Chapter 5 The Periodic Law Section 5 1












- Slides: 20

Chapter 5 The Periodic Law

Section 5. 1 History of the Periodic Table

1860’s • 63 elements discovered so far • no standardized method to compare atomic masses • Different chemists used different methods…very difficult to understand each other’s results

1860 – Conference at Karlsruhe • Stanislao Cannizzaro introduced a method for accurately measuring the relative masses of atoms • chemists now agree on standard values for atomic mass • Now they could focus on finding relationships between atomic mass and other properties of the elements

Dmitri Mendeleev (Russian) • used the new mass values in a textbook that he was writing • tried to organize the elements according to their chemical and physical properties.

Chemical Periodicity • Dmitri noticed that when the elements were arranged in order of increasing atomic mass, certain similarities in their chemical properties appeared at regular intervals

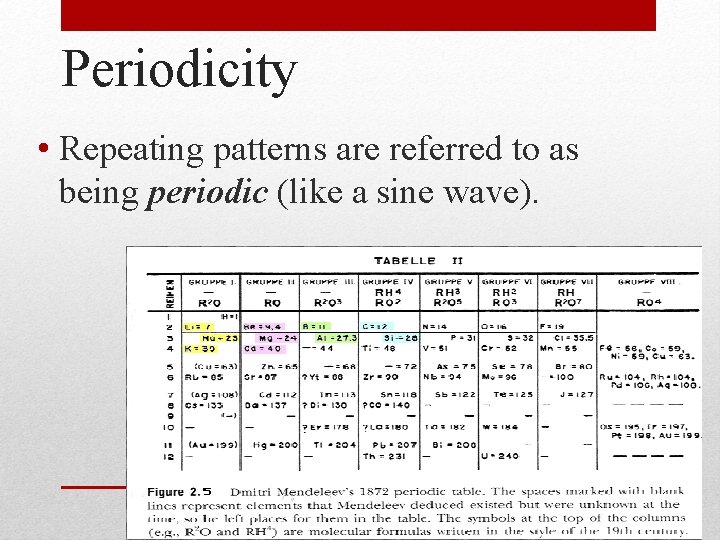
Periodicity • Repeating patterns are referred to as being periodic (like a sine wave).

• Published first periodic table by arranging cards of elements by their properties. • Broke the trend of arranging by mass number to have properties aligned (I[127] after Te[128]; boldly stated that some masses had been miscalculated!!). • Left gaps for predicted unknown elements. (ex. ekasilicon=germanium) Dmitri Mendeleev: (1869)

• By 1886, 3 missing elements were discovered • Mendeleev’s predictions were accurate, most chemists accepted his periodic table • Questions remained – • why did some elements need to be placed out of order of their atomic mass? • What was the reason for chemical periodicity? Dmitri Mendeleev: (1869)

Henry Moseley - 1911 • Examined the spectra of 38 different metals. • Realized that the elements in the periodic table were arranged in order of increasing nuclear charge (i. e. , atomic number) • Consistent with Mendeleev’s ordering of elements by properties (Te – 52; I - 53)

Henry Moseley: (1911) • Established Periodic Law by determining atomic numbers of elements.

Periodic Law • Periodic Law - the physical and chemical properties of the elements are periodic functions of their atomic numbers. • elements are arranged by increasing atomic number, not mass; • elements with similar properties fall in the same group.

The Modern Periodic Table • An arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column, or group.

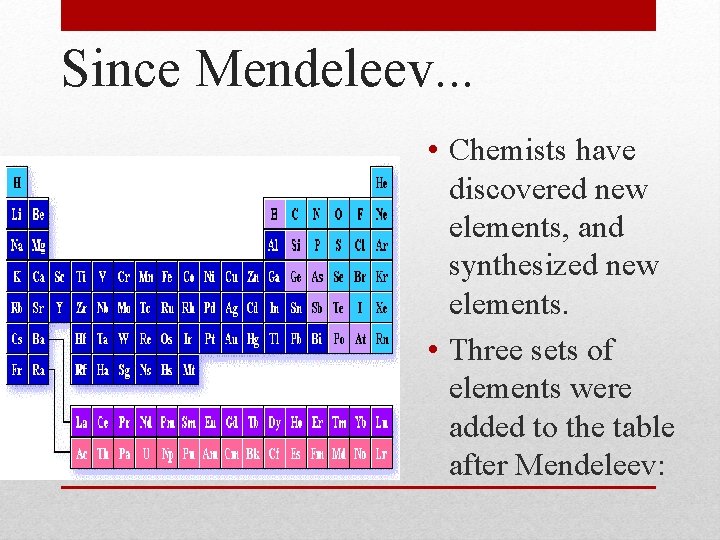
Since Mendeleev. . . • Chemists have discovered new elements, and synthesized new elements. • Three sets of elements were added to the table after Mendeleev:

Noble Gases (Group 18) • 1894 Rayleigh and Ramsay discovered argon, and other noble gases were later discovered. • Due to their unreactivity a new group was proposed.

The Lanthanides (rare earths) • 14 elements with atomic numbers from 58 to 71. • These elements are so similar in their chemical and physical properties that separating and identifying them was difficult.

The Actinides • 14 elements with atomic numbers from 90 to 103. • Extremely rare and those beyond element #92 (U) are synthesized.

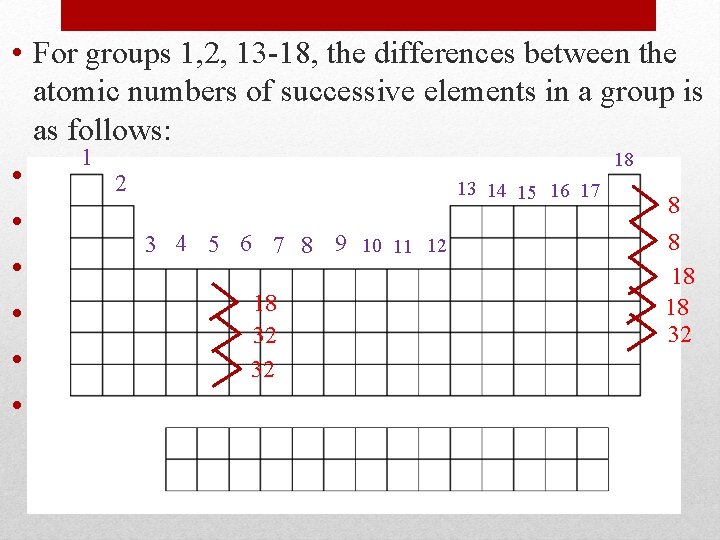
• For groups 1, 2, 13 -18, the differences between the atomic numbers of successive elements in a group is as follows: 1 18 • Li atomic #=3 2 13 14 15 16 17 8 • Na atomic # = 11 (8 protons) 8 3 4 5 6 7 8 9 10 11 12 • K atomic # = 19 (8 protons) 18 18 18 • Rb atomic # = 37 (18 protons) 32 32 • Cs atomic # = 5532 (18 protons) • Fr atomic # = 87 (32 protons) Periodicity

Is explained by the arrangement of the number of electrons around the nucleus (maximum outer level electrons are shown below): • Period 1 - 1 s 2 • period 2 - 2 s 22 p 6 • period 3 - 3 s 23 p 6 • period 4 - 4 s 23 d 104 p 6 • period 5 - 5 s 24 d 105 p 6 • Period 6 – 6 s 24 f 145 d 106 p 6 The Reason for Periodicity

Assignment • 5. 1 Textbook Problems