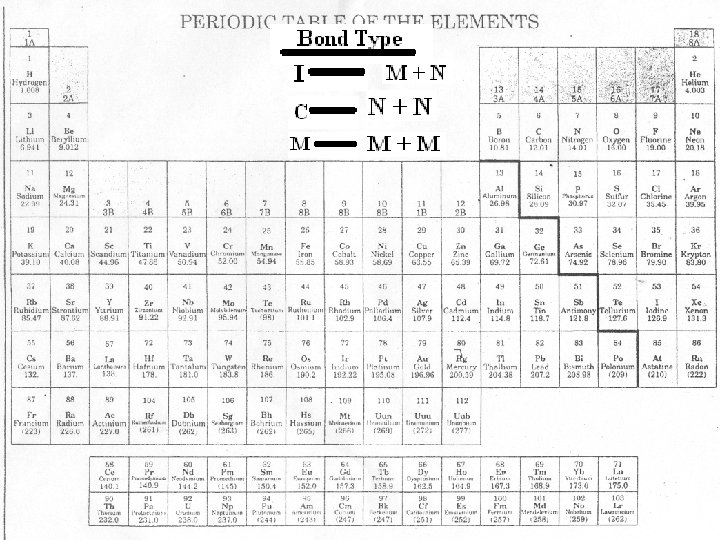
Types of Bonding 1. Ionic Bonding 2. Covalent Bonding • Involves the formation of ions by the transfer of electrons. • Ionic Bonds form between metals and nonmetals. • The + metal ion and the – nonmetal ion are attracted to each other and form a bond. • Involves the sharing of electrons. • Covalent bonds form between nonmetals and nonmetals. • Both nonmetals want to gain electrons. • Instead they will share electrons in an attempt to fill their valence shell! Na Cl

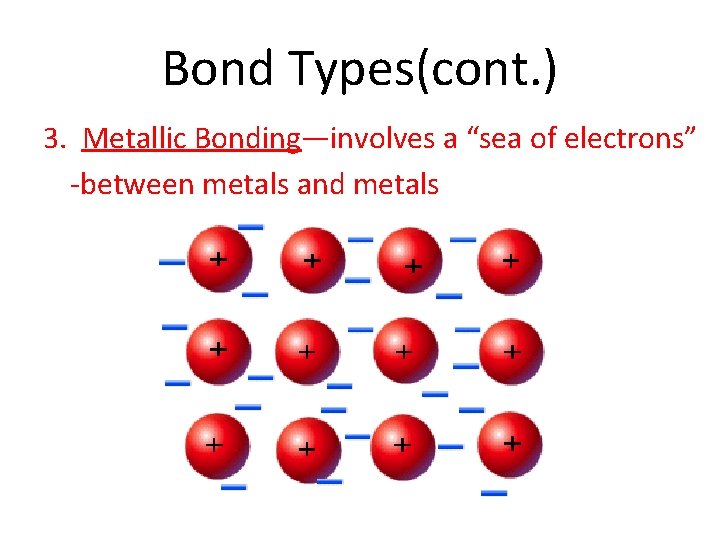
Bond Types(cont. ) 3. Metallic Bonding—involves a “sea of electrons” -between metals and metals

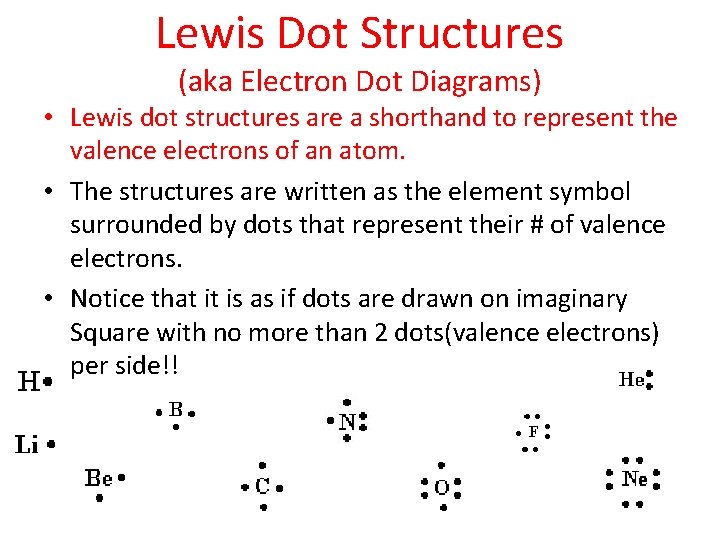
Lewis Dot Structures (aka Electron Dot Diagrams) • Lewis dot structures are a shorthand to represent the valence electrons of an atom. • The structures are written as the element symbol surrounded by dots that represent their # of valence electrons. • Notice that it is as if dots are drawn on imaginary Square with no more than 2 dots(valence electrons) per side!!

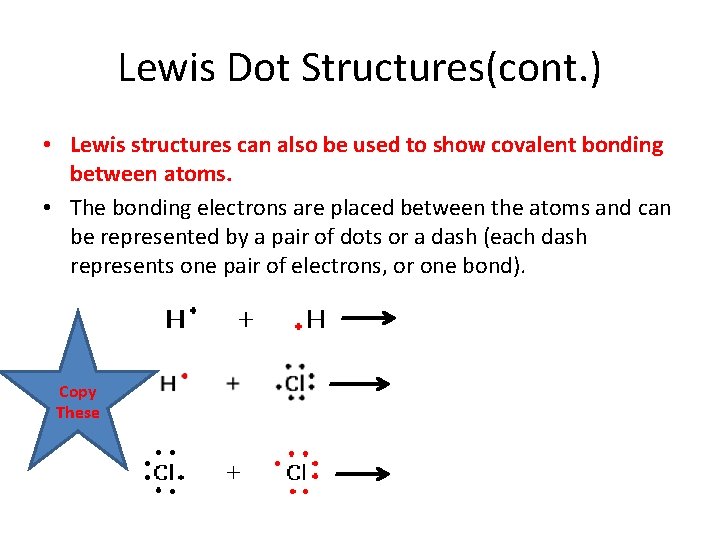
Lewis Dot Structures(cont. ) • Lewis structures can also be used to show covalent bonding between atoms. • The bonding electrons are placed between the atoms and can be represented by a pair of dots or a dash (each dash represents one pair of electrons, or one bond). Copy These

 Phân độ lown
Phân độ lown Block nhĩ thất độ 2 type 1
Block nhĩ thất độ 2 type 1 Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Periodic law states
Periodic law states Chapter 6 the periodic table
Chapter 6 the periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Chapter 5 periodic law
Chapter 5 periodic law What were the 11 free states
What were the 11 free states